

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (10): 2230.doi: 10.7503/cjcu20180434
• Physical Chemistry • Previous Articles Next Articles
LI Jinxing, XING Xiaofeng, QI Zhongnan*( ), AI Hongqi*(
), AI Hongqi*( )
)
Received:2018-06-13
Online:2018-09-14
Published:2018-09-14
Contact:
QI Zhongnan,AI Hongqi
E-mail:chm_qizn@ujn.edu.cn;chm_aihq@ujn.edu.cn
Supported by:CLC Number:
TrendMD:
LI Jinxing,XING Xiaofeng,QI Zhongnan,AI Hongqi. Effects of Three New Modified Molecules on the Structural Stability of Different Aβ42 Fibers†[J]. Chem. J. Chinese Universities, 2018, 39(10): 2230.
| Name | Component | Name | Component |
|---|---|---|---|
| 2BEG | Aβ42 pentamer | 2ME2 | 2MXU+12×E2C |
| 2BE1 | 2BEG+10×E1C | 2MTS | 2MXU+12×TSC |
| 2BE2 | 2BEG+10×E2C | 5KK3 | Aβ42 di-hexamer |
| 2BTS | 2BEG+10×TSC | 5KE1 | 5KK3+24×E1C |
| 2MXU | Aβ42 hexamer | 5KE2 | 5KK3+24×E2C |
| 2ME1 | 2MXU+12×E1C | 5KTS | 5KK3+24×TSC |
Table 1 Definitions for dynamic systems
| Name | Component | Name | Component |
|---|---|---|---|
| 2BEG | Aβ42 pentamer | 2ME2 | 2MXU+12×E2C |
| 2BE1 | 2BEG+10×E1C | 2MTS | 2MXU+12×TSC |
| 2BE2 | 2BEG+10×E2C | 5KK3 | Aβ42 di-hexamer |
| 2BTS | 2BEG+10×TSC | 5KE1 | 5KK3+24×E1C |
| 2MXU | Aβ42 hexamer | 5KE2 | 5KK3+24×E2C |
| 2ME1 | 2MXU+12×E1C | 5KTS | 5KK3+24×TSC |
| Complex | Probability(%) | Complex | Probability(%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coil | β-Sheet | Bend | Turn | Coil | β-Sheet | Bend | Turn | ||
| 2BEG | 29 | 54 | 14 | 1 | 2ME2 | 15 | 74 | 7 | 1 |
| 2BE1 | 30 | 52 | 15 | 1 | 2MTS | 18 | 71 | 7 | 2 |
| 2BE2 | 30 | 51 | 12 | 2 | 5KK3 | 30 | 55 | 12 | 1 |
| 2BTS | 26 | 54 | 13 | 3 | 5KE1 | 28 | 62 | 8 | 0 |
| 2MXU | 19 | 72 | 6 | 1 | 5KE2 | 29 | 56 | 12 | 1 |
| 2ME1 | 14 | 78 | 6 | 1 | 5KTS | 30 | 57 | 10 | 1 |
Table 2 Probability of secondary structures of the 2BEG, 2MXU or 5KK3 in the absence or presence of E1C/E2C/TSC
| Complex | Probability(%) | Complex | Probability(%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coil | β-Sheet | Bend | Turn | Coil | β-Sheet | Bend | Turn | ||
| 2BEG | 29 | 54 | 14 | 1 | 2ME2 | 15 | 74 | 7 | 1 |
| 2BE1 | 30 | 52 | 15 | 1 | 2MTS | 18 | 71 | 7 | 2 |
