

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (6): 1068.doi: 10.7503/cjcu20160952
• Physical Chemistry • Previous Articles Next Articles
HAN Bingyu, LI Yue, LIU Cui*( )
)
Received:2016-12-28
Online:2017-06-10
Published:2017-05-19
Contact:
LIU Cui
E-mail:liuc@lnnu.edu.cn
Supported by:CLC Number:
TrendMD:
HAN Bingyu, LI Yue, LIU Cui. Investigation on the Hydrogen Bonding Interaction Between Amino Acid Side Chains and Base Pairs Containing Oxidized Guanine†[J]. Chem. J. Chinese Universities, 2017, 38(6): 1068.
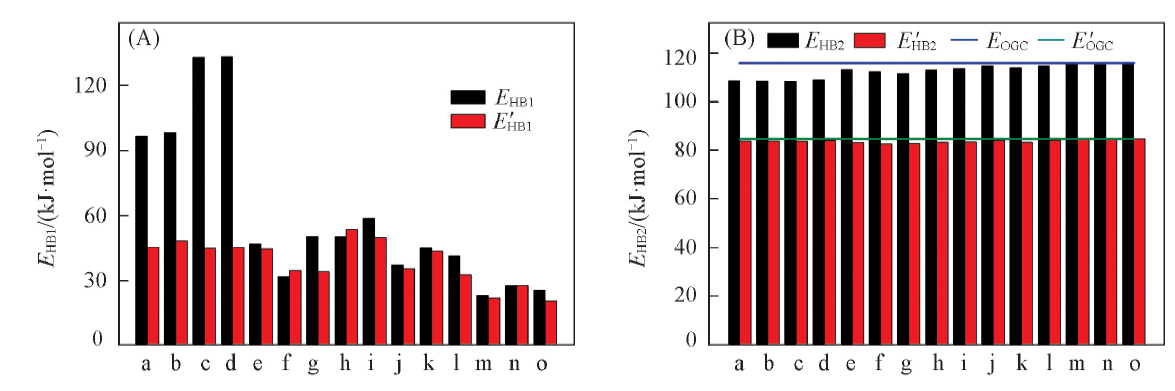
Fig.4 Hydrogen bond energies of region 1(A) and region 2(B) in the ternary hydrogen bond complexesa. OGC1Arg1; b. OGC1Arg2; c. OGC7Arg1; d. OGC7Arg2; e. OGC2Asn; f. OGC3Asn; g. OGC4Asn; h. OGC5Asn; i. OGC6Asn; j. OGC2Ser; k. OGC5Ser; l. OGC6Ser; m. OGC2Cys; n. OGC5Cys; o. OGC6Cys. (B) Straight lines denote hydrogen bond enegies of isolated 8-oxo-G∶C.
| Geometry | ΔL/nm | Δθ/(°) | ΔLgas/nm | Δθgas/(°) | ΔLwater/nm | Δθwater/(°) |
|---|---|---|---|---|---|---|
| Region 1 | -0.0043—0.0224 (0.0071) | -8.5—5.0(2.0) | ||||
| Charged complex | 0.0079—0.0224 (0.0178) | -2.8—2.0(1.2) | ||||
| Neutralcomplex | -0.0043—0.0149 (0.0032) | -8.5—5.0(2.3) | ||||
| Region 2 | -0.0143—0.0125 (0.0030) | -2.2—3.1(1.4) | -0.0130—0.0077 (0.0020) | -3.6—2.4(0.9) | -0.0023—0.0036 (0.0001) | -4.5—1.7(0.4) |
