

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (12): 2645.doi: 10.7503/cjcu20140510
• Physical Chemistry • Previous Articles Next Articles
LIU Cui*( ), ZHANG Qianhui, GONG Lidong, LU Linan, YANG Zhongzhi*(
), ZHANG Qianhui, GONG Lidong, LU Linan, YANG Zhongzhi*( )
)
Received:2014-06-04
Online:2014-12-10
Published:2014-11-29
Contact:
LIU Cui,YANG Zhongzhi
E-mail:liuc@lnnu.edu.cn;zzyang@lnnu.edu.cn
Supported by:CLC Number:
TrendMD:
LIU Cui, ZHANG Qianhui, GONG Lidong, LU Linan, YANG Zhongzhi. Theoretical Studies on the Effect of Fapy-G on Base Pair Hydrogen Bond Complexes†[J]. Chem. J. Chinese Universities, 2014, 35(12): 2645.
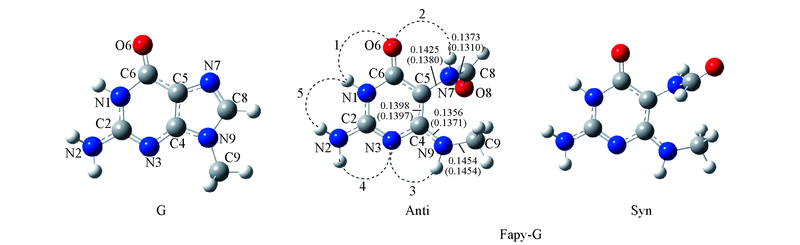
Fig.1 Geometry of G and the syn and anti geometries of Fapy-G The numbers without parentheses are the bond length(nm) in Fapy-G monomer. The numbers in parentheses denote the bond length(nm) in G monomer. The numbers 1—5 denote these regions, at which Fapy-G pairs with A, T, G, or C.
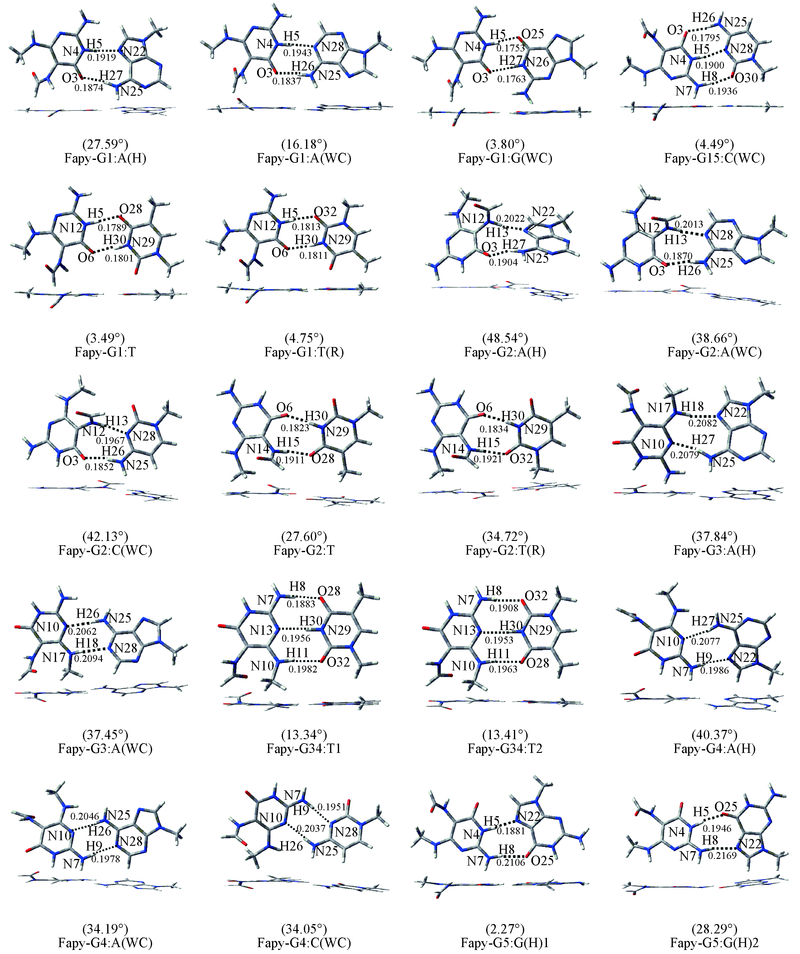
Fig.2 Oxidized base pairs containing Fapy-G WC represents Watson-Crick hydrogen bonding pattern. H represents Hoogsteen hydrogen bonding pattern. R represents reverse Watson-Crick or reverse Hoogsteen hydrogen bonding pattern.The hydrogen bond length are in nm. The number in bracket is the deviation angle.
