

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (4): 758.doi: 10.7503/cjcu20170445
• Physical Chemistry • Previous Articles Next Articles
OUYANG Shunli1, ZHANG Mingzhe1, ZHANG Yongzhao2, HU Qingcheng1, WEI Haiyan1, WU Nannan2,*( ), HUANG Baokun3,*(
), HUANG Baokun3,*( )
)
Received:2017-07-10
Online:2018-04-10
Published:2018-03-27
Contact:
WU Nannan,HUANG Baokun
E-mail:woshinannan04@imust.cn;bkhuang@dicp.ac.cn
Supported by:CLC Number:
TrendMD:
OUYANG Shunli, ZHANG Mingzhe, ZHANG Yongzhao, HU Qingcheng, WEI Haiyan, WU Nannan, HUANG Baokun. Raman Spectroscopic Investigation on the Effect of Hydrogen Bond on Molecular Structure in Ternary Aqueous Solution†[J]. Chem. J. Chinese Universities, 2018, 39(4): 758.
| Sample | n(Water):n(acetonitrile): n(DMSO) | Sample | n(Water):n(acetonitrile): n(DMSO) | Sample | n(Water):n(acetonitrile): n(DMSO) |
|---|---|---|---|---|---|
| a | 1:0:0 | f | 0:1:1 | k | 1.5:1:1 |
| b | 0:0:1 | g | 0.5:1:1 | l | 1.6:1 :1 |
| c | 0:1:0 | h | 1:1:1 | m | 1.8:1:1 |
| d | 1:1:0 | i | 1.2:1:1 | n | 2.0:1:1 |
| e | 1:0:1 | j | 1.4:1:1 | o | 2.5:1:1 |
Table 1 Molar contents of each sample
| Sample | n(Water):n(acetonitrile): n(DMSO) | Sample | n(Water):n(acetonitrile): n(DMSO) | Sample | n(Water):n(acetonitrile): n(DMSO) |
|---|---|---|---|---|---|
| a | 1:0:0 | f | 0:1:1 | k | 1.5:1:1 |
| b | 0:0:1 | g | 0.5:1:1 | l | 1.6:1 :1 |
| c | 0:1:0 | h | 1:1:1 | m | 1.8:1:1 |
| d | 1:1:0 | i | 1.2:1:1 | n | 2.0:1:1 |
| e | 1:0:1 | j | 1.4:1:1 | o | 2.5:1:1 |
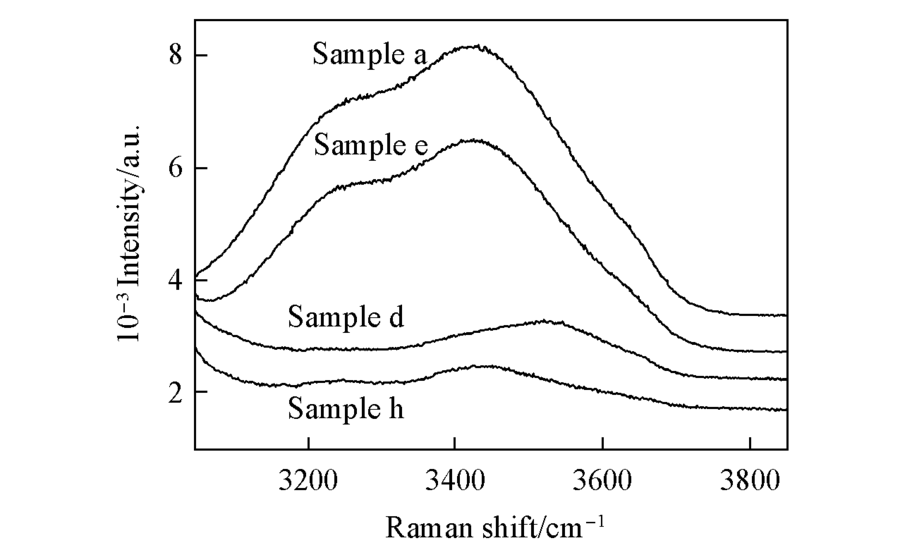
Fig.8 Raman peak of O-H bond in pure water(sample a), DMSO-H2O solution(sample e, 1:1), acetonitrile-H2O solution(sample d, 1:1) and ternary solution(sample h, 1:1:1)
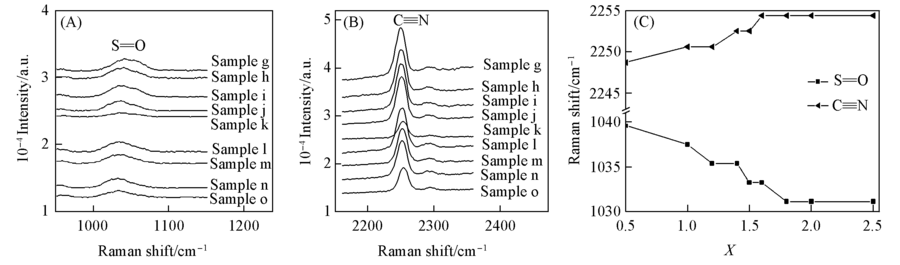
Fig.10 Raman peaks of S=O bond(A) and C≡≡N bond(B) in ternary solutions(X:1:1) with water content(X) from 0.5 to 2.5 and changes of vibration peaks of S=O and C≡≡N bonds with water concentration(C)For samples g-o, X=0.5, 1.0, 1.2, 1.4, 1.5, 1.6, 1.8, 2.0, 2.5, respectively.