| Charged complex | 0.0014—0.0050 (0.0011) | -0.7—2.2(1.5) | -0.0130—0.0077 (0.0061) | -3.4—2.4(1.4) | -0.0023—0.0008 (0.0001) | -0.7—0(0.3) |
| Neutral complex | -0.0143—0.0125 (0.0037) | -2.2—3.1(1.4) | -0.0046—0.0072 (0.0005) | -3.6—2.4(0.7) | -0.0008—0.0036 (0.0001) | -4.5—1.7(0.4) |
Table 1 Statistical of hydrogen bond length and hydrogen bond angle in different regions*
| Geometry | ΔL/nm | Δθ/(°) | ΔLgas/nm | Δθgas/(°) | ΔLwater/nm | Δθwater/(°) |
|---|---|---|---|---|---|---|
| Region 1 | -0.0043—0.0224 (0.0071) | -8.5—5.0(2.0) | ||||
| Charged complex | 0.0079—0.0224 (0.0178) | -2.8—2.0(1.2) | ||||
| Neutralcomplex | -0.0043—0.0149 (0.0032) | -8.5—5.0(2.3) | ||||
| Region 2 | -0.0143—0.0125 (0.0030) | -2.2—3.1(1.4) | -0.0130—0.0077 (0.0020) | -3.6—2.4(0.9) | -0.0023—0.0036 (0.0001) | -4.5—1.7(0.4) |
| Charged complex | 0.0014—0.0050 (0.0011) | -0.7—2.2(1.5) | -0.0130—0.0077 (0.0061) | -3.4—2.4(1.4) | -0.0023—0.0008 (0.0001) | -0.7—0(0.3) |
| Neutral complex | -0.0143—0.0125 (0.0037) | -2.2—3.1(1.4) | -0.0046—0.0072 (0.0005) | -3.6—2.4(0.7) | -0.0008—0.0036 (0.0001) | -4.5—1.7(0.4) |
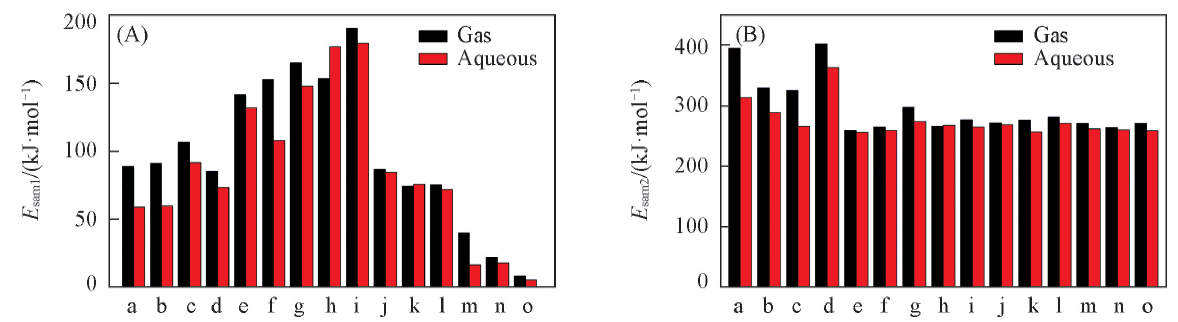
Fig.5 Second-order stabilization energies of triple-body complexes in region 1(A) and region 2(B)a. OGC1Arg1; b. OGC1Arg2; c. OGC7Arg1; d. OGC7Arg2; e. OGC2Asn; f. OGC3Asn; g. OGC4Asn; h. OGC5Asn; i. OGC6Asn; j. OGC2Ser; k. OGC5Ser; l. OGC6Ser; m. OGC2Cys; n. OGC5Cys; o. OGC6Cys.