| Molecule | ν1/cm-1 | ν6a/cm-1 | ν8a/cm-1 | ν12/cm-1 |
|---|---|---|---|---|
| Fapy-G | 1058.65 | 610.98 | 1640.02 | 1293.97 |
| Fapy-G3:A(H) | 1060.63 | 612.45 | 1641.10 | 1299.20 |
| Fapy-G3:A(WC) | 1061.16 | 612.44 | 1641.30 | 1300.55 |
| Fapy-G4:A(H) | 1064.21 | 612.71 | 1633.37 | 1288.29 |
| Fapy-G4:A(WC) | 1064.98 | 614.25 | 1634.57 | 1289.78 |
| Fapy-G4:C(WC) | 1066.50 | 614.12 | 1636.79 | 1289.73 |
| Fapy-G34:T1 | 1071.58 | 615.72 | 1636.70 | 1303.04 |
| Fapy-G34:T2 | 1071.49 | 615.50 | 1637.90 | 1303.91 |
Table 1 Frequencies of the four kinds of infrared vibrational modes in monomer Fapy-G and the base pairs formed hydrogen bond at 3,4 regions
| Molecule | ν1/cm-1 | ν6a/cm-1 | ν8a/cm-1 | ν12/cm-1 |
|---|---|---|---|---|
| Fapy-G | 1058.65 | 610.98 | 1640.02 | 1293.97 |
| Fapy-G3:A(H) | 1060.63 | 612.45 | 1641.10 | 1299.20 |
| Fapy-G3:A(WC) | 1061.16 | 612.44 | 1641.30 | 1300.55 |
| Fapy-G4:A(H) | 1064.21 | 612.71 | 1633.37 | 1288.29 |
| Fapy-G4:A(WC) | 1064.98 | 614.25 | 1634.57 | 1289.78 |
| Fapy-G4:C(WC) | 1066.50 | 614.12 | 1636.79 | 1289.73 |
| Fapy-G34:T1 | 1071.58 | 615.72 | 1636.70 | 1303.04 |
| Fapy-G34:T2 | 1071.49 | 615.50 | 1637.90 | 1303.91 |
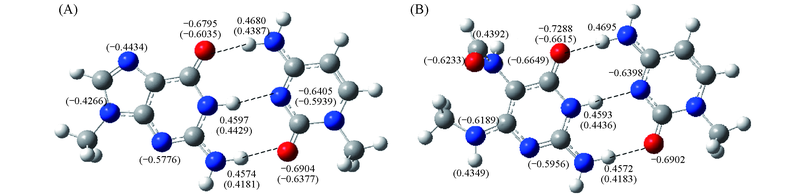
Fig.4 Natural atomic charges(e) formed hydrogen bond in G:C(A) and Fapy-G15:C(WC)(B) base pairs The numbers in parentheses mean natural atomic charges(e) in G, Fapy-G or C monomer. The numbers without parentheses mean natural atomic charges(e) of base pairs.
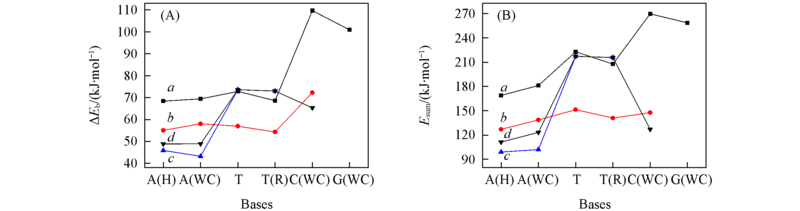
Fig.5 Order of binding energy(ΔEb)(A) and second-order stabilization energies (Esum)(B) of base pairs a. Region 1; b. region 2; c. region 3; d. region 4.
