

Chem. J. Chinese Universities ›› 2022, Vol. 43 ›› Issue (3): 20210838.doi: 10.7503/cjcu20210838
• Physical Chemistry • Previous Articles
CUI Shaoli1, ZHANG Weijia1, SHAO Xueguang1,2( ), CAI Wensheng1(
), CAI Wensheng1( )
)
Received:2021-12-17
Online:2022-03-10
Published:2022-01-11
Contact:
SHAO Xueguang,CAI Wensheng
E-mail:xshao@nankai.edu.cn;wscai@nankai.edu.cn
Supported by:CLC Number:
TrendMD:
CUI Shaoli, ZHANG Weijia, SHAO Xueguang, CAI Wensheng. Revealing the Effect of Threonine on the Binding Ability of Antifreeze Proteins with Ice Crystals by Free-energy Calculations[J]. Chem. J. Chinese Universities, 2022, 43(3): 20210838.
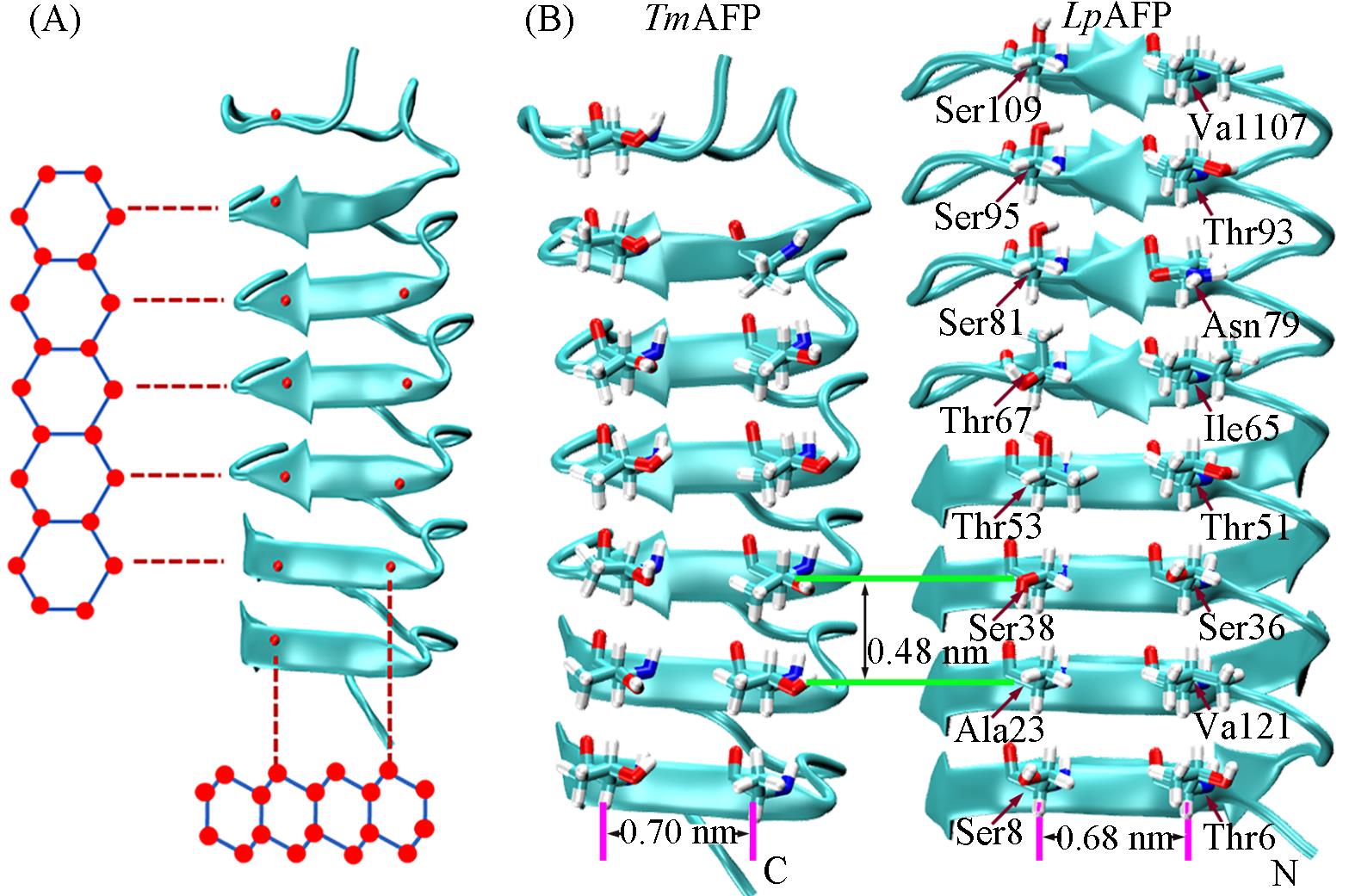
Fig.1 Schematic representation of TmAFP(PDB ID: 1EZG) aligned to the basal plane of ice(A), crystal structures of TmAFP and LpAFP(PDB ID: 3ULT)(B)(A) Red