| [1] | Klemperer W., Science, 1992, 257(5072), 887-888 |
| [2] | Hunter C. A., Meah M. N., Sanders J. K. M., J. Am. Chem. Soc., 1990, 112(15), 5773-5780 |
| [3] | Das A., Das R., Kumar K., Spectrochimica Acta Part A Molecular & Biomolecular Spectroscopy,2002, 58(8), 1583-1588 |
| [4] | Ouyang S. L., Li Z. Q., Wu N. N., Li Z. W., Sun C. L., Fan L. M., Spectroscopy and Spectral Analysis, 2013, 33(9), 2425-2428 |
| (欧阳顺利, 李正强, 吴楠楠, 里佐威, 孙成林, 范丽梅. 光谱学与光谱分析, 2013, 33(9), 2425-2428) | |
| [5] | Ouyang S. L., Zhou M., Cao B., Lu G. H., Gao S. Q., Li Z. W., Chem. J. Chinese Universities, 2008, 29(10), 2055-2058 |
| (欧阳顺利, 周密, 曹彪, 陆国会, 高淑琴, 里佐威. 高等学校化学学报, 2008, 29(10), 2055-2058) | |
| [6] | Ouyang S. L., Wu N. N., Lu J. Y., Sun C. L., Li Z. W., Gao S. Q., Chin. Phys. B, 2010, 19(12), 154-160 |
| [7] | Ouyang S. L., Wu N. N., Lu J. Y., Sun C. L., LiuJ. Y., Li Z. W., Gao S. Q., Chin. Phys. B, 2010, 19(9), 317-322 |
| [8] | Ouyang S. L., Zhang X. F., Liu J. Y., Li Z. W., Wu N. N., Asian J. Chem., 2013, 25(8), 4512-4516 |
| [9] | Wu N. N., Zhang X. F., Ouyang S. L., Han X. G., Spectroscopy and Spectral Analysis, 2012, 32(11), 3031-3034 |
| (吴楠楠, 张雪峰, 欧阳顺利, 韩向刚. 光谱学与光谱分析, 2012, 32(11), 3031-3034) | |
| [10] | Hu Q. C., Ouyang S. L., Li J., Cao Z., J. Raman Spectrosc., 2017, 19(32), 21540-21547 |
| [11] | Ivana D., Rémy C., Patrice B., Mario M., Jean-Marie C., Marc D. F., J. Raman Spectrosc., 2011, 42(6), 1408-1412 |
| [12] | Gojło E., Gampe T., Krakowiak J., Stangret J., J. Phys. Chem. A, 2007, 111(10), 1827-1834 |
| [13] | Solomonov B. N., Varfolomeev M. A., Abaidullina D. I., Vib. Spectrosc., 2007, 43(2), 380-386 |
| [14] | Alves W. A., Antunes O. A. C., Spectrochimica Acta Part A Molecular & Biomolecular Spectroscopy,2007, 67(3/4), 847-851 |
| [15] | Umebayashi Y., Matsumoto K., Mekata I., Ishiguro S. I., Phys. Chem. Chem. Phys., 2002, 4(22), 5599-5605 |
| [16] | Weng S. F., Fourier Transform Infrared Spectrum Analysis, 2nd Ed., Chemical Industry Press, Beijing, 2010, 2-41 |
| (翁诗甫. 傅里叶变换红外光谱分析, 第二版, 北京: 化学工业出版社, 2010, 2-41) | |
| [17] | He Z. F., Gu R. A., Chem. J. Chinese Universities, 1993, 14(1), 102-105 |
| (何张飞, 顾仁敖. 高等学校化学学报, 1993, 14(1), 102-105) | |
| [18] | Zhang L. Y., Li Z. W., Lu G. H., Gao S. Q., Jiang Y. H., Spectroscopy and Spectral Analysis, 2009, 29(5), 1296-1299 |
| (张留洋, 里佐威, 陆国会, 高淑琴, 姜永恒. 光谱学与光谱分析, 2009, 29(5), 1296-1299) | |
| [19] | MadigoskyW. M., Warfield R. W., J. Chem. Phys., 1983, 78(4), 1912-1916 |
| [20] | Yang Y., Zhang W. J., Pei S. X., Shao J., Huang W., Gao X. M., Sci. China Seri. B, 2006, 36(3), 215-226 |
| (杨颙, 张为俊, 裴世鑫, 邵杰, 黄伟, 高晓明. 中国科学 B: 化学, 2006, 36(3), 215-226) | |
| [21] | Wang S. W., Li An. Y., Tan H. W., Chem. J. Chinese Universities, 2007, 28(10), 1962-1967 |
| (王素纹, 黎安勇, 谭宏伟. 高等学校化学学报, 2007, 28(10), 1962-1967) | |
| [22] | Nickolov Z. S., Goutev A. N., Matsuura H., J. Phys. Chem. A, 2001, 105(48), 10884-10889 |
| [1] | MIN Jing, WANG Liyan. 1H NMR Study on the Conformation of Aromatic Amides Limited by Three-center Hydrogen Bonds [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220084. |