| [1] | Greenberg M. M., Acc. Chem. Res., 2012, 45(4), 588—597 |
| [2] | Lord C. J., Ashworth A., Nature,2012, 481, 287—294 |
| [3] | Crenshaw C. M., Wade J. E., Arthanari H., Frueh D., Lane B. F., Núñez M. E., Biochemistry, 2011, 50(39), 8463—8477 |
| [4] | Naôme A., Schyman P., Laaksonen A., Vercauteren D. P., J. Phys. Chem. B, 2010, 114(14), 4789—4801 |
| [5] | Hamm M. L., Crowley K. A., Ghio M., Lindell M. A. M., McFadden E. J., Silberg J. S. L., Weaver A. M., Chem. Res. Toxicol., 2012, 25(11), 2577—2588 |
| [6] | Lindahl T., Nature, 1993, 362, 709—715 |
| [7] | Volle C. B., Jarem D. A., Delaney S., Biochemistry,2012, 51(1), 52—62 |
| [8] | Crenshaw C. M., Wade J. E., Arthanari H., Frueh D., Lane B. F., Núñez M. E., Biochemistry, 2011, 50(39), 8463—8477 |
| [9] | Demple B., Annu. Rev. Biochem., 1994, 63, 915—948 |
| [10] | Cysewskia P., Jeziorek D., Olifiskia R., J. Mol. Struct.(Theochem.), 1996, 369, 93—104 |
| [11] | Sun Y. P., Ren X. H., Wang H. J., Struct. Chem., 2009, 20(2), 213—220 |
| [12] | Gurena C. F., Bickelhaupt F. M., Baerends E. J., Crystal Growth & Design, 2002, 2(3), 239—245 |
| [13] | He Q., Zhou L. X., Acta Phys.-Chim. Sin., 2005, 21(8), 846—851 |
| (和芹, 周立新. 物理化学学报, 2005, 21(8), 846—851) | |
| [14] | Liu T. T., Zhao L. J., Zhong R. G., Chem. J. Chinese Universities, 2010, 31(5), 957—963 |
| (刘婷婷, 赵丽娇, 钟儒刚. 高等学校化学学报, 2010, 31(5), 957—963) | |
| [15] | Qiu Z. M., Xia Y. M., Wang H. J., Diao K. S., J. Mol. Struct.(Theochem.), 2009, 915, 33—37 |
| [16] | Qiu Z. M., Xia Y. M., Wang H. J., Diao K. S., Struct. Chem., 2010, 21(1), 99—105 |
| [17] | Qiu Z. M., Wang H. J., Xia Y. M., Struct. Chem., 2010, 21(5), 931—937 |
| [18] | Lin X. F., Sun C. K., Yang S. Y., Yu S. W., Yao L. F., Chen Y. S., Acta Chim. Sinica, 2011, 69(23), 2787—2795 |
| (林雪飞, 孙成科, 杨思娅, 余仕问, 姚立峰, 陈益山. 化学学报, 2011, 69(23), 2787—2795) | |
| [19] | Suzuki M., Kino K., Morikawa M., Kobayashi T., Komori R., Miyazawa H., Molecules,2012, 17(6), 6705—6715 |
| [20] | Mukherjee S., Majumdar M., Bhattacharyya D., J. Phys. Chem. B, 2005, 109(20), 10484—10492 |
| [21] | Rutledge L. R., Navarro-Whyte L., Peterson T. L., Wetmore S. D., J. Phys. Chem. A, 2011, 115(45), 12646—12658 |
| [22] | Huo H. J., Zhao D. X., Yang Z. Z., Chem. J. Chinese Universities, 2011, 32(12), 2877—2884 |
| (霍红洁, 赵东霞, 杨忠志. 高等学校化学学报, 2011, 32(12), 2877—2884) | |
| [23] | Wang C. S., Liu P., Yu N., Acta Phys.-Chim. Sin., 2013, 29(6), 1173—1182 |
| (王长生, 刘朋, 于楠. 物理化学学报, 2013, 29(6), 1173—1182) | |
| [24] | Liu C.,Wang Y., Zhao D. X., Gong L. D., Yang Z. Z., Theor. Chem. Acc., 2014, 133, 1469—1482 |
| [25] | Li A. Y., Cao L. J., Ji H. B., Xu L., Zhang Y., J. Mol. Struct.(Theochem.), 2010, 959, 80—86 |
| [26] | Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Balkken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas ϕ., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision A. 02, Gaussian Inc., Wallingford CT, 2009 |
| [27] | Glendening E. D., Reed A. E., Carpenter J. E., Weinhold F., NBO Version 3.1 Gaussian 09, Revision A. 02, Gaussian In., Wallingford CT, 2009 |
| [1] | MIN Jing, WANG Liyan. 1H NMR Study on the Conformation of Aromatic Amides Limited by Three-center Hydrogen Bonds [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220084. |