spheres indicate oxygen atoms in the ice lattice and within the hydroxyl groups of threonine. Horizontal dotted red lines highlight the near?perfect two?dimensional match of the interstrand threonine residues to the basal plane. Vertical dotted red lines highlight the match of the intrastrand threonine residues to a section of the basal plane. (B) TmAFP and LpAFP, with 0.48 nm between adjacent β?strands and 0.70 and 0.68 nm, between residues separated by two positions on the same β?strand, respectively. AFP backbones and IBSs are shown in cartoon and stick representations, respectively. The front view is the ice?binding face of the AFPs.
| Molecular assembly | Number of atom | Box/nm3 | Simulation time/ns |
|---|---|---|---|
| LpAFP | 57223 | 7.1×8.8×7.3 | 400 |
| Mutant1 a | 52610 | 7.1×8.1×7.3 | 400 |
| Mutant2 b | 52578 | 7.1×8.1×7.3 | 400 |
| Mutant3 c | 52545 | 7.1×8.1×7.3 | 400 |
| Mutant4 d | 52516 | 7.1×8.1×7.3 | 400 |
Table 1 Details of the molecular assemblies investigated in this study
| Molecular assembly | Number of atom | Box/nm3 | Simulation time/ns |
|---|---|---|---|
| LpAFP | 57223 | 7.1×8.8×7.3 | 400 |
| Mutant1 a | 52610 | 7.1×8.1×7.3 | 400 |
| Mutant2 b | 52578 | 7.1×8.1×7.3 | 400 |
| Mutant3 c | 52545 | 7.1×8.1×7.3 | 400 |
| Mutant4 d | 52516 | 7.1×8.1×7.3 | 400 |
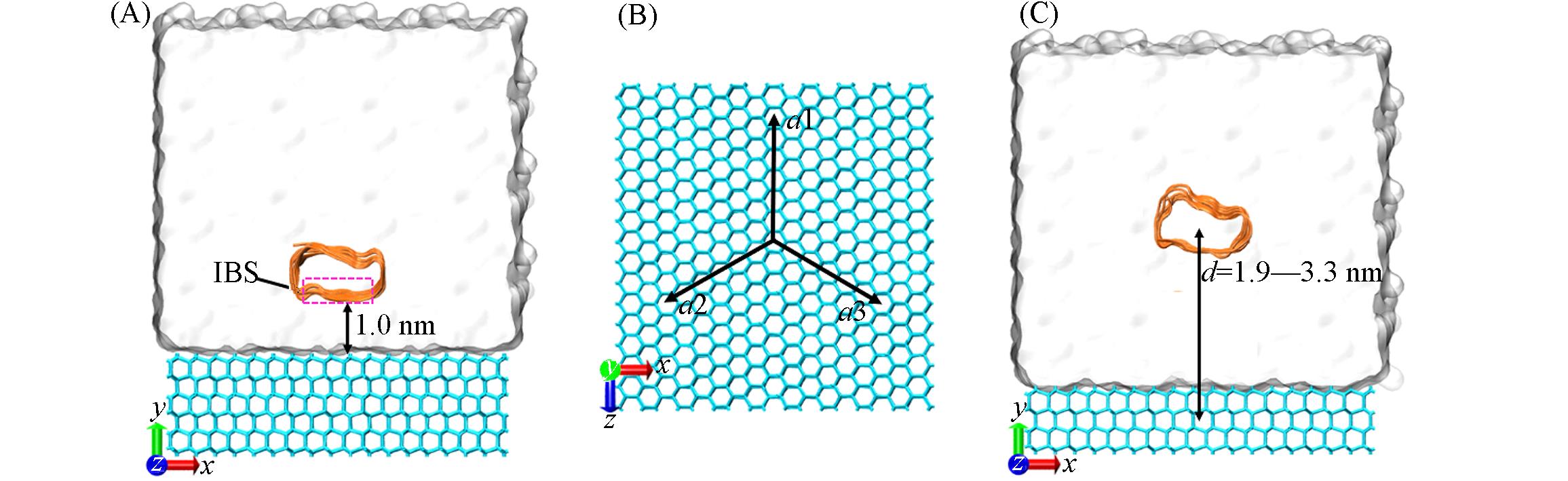
Fig.2 LpAFP/mutant?ice?water assemblies for MD(A), schematic diagram of the a?axis of ice(B) and LpAFP/mutant?ice?water assemblies for free?energy calculations(C)AFP backbones are shown in cartoon. (A) The distance between the IBS and the basal plane of ice crystals is 1.0 nm. (C) d denotes the projection along the y direction of Cartesian space of the vector connecting the center of mass of the oxygen atoms of the ice and that of the AFP.