| 2BE2 | 30 | 51 | 12 | 2 | 5KK3 | 30 | 55 | 12 | 1 |
| 2BTS | 26 | 54 | 13 | 3 | 5KE1 | 28 | 62 | 8 | 0 |
| 2MXU | 19 | 72 | 6 | 1 | 5KE2 | 29 | 56 | 12 | 1 |
| 2ME1 | 14 | 78 | 6 | 1 | 5KTS | 30 | 57 | 10 | 1 |
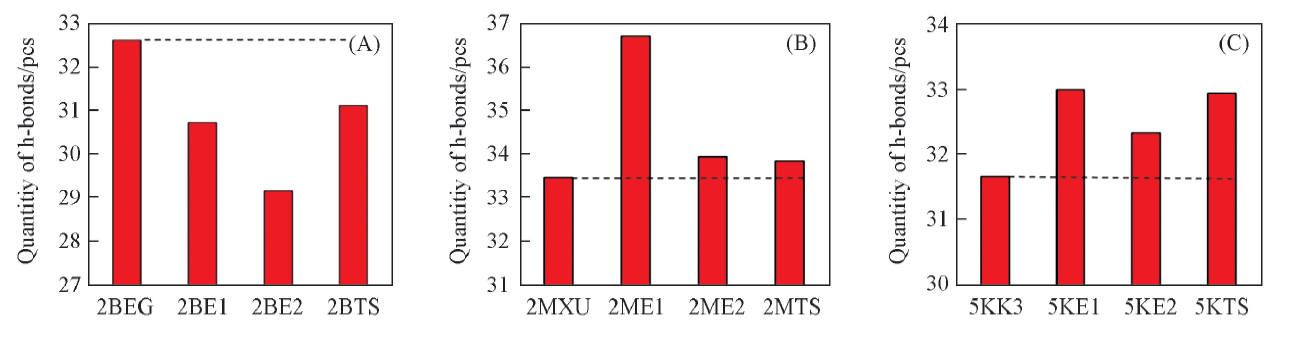
Fig.3 Quantity of hydrogen bonds of each monomer in 2BEG, 2BE1, 2BE2 and 2BTS(A), 2MXU, 2ME1, 2ME2 and 2MTS(B) or 5KK3, 5KE1, 5KE2 and 5KTS(C), obtained by averaging the values of all the fibrous monomers
| Complex | ΔEvdw/(kJ·mol-1) | ΔEeler/(kJ·mol-1) | ΔEGB/(kJ·mol-1) | ΔEnp/(kJ·mol-1) | ΔEb/(kJ·mol-1) |
|---|---|---|---|---|---|
| 2BE1 | -129.5 | 275.3 | 218.3 | -16.1 | 348.0 |
| 2BE2 | -297.3 | 388.8 | 167.5 | -27.8 | 231.2 |
| 2BTS | -260.0 | -19.5 | 117.9 | -25.5 | -187.1 |
| 2ME1 | -128.5 | 302.9 | 263.3 | -15.4 | 422.2 |
| 2ME2 | -197.5 | 362.7 | 173.8 | -18.4 | 320.7 |
| 2MTS | -232.4 | -53.2 | 126.3 | -20.5 | -179.8 |
| 5KE1 | -123.4 | 493.9 | 156.1 | -15.4 | 511.2 |
| 5KE2 | -241.7 | 677.1 | 119.9 | -22.2 | 533.1 |
| 5KTS | -225.6 | -32.4 | 134.8 | -21.4 | -144.6 |
Table 3 Binding free energies between E1C/E2C/TSC and each monomer of 2BEG, 2MXU or 5KK3, obtained by the MM-PBSA method
| Complex | ΔEvdw/(kJ·mol-1) | ΔEeler/(kJ·mol-1) | ΔEGB/(kJ·mol-1) | ΔEnp/(kJ·mol-1) | ΔEb/(kJ·mol-1) |
|---|---|---|---|---|---|
| 2BE1 | -129.5 | 275.3 | 218.3 | -16.1 | 348.0 |
| 2BE2 | -297.3 | 388.8 | 167.5 | -27.8 | 231.2 |
| 2BTS | -260.0 | -19.5 | 117.9 | -25.5 | -187.1 |
| 2ME1 | -128.5 | 302.9 | 263.3 | -15.4 | 422.2 |
| 2ME2 | -197.5 | 362.7 | 173.8 | -18.4 | 320.7 |
| 2MTS | -232.4 | -53.2 | 126.3 | -20.5 | -179.8 |
| 5KE1 | -123.4 | 493.9 | 156.1 | -15.4 | 511.2 |
| 5KE2 | -241.7 | 677.1 | 119.9 | -22.2 | 533.1 |
| 5KTS | -225.6 | -32.4 | 134.8 | -21.4 | -144.6 |
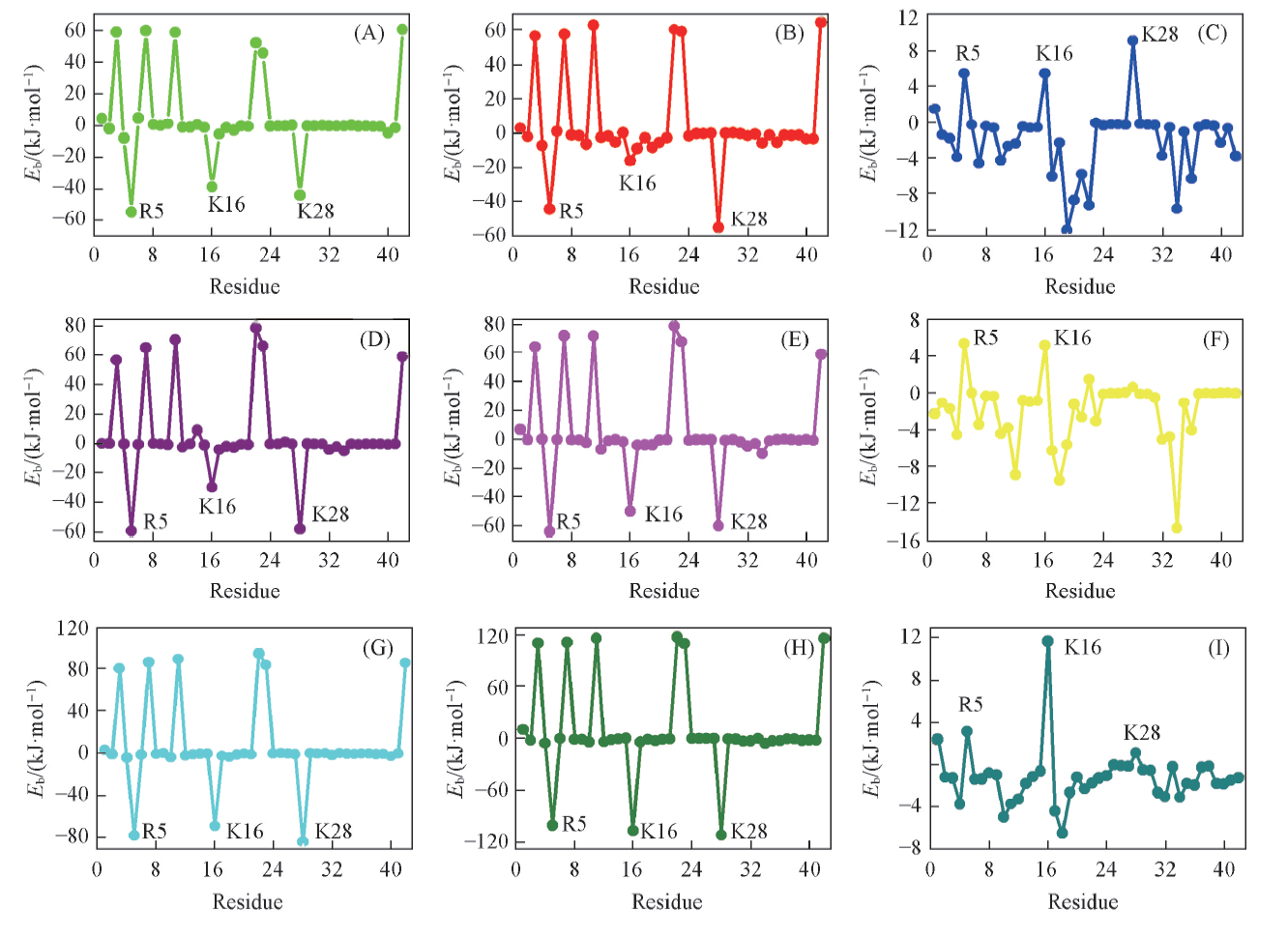
Fig.4 Contribution of each residue to the binding free energy in a Aβ42 monomer of 2BE1(A), 2BE2(B), 2BTS(C), 2ME1(D), 2ME2(E), 2MTS(F), 5KE1(G), 5KE2(H) and 5KTS(I), obtained by averaging the values of all the fibrous monomers
| [1] | Selkoe D.J., Science, 2002, 298(5594), 789—791 |