| Complex | Charge(OG)/e | Charge(C)/e | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H7 | H7' | N9 | N9' | O11 | O11' | O21 | O21' | H23 | H23' | H26 | H26' | |
| OGC | 0.468 | 0.457 | -0.641 | -0.654 | -0.691 | -0.733 | -0.716 | -0.735 | 0.462 | 0.465 | 0.459 | 0.459 |
| OGC1Arg1 | 0.463 | 0.456 | -0.649 | -0.654 | -0.707 | -0.733 | -0.668 | -0.721 | 0.470 | 0.467 | 0.468 | 0.462 |
| OGC1Arg2 | 0.463 | 0.456 | -0.649 | -0.654 | -0.707 | -0.733 | -0.668 | -0.721 | 0.470 | 0.467 | 0.469 | 0.462 |
| OGC7Arg1 | 0.463 | 0.456 | -0.646 | -0.654 | -0.695 | -0.733 | -0.686 | -0.727 | 0.468 | 0.466 | 0.465 | 0.460 |
| OGC7Arg2 | 0.462 | 0.456 | -0.646 | -0.654 | -0.695 | -0.733 | -0.686 | -0.727 | 0.468 | 0.466 | 0.465 | 0.460 |
| OGC2Asn | 0.468 | 0.457 | -0.641 | -0.654 | -0.683 | -0.732 | -0.713 | -0.734 | 0.464 | 0.465 | 0.455 | 0.454 |
| OGC3Asn | 0.468 | 0.455 | -0.636 | -0.649 | -0.718 | -0.743 | -0.723 | -0.737 | 0.459 | 0.463 | 0.444 | 0.453 |
| OGC4Asn | 0.447 | 0.447 | -0.653 | -0.656 | -0.702 | -0.736 | -0.744 | -0.744 | 0.464 | 0.465 | 0.460 | 0.458 |
| OGC5Asn | 0.467 | 0.456 | -0.640 | -0.654 | -0.689 | -0.732 | -0.753 | -0.748 | 0.461 | 0.464 | 0.457 | 0.459 |
| OGC6Asn | 0.469 | 0.457 | -0.640 | -0.654 | -0.692 | -0.733 | -0.700 | -0.734 | 0.461 | 0.464 | 0.458 | 0.459 |
| OGC2Ser | 0.468 | 0.456 | -0.641 | -0.654 | -0.688 | -0.733 | -0.710 | -0.730 | 0.464 | 0.466 | 0.458 | 0.459 |
| OGC5Ser | 0.468 | 0.457 | -0.641 | -0.654 | -0.691 | -0.733 | -0.732 | -0.730 | 0.463 | 0.466 | 0.459 | 0.459 |
| OGC6Ser | 0.468 | 0.456 | -0.641 | -0.654 | -0.692 | -0.733 | -0.707 | -0.732 | 0.462 | 0.465 | 0.459 | 0.459 |
| OGC2Cys | 0.466 | 0.457 | -0.641 | -0.654 | -0.689 | -0.733 | -0.713 | -0.735 | 0.463 | 0.465 | 0.458 | 0.458 |
| OGC5Cys | 0.468 | 0.457 | -0.641 | -0.654 | -0.691 | -0.733 | -0.737 | -0.738 | 0.462 | 0.465 | 0.459 | 0.459 |
| OGC6Cys | 0.468 | 0.457 | -0.641 | -0.654 | -0.692 | -0.733 | -0.709 | -0.734 | 0.462 | 0.465 | 0.459 | 0.459 |
Table 2 Charge of atoms in region 2
| Complex | Charge(OG)/e | Charge(C)/e | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H7 | H7' | N9 | N9' | O11 | O11' | O21 | O21' | H23 | H23' | H26 | H26' | |
| OGC | 0.468 | 0.457 | -0.641 | -0.654 | -0.691 | -0.733 | -0.716 | -0.735 | 0.462 | 0.465 | 0.459 | 0.459 |
| OGC1Arg1 | 0.463 | 0.456 | -0.649 | -0.654 | -0.707 | -0.733 | -0.668 | -0.721 | 0.470 | 0.467 | 0.468 | 0.462 |
| OGC1Arg2 | 0.463 | 0.456 | -0.649 | -0.654 | -0.707 | -0.733 | -0.668 | -0.721 | 0.470 | 0.467 | 0.469 | 0.462 |
| OGC7Arg1 | 0.463 | 0.456 | -0.646 | -0.654 | -0.695 | -0.733 | -0.686 | -0.727 | 0.468 | 0.466 | 0.465 | 0.460 |
| OGC7Arg2 | 0.462 | 0.456 | -0.646 | -0.654 | -0.695 | -0.733 | -0.686 | -0.727 | 0.468 | 0.466 | 0.465 | 0.460 |