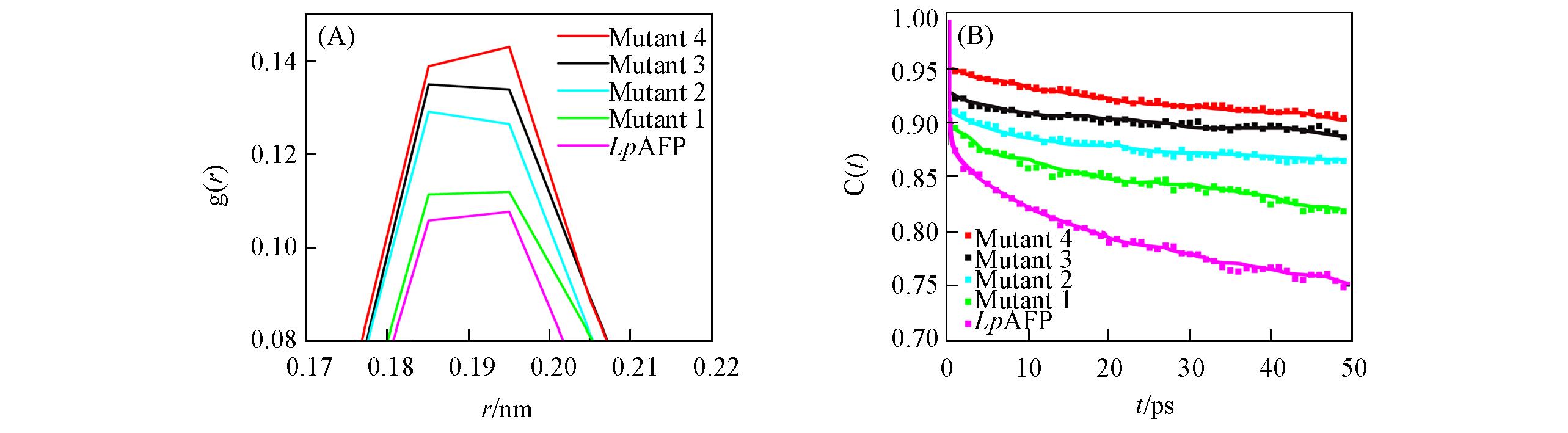
Fig.3 Radial distribution function of water(oxygen atoms) around the IBS of LpAFP and its mutants obtained from MD simulations(A), hydrogen bond lifetime correlation functions between any water molecules and amino acid residues at the IBS for wild?type LpAFP and its mutants adsorbed on ice crystals(B)
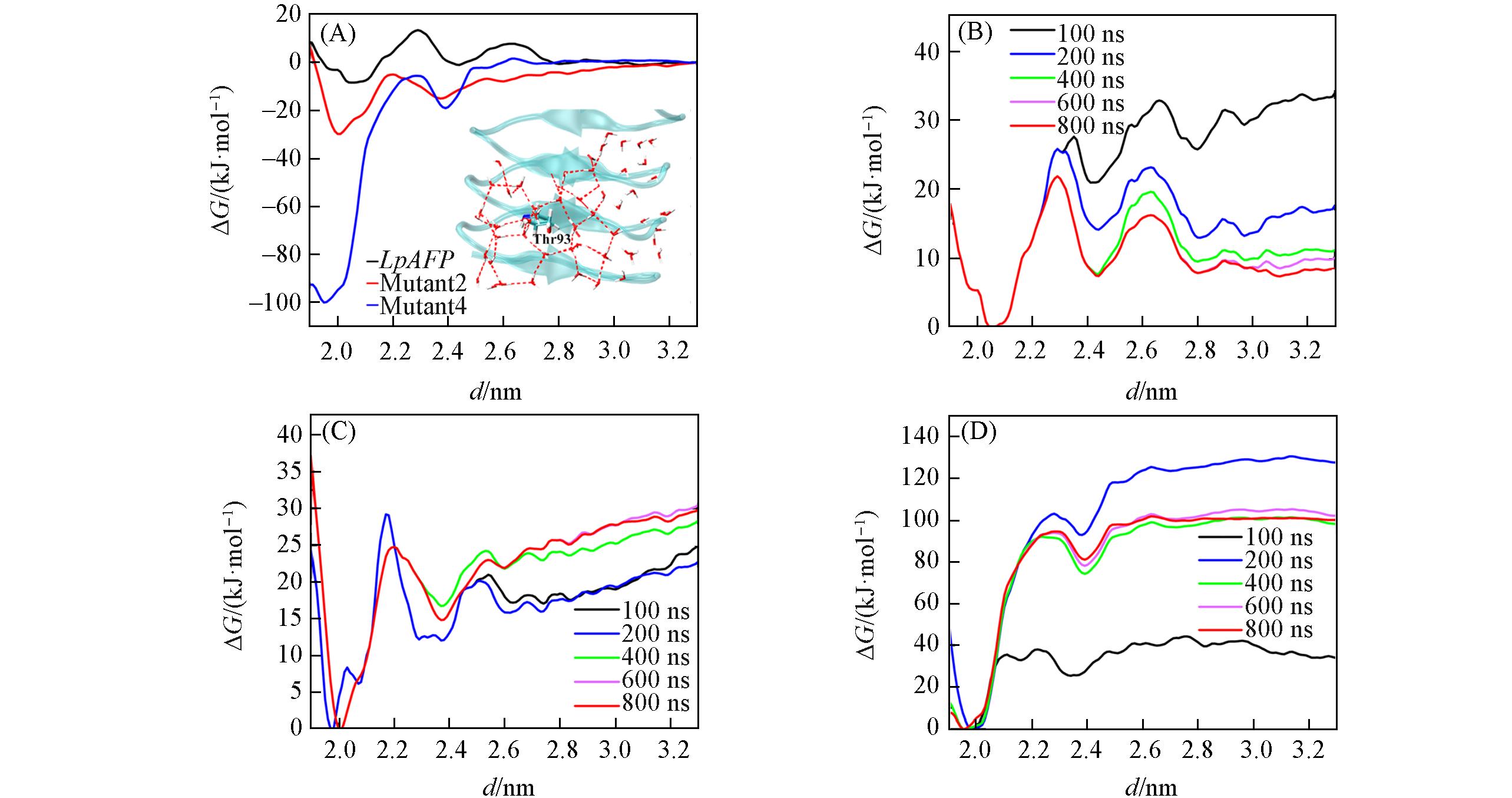
Fig.4 Free?energy profiles characterizing the adsorption process for LpAFP, Mutant 2 and Mutant 4(A), free?energy profiles at different simulation times for the LpAFP(B), Mutant 2(C) and Mutant 4(D)Inset of (A): water molecules around threonine form water cages. The results show that the calculations have converged within 800 ns.