| [2] | Andreini C., Bertini I., Cavallaro G., Holliday G.L., Thornton J. M., J. Biol. Inorg. Chem., 2008, 13(8), 1205—1218 |
| [3] | Kang J., Lemaire H.G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B., Nature, 1987, 325(6106), 733—736 |
| [4] | Dahlgren K.N., Manelli A. M., Stine W. B., Baker L. K., Krafft G. A., LaDu M. J., J. Biol. Chem., 2002, 277(35), 32046—32053 |
| [5] | Petkova A.T., Ishii Y., Balbach J. J., Antzutkin O. N., Leapman R. D., Delaglio F., Tycko R., Proc. Natl. Acad. Sci.USA, 2002, 99(26), 16742—16747 |
| [6] | Xu L., Nussinov R., Ma B., Chem. Commun., 2016, 52(8), 1733—1736 |
| [7] | Adler J., Scheidt H.A., Lemmnitzer K., Krueger M., Huster D., Phys. Chem. Chem. Phys., 2017, 19(3), 1839—1846 |
| [8] | Szczepankiewicz O., Linse B., Meisl G., Thulin E., Frohm B., Sala Frigerio C., Colvin M.T., Jacavone A. C., Griffin R. G., Knowles T., Walsh D. M., Linse S., J. Am. Chem. Soc., 2015, 137(46), 14673—14685 |
| [9] | Lee J., Kwon I., Jang S.S., Cho A. E., J. Mol. Model., 2016, 22(4), 1—9 |
| [10] | Dong M., Zhao W., Hu D., Ai H., Kang B., ACS Chem.Neurosci., 2017, 8(7), 1577—1588 |
| [11] | Geng J., Li M., Wu L., Ren J., Qu X., J. Med. Chem., 2012, 55(21), 9146—9155 |
| [12] | Braymer J.J., Detoma A. S., Choi J. S., Ko Kristin S., Lim Mi H., Int. J. Alzheimers Dis., 2011, 2011(5), 623051 |
| [13] | Dong M., Li H., Hu D., Zhao W., Zhu X., Ai H., ACS Chem.Neurosci., 2016, 7(5), 599—614 |
| [14] | Luhrs T., Ritter C., Adrian M., Riek-Loher D., Bohrmann B., Dobeli H., Schubert D., Riek R., Proc. Natl. Acad. Sci. USA, 2005, 102(48), 17342—17347 |
| [15] | Xiao Y., Ma B., Nat. Struct. Mol. Biol., 2015, 22(6), 499—505 |
| [16] | Colvin M.T., Silvers R., Ni Q. Z., Can T. V., Sergeyev I., Rosay M., Donovan K. J., Michael B., Wall J., Linse S., Griffin R. G., J. Am. Chem. Soc., 2016, 138(30), 9663—9674 |
| [17] | Pettersen E.F., Goddard T. D., Huang C. C., J. Comput. Chem., 2004, 25(13), 1605—1612 |
| [18] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision A.02, Gaussian Inc.,Wallingford CT, 2009 |
| [19] | Malde A.K., Zuo L., Breeze M., Stroet M., Poger D., Nair P. C., Oostenbrink C., Mark A. E., J. Chem. Theory Comput., 2011, 7(12), 4026—4037 |