| OGC2Asn | 0.468 | 0.457 | -0.641 | -0.654 | -0.683 | -0.732 | -0.713 | -0.734 | 0.464 | 0.465 | 0.455 | 0.454 |
| OGC3Asn | 0.468 | 0.455 | -0.636 | -0.649 | -0.718 | -0.743 | -0.723 | -0.737 | 0.459 | 0.463 | 0.444 | 0.453 |
| OGC4Asn | 0.447 | 0.447 | -0.653 | -0.656 | -0.702 | -0.736 | -0.744 | -0.744 | 0.464 | 0.465 | 0.460 | 0.458 |
| OGC5Asn | 0.467 | 0.456 | -0.640 | -0.654 | -0.689 | -0.732 | -0.753 | -0.748 | 0.461 | 0.464 | 0.457 | 0.459 |
| OGC6Asn | 0.469 | 0.457 | -0.640 | -0.654 | -0.692 | -0.733 | -0.700 | -0.734 | 0.461 | 0.464 | 0.458 | 0.459 |
| OGC2Ser | 0.468 | 0.456 | -0.641 | -0.654 | -0.688 | -0.733 | -0.710 | -0.730 | 0.464 | 0.466 | 0.458 | 0.459 |
| OGC5Ser | 0.468 | 0.457 | -0.641 | -0.654 | -0.691 | -0.733 | -0.732 | -0.730 | 0.463 | 0.466 | 0.459 | 0.459 |
| OGC6Ser | 0.468 | 0.456 | -0.641 | -0.654 | -0.692 | -0.733 | -0.707 | -0.732 | 0.462 | 0.465 | 0.459 | 0.459 |
| OGC2Cys | 0.466 | 0.457 | -0.641 | -0.654 | -0.689 | -0.733 | -0.713 | -0.735 | 0.463 | 0.465 | 0.458 | 0.458 |
| OGC5Cys | 0.468 | 0.457 | -0.641 | -0.654 | -0.691 | -0.733 | -0.737 | -0.738 | 0.462 | 0.465 | 0.459 | 0.459 |
| OGC6Cys | 0.468 | 0.457 | -0.641 | -0.654 | -0.692 | -0.733 | -0.709 | -0.734 | 0.462 | 0.465 | 0.459 | 0.459 |
| [1] | Mazurek A., Berardini M., Fishel R., J. Biol. Chem., 2002, 277(10), 8260—8266 |
| [2] | Greenberg M.M., Acc. Chem. Res., 2012, 45(4), 588—597 |
| [3] | Lord C.J., Ashworth A., Nature, 2012, 481(7381), 287—294 |
| [4] | Shukla L.I., Adhikary A., Pazdro R., Becker D., Sevilla M. D., Nucleic Acids Res., 2004, 32(22), 6565—6574 |
| [5] | Crenshaw C.M., Wade J. E., Arthanari H., Frueh D., Lane B. F., Núñez M. E., Biochemistry, 2011, 50, 8463—8477 |
| [6] | Naômé A., Schyman P., Laaksonen A., Vercauteren D.P., J. Phys. Chem. B, 2010, 114(14), 4789—4801 |
| [7] | Hamm M.L., Crowley K. A., Ghio M., Lindell M. A. M., McFadden E. J., Silberg J. S. L., Weaver A. M., Chem. Res. Toxicol., 2012, 25(11), 2577—2588 |
| [8] | David S.S., O'Shea V. L., Kundu S., Nature, 2007, 447(7147), 941—950 |
| [9] | Svozil D., Hobza P., Šponer J., J. Phys. Chem. B, 2010, 114(2), 1191—1203 |
| [10] | Thiviyanathan V., Somasunderam A., Volk D.E., Hazra T. K., Mitra S., Gorenstein D. G., Biochem. Biophys. Res. Commun., 2008, 366(3), 752—757 |
| [11] | Manas E.S., Getahun Z., Wright W. W., DeGrado W. F., Vanderkooi J. M., J. Am. Chem. Soc., 2000, 122(41), 9883—9890 |
| [12] | Shukla A., Barbiellini B., Buslaps T., Suortti P., Phys. Chem.(Munich), 2001, 215(10), 1315—1321 |
| [13] | Guindon-Kezis K.A., Mulder J. E., Massey T. E., Toxicology, 2014, 321, 21—26 |
| [14] | Dracínsky M., Šála M., Klepetáǐová B., Šebera J., Fukal J., Holecková V., Tanaka Y., Nencka R., Sychrovsky V., J. Phys. Chem. B, 2016, 120(5), 915—925 |