| [2] | ZHANG Yong, XU Jun, BAO Yu, CUI Shuxun. Quantifying the Degree of Weakening Effect of Nonpolar Organic Solvent on the Strength of Intramolecular Hydrogen Bonding by Single-molecule Force Spectroscopy [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210863. |
| [3] | CUI Shaoli, ZHANG Weijia, SHAO Xueguang, CAI Wensheng. Revealing the Effect of Threonine on the Binding Ability of Antifreeze Proteins with Ice Crystals by Free-energy Calculations [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210838. |
| [4] | HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614. |
| [5] | GAO Huiling, CAO Zhenzhen, GU Fang, WANG Haijun. Monte Carlo Simulation on Self-healing Behaviour of Hydrogen-bonded Hydrogel [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220482. |
| [6] | WANG Le, QIN Liulei, LIU Yang, REN Li, XU Huiting, LIU Zunqi. Synthesis, Structure and Dielectric Properties of One-dimensional Chain Hydrogen Glycine Supramolecular Compound [(Gly)2+(18-crown-6)2(MnCl4)2‒] [J]. Chem. J. Chinese Universities, 2021, 42(3): 691. |
| [7] | NI Qingsheng, DU Miao, SHAN Guorong, SONG Yihu, WU Ziliang, ZHENG Qiang. Regulation of Rheological Behavior of Polyvinyl Alcohol Aqueous Solution by One-dimensional Particles [J]. Chem. J. Chinese Universities, 2021, 42(12): 3738. |
| [8] | GONG Shanshan, WU Tong, WANG Guange, HUANG Qing, SU Yuefeng, WU Feng. Screening of Deep Eutectic Solvent Based on Efficient Recovery of Spent Lithium⁃ion Battery Cathode Materials [J]. Chem. J. Chinese Universities, 2021, 42(10): 3151. |
| [9] | BAI Lan, ZHAI Lei, WANG Changou, HE Minhui, MO Song, FAN Lin. Thermal Expansion Behavior of Amide-containing Polyimide Films with Ultralow Thermal Expansion Coefficient † [J]. Chem. J. Chinese Universities, 2020, 41(4): 795. |
| [10] | QIN Liulei,LIU Yang,GUAN Xiaoqin,ZHENG Xiaoyuan,ZHANG Ziyu,LIU Zunqi. Synthesis and Switchable Dielectric Properties of an Inorganic-organic Hybrid Complex [H2(DABCO)CuCl4]·H2O † [J]. Chem. J. Chinese Universities, 2020, 41(1): 70. |
| [11] | XU Yan,LIU Cui,HAN Chengjuan,PAN Mingyu,SUN Zhaoqi,HAN Bingyu,YANG Zhongzhi. Development of Polarization Force Field for Guanine and Amino Acid Residues Systems† [J]. Chem. J. Chinese Universities, 2019, 40(2): 288. |
| [12] | XU Yu,HUA Er. Hydrogen Bonding Study on Protic Ionic Liquids Composed of N-Alkyl Ethylenediaminum Cations with Trifluoroacetic Anion† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1954. |
| [13] | OUYANG Shunli, ZHANG Mingzhe, ZHANG Yongzhao, HU Qingcheng, WEI Haiyan, WU Nannan, HUANG Baokun. Raman Spectroscopic Investigation on the Effect of Hydrogen Bond on Molecular Structure in Ternary Aqueous Solution† [J]. Chem. J. Chinese Universities, 2018, 39(4): 758. |
| [14] | LI Jinxing,XING Xiaofeng,QI Zhongnan,AI Hongqi. Effects of Three New Modified Molecules on the Structural Stability of Different Aβ42 Fibers† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2230. |
| [15] | HAN Bingyu, LI Yue, LIU Cui. Investigation on the Hydrogen Bonding Interaction Between Amino Acid Side Chains and Base Pairs Containing Oxidized Guanine† [J]. Chem. J. Chinese Universities, 2017, 38(6): 1068. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||