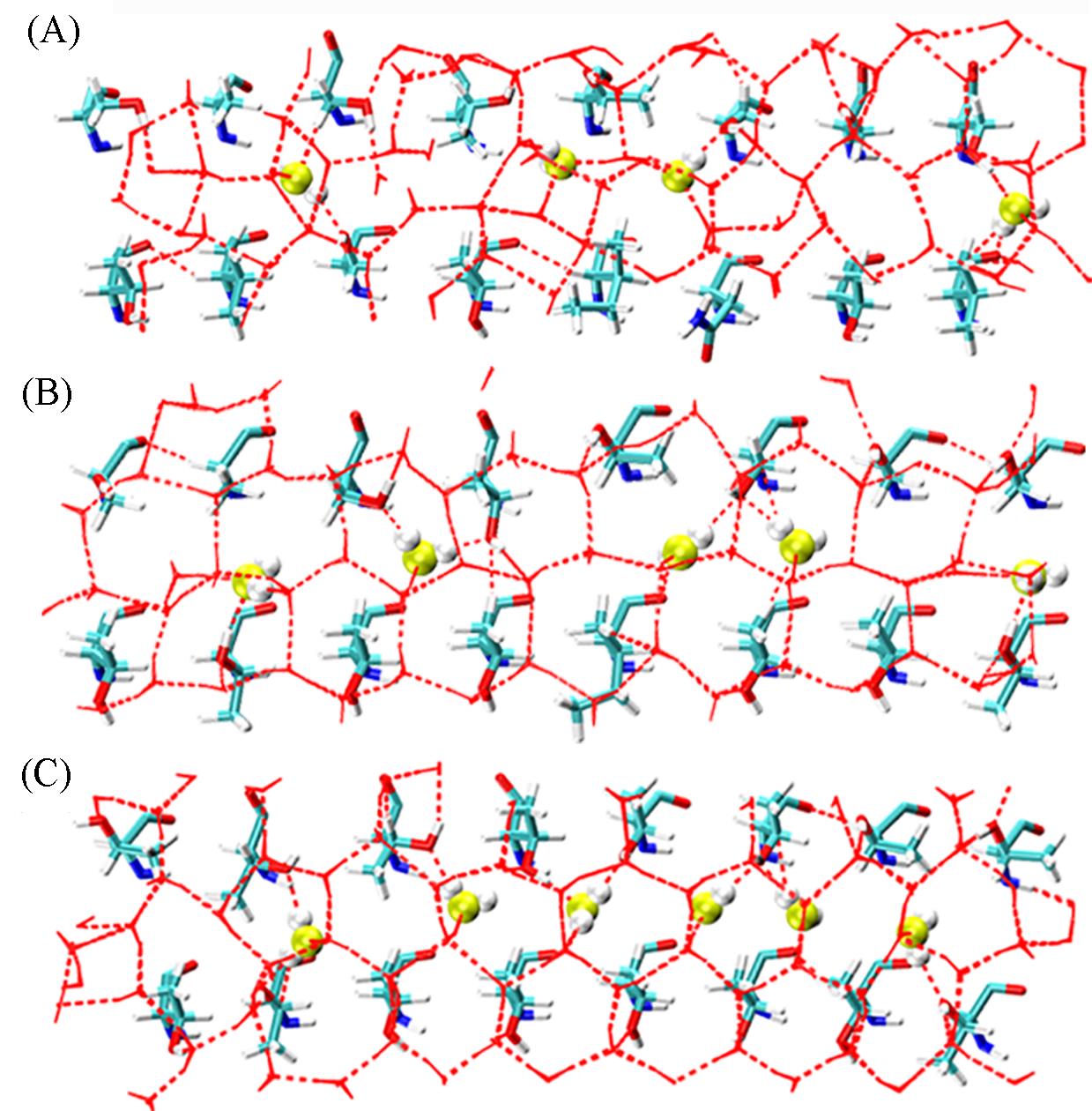
Fig.5 Hydrogen bond structures of water molecules within 0.5 nm below the IBS of LpAFP(A), Mutant 2(B) andMutant 4(C) adsorbed on ice crystalsCW is shown in vdW representation(oxygen atoms shown in yellow).