| [20] | Van Der Spoel D., Lindahl E., Hess B., Groenhof G., Mark A. E., Berendsen H. J., J. Comput. Chem., 2005, 26(16), 1701—1718 |
| [21] | Darden T.A., York D. M., Pedersen L. G., J. Chem. Phys., 1993, 98, 10089—10092 |
| [22] | Hockney R.W., Goel S. P., Eastwood J. W., J. Comput. Phys., 1974, 14(2), 148—158 |
| [23] | Hess B., J. Chem.Theory Comput., 2008, 4(1), 116—122 |
| [24] | Bussi G., Donadio D., Parrinello M., J. Chem. Phys., 2007, 126(1), 014101 |
| [25] | Berendsen H., van Postma J. P. M., van Gunsteren W., DiNola A. D., Haak J. R., J. Chem. Phys., 1984, 81, 3684—3690 |
| [26] | Khandogin J., Brooks C.L., Proc. Natl. Acad. Sci.USA, 2007, 104(43), 16880—16885 |
| [27] | Wang Q., Yu X., Patal K., Hu R., Chuang S., Zhang G., Zheng J., ACS Chem.Neurosci., 2013, 4(6), 1004—1015 |
| [28] | Cairo C.W., Strzelec A., Murphy R. M., Kiessling L. L., Biochemistry, 2002, 41(27), 8620—8629 |
| [29] | Thai N.Q., Bednarikova Z., Gancar M., Linh H. Q., Hu C. K., Li M. S., Gazova Z., ACS Chem. Neurosci., 2018, DOI: 10.1021/acschemneuro.8b00091 |
| [30] | Irwin J.A., Wong H. E., Kwon I., Biomacromolecules, 2013, 14(1), 264—274 |
| [1] | WEN Jiali, ZHANG Junhao, JIANG Jiuxing. Extra-large Pore Zeolites: a Ten-year Updated Review [J]. Chem. J. Chinese Universities, 2021, 42(1): 101. |
| [2] | JIAO Meichen, JIANG Jingang, XU Hao, WU Peng. Structural Stabilization, Modification and Catalytic Applications of Germanosilicates [J]. Chem. J. Chinese Universities, 2021, 42(1): 29. |
| [3] | MIAO Xiaomei, MAO Keyu, PEI Yongbing, JIANG Jianxiong, YAN Yue, WU Lianbin. Preparation of Superhydrophobic Porous Silicon and Its Surface Stability† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1499. |
| [4] | LI Lei,HUANG Cuiying,JIANG Xiaonan,GAO Xichan,WANG Changsheng. Ionic Hydrogen Bonding Between Arginine Side Chain and Nucleic Acid Bases† [J]. Chem. J. Chinese Universities, 2016, 37(8): 1460. |
| [5] | SUN Xiaoli, HUO Ruiping, BU Yuxiang, LI Jilai. Benchmark Studies of Density Functional Theory on the Hydrogen Adsorption† [J]. Chem. J. Chinese Universities, 2015, 36(8): 1570. |