| [2] | CHEN Jiamin, QU Xiaozhang, QI Guohua, XU Weiqing, JIN Yongdong, XU Shuping. SERS Nanoprobe for the Detection of Reactive Oxygen Species in Cells Produced by Electrostimulus [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220033. |
| [3] | ZHANG Yong, XU Jun, BAO Yu, CUI Shuxun. Quantifying the Degree of Weakening Effect of Nonpolar Organic Solvent on the Strength of Intramolecular Hydrogen Bonding by Single-molecule Force Spectroscopy [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210863. |
| [4] | CUI Shaoli, ZHANG Weijia, SHAO Xueguang, CAI Wensheng. Revealing the Effect of Threonine on the Binding Ability of Antifreeze Proteins with Ice Crystals by Free-energy Calculations [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210838. |
| [5] | HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614. |
| [6] | GAO Huiling, CAO Zhenzhen, GU Fang, WANG Haijun. Monte Carlo Simulation on Self-healing Behaviour of Hydrogen-bonded Hydrogel [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220482. |
| [7] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [8] | XUE Jin, CAO Xiaowei, LIU Yifan, WANG Min. Preparation of Paper Hollow Gold Nanocage SERS Sensor and Its Rapid and Highly Sensitive Detection for miRNAs in Sputum of Patients with Non-small Cell Lung Cancer [J]. Chem. J. Chinese Universities, 2021, 42(8): 2393. |
| [9] | CHEN Feng, CHENG Na, ZHAO Jianwei, SONG Yitian, SUN Yanyan, LOU Xinli, TONG Xiayan. Electrodeposition Mechanism and Surface-enhanced Raman Spectroscopic Effect of Nano-sized Silver Layer [J]. Chem. J. Chinese Universities, 2021, 42(6): 1891. |
| [10] | WANG Le, QIN Liulei, LIU Yang, REN Li, XU Huiting, LIU Zunqi. Synthesis, Structure and Dielectric Properties of One-dimensional Chain Hydrogen Glycine Supramolecular Compound [(Gly)2+(18-crown-6)2(MnCl4)2‒] [J]. Chem. J. Chinese Universities, 2021, 42(3): 691. |
| [11] | PAN Jing, XU Minmin, YUAN Yaxian, YAO Jianlin. Rapid Detection of Banned Dyes in Textiles Based on Surface-enhanced Raman Spectroscopy [J]. Chem. J. Chinese Universities, 2021, 42(12): 3716. |
| [12] | NI Qingsheng, DU Miao, SHAN Guorong, SONG Yihu, WU Ziliang, ZHENG Qiang. Regulation of Rheological Behavior of Polyvinyl Alcohol Aqueous Solution by One-dimensional Particles [J]. Chem. J. Chinese Universities, 2021, 42(12): 3738. |
| [13] | GONG Shanshan, WU Tong, WANG Guange, HUANG Qing, SU Yuefeng, WU Feng. Screening of Deep Eutectic Solvent Based on Efficient Recovery of Spent Lithium⁃ion Battery Cathode Materials [J]. Chem. J. Chinese Universities, 2021, 42(10): 3151. |
| [14] | WANG Ruixue, YIN Dongmei, SONG Yongxin, SHAN Guiye. Preparation of CuS/Ag2S Nanocomposite and the Peroxidase-like Properties † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1218. |
| [15] | LI Wenshuai, WU Guorui, ZHANG Xijing, YUE Aiqin, DU Weijun, ZHAO Jinzhong, LIU Dingbin. Advances in Bacterial Detection Based on Raman Spectroscopy † [J]. Chem. J. Chinese Universities, 2020, 41(5): 872. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||