| 1 | Duman J. G., Annu. Rev. Physiol., 2001, 63(1), 327—357 |
| 2 | Zhang W. J., Shao X. G., Cai W. S., Prog. Chem., 2021, 33(10), 1797—1811(张维佳, 邵学广, 蔡文生. 化学进展, 2021, 33(10), 1797—1811) |
| 3 | Davies P. L., Trends Biochem. Sci., 2014, 39(11), 548—555 |
| 4 | Voets I. K., Soft Matter., 2017, 13(28), 4808—4823 |
| 5 | Kozuch D. J., Stillinger F. H., Debenedetti P. G., Proc. Natl. Acad. Sci., 2018, 115(52), 13252—13257 |
| 6 | Drori R., Davies P. L., Braslavsky I., Langmuir, 2015, 31(21), 5805—5811 |
| 7 | Rubinsky B., Arav A., Devries A. L., Cryobiology, 1992, 29(1), 69—79 |
| 8 | Griffith M., Ewart K., Biotechnol. Adv., 1995, 13(3), 375—402 |
| 9 | Liu K., Wang C. L., Ma J., Shi G. S., Yao X., Fang H. P., Song Y. L., Wang J. J., Proc. Natl. Acad. Sci., 2016, 113(51), 14739—14744 |
| 10 | Shi M. H., Liu J. J., Zhao X. J., Liu J. P., J. Inner Mongolia University, 2017, 48(5), 515—521(史明卉, 刘俊杰, 赵雪珺, 刘俊平. 内蒙古大学学报, 2017, 48(5), 515—521) |
| 11 | Wang C., Liu J. J., J. Inner Mongolia University, 2019, 50(1), 66—73(王超, 刘俊杰. 内蒙古大学学报, 2019, 50(1), 66—73) |
| 12 | Garnham C. P., Campbell R. L., Davies P. L., Proc. Natl. Acad. Sci., 2011, 108(18), 7363—7367 |
| 13 | Hudait A., Odendahl N., Qiu Y., Paesani F., Molinero V., J. Am. Chem. Soc., 2018, 140(14), 4905—4912 |
| 14 | Hakim A., Nguyen J. B., Basu K., Zhu D. F., Thakral D., Davies P. L., Isaacs F. J., Modis Y., Meng W. Y., J. Biol. Chem., 2013, 288(17), 12295—12304 |
| 15 | Chakraborty S., Jana B., Langmuir, 2017, 33(28), 7202—7214 |
| 16 | Chakraborty S., Jana B., J. Phys. Chem. B, 2018, 122(12), 3056—3067 |
| 17 | Scotter A. J., Marshall C. B., Graham L. A., Gilbert J. A., Garnham C. P., Davies P. L., Cryobiology, 2006, 53(2), 229—239 |
| 18 | Bar M., Celik Y., Fass D., Braslavsky I., Cryst. Growth Des., 2008, 8(8), 2954—2963 |
| 19 | Zhou Y. X., Zhang Y., Tan H. W., Jia Z. C., Chen G. J., Chem. J. Chinese Universities, 2007, 28(3), 526—529(周艳霞, 张勇, 谭宏伟, 贾宗超, 陈光巨. 高等学校化学学报, 2007, 28(3), 526—529) |
| 20 | Middleton A. J., Marshall C. B., Faucher F., Bar⁃Dolev M., Braslavsky I., Campbell R. L., Walker V. K., Davies P. L., J. Mol. Biol., 2012, 416(5), 713—724 |
| 21 | Zhang H., Cai W. S., Shao X. G., Chem. J. Chinese Universities, 2018, 39(6), 1205—1211(张宏, 蔡文生, 邵学广. 高等学校化学学报, 2018, 39(6), 1205—1211) |
| 22 | Fu H. H., Chen H. C., Zhang H., Shao X. G., Cai W. S., Acta Chim. Sinica, 2021, 79(4), 472—480(付浩浩, 陈淏川, 张宏, 邵学广, 蔡文生. 化学学报, 2021, 79(4), 472—480) |
| 23 | Miao M. Y., Guo Y. C., Shao X. G., Cai W. S., Chem. J. Chinese Universities, 2021, 42(10), 3116—3124(妙孟姚, 郭一畅, 邵学广, 蔡文生. 高等学校化学学报, 2021, 42(10), 3116—3124) |
| 24 | Shao Q., Zhu W. L., Phys. Chem. Chem. Phys., 2019, 21(22), 11924—11936 |
| 25 | Liu P., Hao W. Q., Bian X. H., Mei D. H., Phys. Chem. Chem. Phys., 2020, 22(23), 12967—12972 |
| 26 | Liu Y. Z., Lai W. P., Yu T., Ma Y. D., Guo W. J., Ge Z. X., ACS Omega, 2019, 4(2), 4320—4324 |