| [6] | WANG Xiaowen, LI Shuang, JIANG Xiaonan, WANG Changsheng. Site-preference of Quercetin Hydrogen Bonding to Adenine† [J]. Chem. J. Chinese Universities, 2015, 36(5): 932. |
| [7] | LIU Cui, ZHANG Qianhui, GONG Lidong, LU Linan, YANG Zhongzhi. Theoretical Studies on the Effect of Fapy-G on Base Pair Hydrogen Bond Complexes† [J]. Chem. J. Chinese Universities, 2014, 35(12): 2645. |
| [8] | LIU Peng, LI Shushi, WANG Changsheng. Effects of Substituents on the Binding Energy in Hydrogen-bonded Complexes Containing Adenine and Thymine† [J]. Chem. J. Chinese Universities, 2014, 35(1): 154. |
| [9] | ZHANG Ji, LI Hai-Bin, WU Yong, GENG Yun, DUAN Yu-Ai, LIAO Yi*, SU Zhong-Min*. TD-DFT Studies on Phenothiazine-based Dyes with Different Donor in Dye-sensitized Solar Cells [J]. Chem. J. Chinese Universities, 2011, 32(6): 1343. |
| [10] | HUO Hong-Jie, ZHAO Dong-Xia*, YANG Zhong-Zhi* . Theoretical Study on the Interaction between Bases and NMA by Ab initio and ABEEMσπ Methods [J]. Chem. J. Chinese Universities, 2011, 32(12): 2877. |
| [11] | GAO Feng, ZHAO Jiang*. Effect of Tension on the Microvoids Structure of Polyacylonitrile(PAN) Fibers During the Thermal Stabilization Stage [J]. Chem. J. Chinese Universities, 2011, 32(12): 2711. |
| [12] | ZHANG Wen-Long, CHEN Shu-Ling, YANG Zhong-Zhi*. Calculation of Complexes of the Recombinant Kringle 1 Domain of Human Plasminogen and Its Ligands by ABEEMσπ/MM Method [J]. Chem. J. Chinese Universities, 2010, 31(8): 1630. |
| [13] | NI Zhe-Ming, YAO Ping, LIU Xiao-ing, WANG Qiao-iao, XU Qian. Theory Study on the Distorted Structure and Stability of Copper\|Zinc\|Aluminum Layered Double Hydroxides [J]. Chem. J. Chinese Universities, 2010, 31(12): 2438. |
| [14] | LÜ Yan-Yan, TAN Hong-Wei*, CHEN Guang-Ju, LIU Ruo-Zhuang*. Theoretical Study on Ions Selectivity of Calmodulin [J]. Chem. J. Chinese Universities, 2008, 29(12): 2345. |
| [15] | JIN Lian-Ji, ZHANG Min, SU Zhong-Min*, SHI Li-Li, ZHAO Liang. Theoretical Study on Hydrocarbon Molecule(Acetylene, Ethylene, Ethane) Doped Armchair Single-Walled Carbon Nanotube [J]. Chem. J. Chinese Universities, 2007, 28(4): 755. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||