| 27 | Hudait A., Qiu Y., Odendahl N., Molinero V., J. Am. Chem. Soc., 2019, 141(19), 7887—7898 |
| 28 | Mochizuki K., Molinero V., J. Am. Chem. Soc., 2018, 140(14), 4803—4811 |
| 29 | Jo S., Kim T., Iyer V. G., Im W., J. Comput. Chem., 2008, 29(11), 1859—1865 |
| 30 | Matsumoto M., Yagasaki T., Tanaka H., J. Comput. Chem., 2018, 39(1), 61—64 |
| 31 | Grabowska J., Kuffel A., Zielkiewicz J., Phys. Chem. Chem. Phys., 2018, 20(39), 25365—25376 |
| 32 | Bjelkmar P., Larsson P., Cuendet M. A., Hess B., Lindahl E., J. Chem. Theory Comput., 2010, 6(2), 459—466 |
| 33 | Fernández R. G., Abascal J. L. F., Vega C., J. Chem. Phys., 2006, 124(14), 144506 |
| 34 | Abascal J. L. F., Sanz E., Fernández R. G., Vega C., J. Chem. Phys., 2005, 122(23), 234511 |
| 35 | Kumari S., Muthachikavil A. V., Tiwari J. K., Punnathanam S. N., Langmuir, 2020, 36(9), 2439—2448 |
| 36 | Bussi G., Donadio D., Parrinello M., J. Chem. Phys., 2007, 126(1), 014101 |
| 37 | Nosé S., Klein M. L., Mol. Phys., 1983, 50(5), 1055—1076 |
| 38 | Hess B., Bekker H., Berendsen H. J. C., Fraaije J. G. E. M., J. Comput. Chem., 1997, 18(12), 1463—1472 |
| 39 | Essmann U., Perera L., Berkowitz M. L., Darden T., Lee H., Pedersen L. G., J. Chem. Phys., 1995, 103(19), 8577—8593 |
| 40 | Humphrey W., Dalke A., Schulten K., J. Mol. Graphics, 1996, 14(1), 33—38 |
| 41 | Darve E., Pohorille A., J. Chem. Phys., 2001, 115(20), 9169—9183 |
| 42 | Darve E., Wilson M. A., Pohorille A., Mol. Simulat., 2002, 28(1/2), 113—144 |
| 43 | Fu H. H., Shao X. G., Chipot C., Cai W. S., J. Chem. Theory Comput., 2016, 12(8), 3506—3513 |
| 44 | Fu H. H., Shao X. G., Cai W. S., Chipot C., Acc. Chem. Res., 2019, 52(11), 3254—3264 |
| 45 | Fu H. H., Chen H. C., Wang X. A., Chai H., Shao X. G., Cai W. S., Chipot C., J. Chem. Inf. Model., 2020, 60(11), 5366—5374 |
| 46 | Barducci A., Bussi G., Parrinello M., Phys. Rev. Lett., 2008, 100(2), 20603 |
| 47 | Dama J. F., Parrinello M., Voth G. A., Phys. Rev. Lett., 2014, 112(24), 240602 |
| 48 | Fu H. H., Zhang H., Chen H. C., Shao X. G., Chipot C., Cai W. S., J. Phys. Chem. Lett., 2018, 9(16), 4738—4745 |
| 49 | Guo Y. C., Fu H. H., Shao X. G., Cai W. S., Chem. Res. Chinese Universities, 2020, 36(5), 748—754 |
| 50 | Cui S. L., Zhang W. J., Shao X. G., Cai W. S., J. Chem. Inf. Model., doi: 10.1021/acs.jcim.1c00915 |
| 51 | Luzar A., Chandler D., Nature, 1996, 379(6560), 55—57 |
| 52 | Chakraborty S., Jana B., Phys. Chem. Chem. Phys., 2019, 21(35), 19298—19310 |
| [1] | MIN Jing, WANG Liyan. 1H NMR Study on the Conformation of Aromatic Amides Limited by Three-center Hydrogen Bonds [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220084. |
| [2] | ZHANG Yong, XU Jun, BAO Yu, CUI Shuxun. Quantifying the Degree of Weakening Effect of Nonpolar Organic Solvent on the Strength of Intramolecular Hydrogen Bonding by Single-molecule Force Spectroscopy [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210863. |
| [3] | HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614. |
| [4] | GAO Huiling, CAO Zhenzhen, GU Fang, WANG Haijun. Monte Carlo Simulation on Self-healing Behaviour of Hydrogen-bonded Hydrogel [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220482. |
| [5] | WANG Le, QIN Liulei, LIU Yang, REN Li, XU Huiting, LIU Zunqi. Synthesis, Structure and Dielectric Properties of One-dimensional Chain Hydrogen Glycine Supramolecular Compound [(Gly)2+(18-crown-6)2(MnCl4)2‒] [J]. Chem. J. Chinese Universities, 2021, 42(3): 691. |
| [6] | NI Qingsheng, DU Miao, SHAN Guorong, SONG Yihu, WU Ziliang, ZHENG Qiang. Regulation of Rheological Behavior of Polyvinyl Alcohol Aqueous Solution by One-dimensional Particles [J]. Chem. J. Chinese Universities, 2021, 42(12): 3738. |
| [7] | LIU Ke, JIN Yu, LIANG Jiangong, WU Yuan. Research Progress on Improving the Binding Affinity of Aptamers through Chemical Modification [J]. Chem. J. Chinese Universities, 2021, 42(11): 3477. |
| [8] | MIAO Mengyao, GUO Yichang, SHAO Xueguang, CAI Wensheng. Mechanism of Ion Transport Across Membranes Assisted by Molecular Shuttles [J]. Chem. J. Chinese Universities, 2021, 42(10): 3116. |
| [9] | GONG Shanshan, WU Tong, WANG Guange, HUANG Qing, SU Yuefeng, WU Feng. Screening of Deep Eutectic Solvent Based on Efficient Recovery of Spent Lithium⁃ion Battery Cathode Materials [J]. Chem. J. Chinese Universities, 2021, 42(10): 3151. |
| [10] | BAI Lan, ZHAI Lei, WANG Changou, HE Minhui, MO Song, FAN Lin. Thermal Expansion Behavior of Amide-containing Polyimide Films with Ultralow Thermal Expansion Coefficient † [J]. Chem. J. Chinese Universities, 2020, 41(4): 795. |
| [11] | QIN Liulei,LIU Yang,GUAN Xiaoqin,ZHENG Xiaoyuan,ZHANG Ziyu,LIU Zunqi. Synthesis and Switchable Dielectric Properties of an Inorganic-organic Hybrid Complex [H2(DABCO)CuCl4]·H2O † [J]. Chem. J. Chinese Universities, 2020, 41(1): 70. |
| [12] | GAO Yang, LI Daixi, LIU Baolin, GUO Baisong, WEI Dongqing. Inhibitory Mechanism of Glycerol on the Growth of Ice Crystals by Molecular Dynamics† [J]. Chem. J. Chinese Universities, 2019, 40(4): 763. |
| [13] | XU Yan,LIU Cui,HAN Chengjuan,PAN Mingyu,SUN Zhaoqi,HAN Bingyu,YANG Zhongzhi. Development of Polarization Force Field for Guanine and Amino Acid Residues Systems† [J]. Chem. J. Chinese Universities, 2019, 40(2): 288. |
| [14] | XU Yu,HUA Er. Hydrogen Bonding Study on Protic Ionic Liquids Composed of N-Alkyl Ethylenediaminum Cations with Trifluoroacetic Anion† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1954. |
| [15] | ZHANG Hong, CAI Wensheng, SHAO Xueguang. Effect of Different Force Fields on B-DNA to A-DNA Conversion† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1205. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||