| [15] | Cadet J., Douki T., Ravanat J.L., Photochem. Photobio., 2015, 91(1), 140—155 |
| [16] | Zhao J., Du J., Liu S., Yang Z.Z., Zhao D. X., Liu C., Chem. J. Chinese Universities, 2016, 37(9), 1686—1693 |
| (赵健, 都京, 刘硕, 杨忠志, 赵东霞, 刘翠. 高等学校化学学报, 2016, 37(9), 1686—1693) | |
| [17] | Zhang Q.H., Wang Y., Liu C., Yang Z. Z., Acta Chim. Sinica, 2014, 72(8), 956—962 |
| (张千慧, 王阳, 刘翠, 杨忠志. 化学学报, 2014, 72(8), 956—962) | |
| [18] | Liu C., Wang Y., Zhao D.X., Gong L. D., Yang Z. Z., J. Mol. Graph. Model., 2014, 47, 62—76 |
| [19] | Liu C., Zhang Q.H., Gong L. D., Lu L. N., Yang Z. Z., Chem. J. Chinese Universities, 2014, 35(12), 2645—2653 |
| (刘翠, 张千慧, 宫利东, 卢丽男, 杨忠志. 高等学校化学学报, 2014, 35(12), 2645—2653) | |
| [20] | Suzuki M., Kino K., Morikawa M., Kobayashi T., Komori R., Miyazawa H., Molecules, 2012, 17(6), 6705—6715 |
| [21] | González J., Baños I., León I., Contreras-Garcia J., Cocinero E.J., Lesarri A., Fernández J. A., Millán J., J. Chem. Theory Comput., 2016, 12(2), 523—534 |
| [22] | Tomasi J., Mennucci B., Cammi R., Chem. Rev., 2005, 105(8), 2999—3094 |
| [23] | Paukku Y., Hill G., J. Phys. Chem. A, 2011, 115(18), 4804—4810 |
| [24] | Czyznikowskaz., Lipkowski P., Góra R. W., Zalesny R., Cheng C. A., J. Phys. Chem. B, 2009, 113(33), 11511—11520 |
| [25] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Balkken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision A.02, Gaussian Inc., Wallingford CT, 2009 |
| [26] | Zhao Y., Truhlar D.G., Theor. Chem. Acc., 2008, 120(1), 215—241 |
| [27] | Zhao Y., Truhlar D.G., Acc. Chem. Res., 2008, 41(2), 157—167 |
| [28] | Hohenstein E.G., Chill S. T., Sherrill C. D., J. Chem. Theory Comput., 2008, 4(12), 1996—2000 |
| [29] | Liu C., Zhao D.X., Yang Z. Z., J. Comput. Chem., 2012, 33(4), 379—390 |
| [1] | XU Yu,HUA Er. Hydrogen Bonding Study on Protic Ionic Liquids Composed of N-Alkyl Ethylenediaminum Cations with Trifluoroacetic Anion† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1954. |
| [2] | ZHAO Jian, DU Jing, LIU Shuo, YANG Zhongzhi, ZHAO Dongxia, LIU Cui. Theoretical Studies on the Effect of Amino Acid Side Chains on Hydrogen Bonding for G:C in Aqueous Solution† [J]. Chem. J. Chinese Universities, 2016, 37(9): 1686. |
| [3] | SANG Yuan-Mei, YAN Li-Kai, WEN Shi-Zheng, CONG Sha, SU Zhong-Min. Density Functional Theoretical Study on Electronic Properties of Lindquist-type Anions [M6-nMonO19]p-(M=W, Nb, Ta) [J]. Chem. J. Chinese Universities, 2012, 33(06): 1285. |
| [4] | XIE Mei-Xiang, XU Xuan*. Theoretical Studies on the Fe—Hg Interactions and the 31P NMR in [Fe(CO)x(Ph2Ppy)y(HgCl2)z](x=3, 4; y=1, 2; z=0, 1, 2) [J]. Chem. J. Chinese Universities, 2009, 30(9): 1861. |
| [5] | WAN Hui, WANG Xiao-Lu, GUAN Guo-Feng*. Interaction Between Cation and Anion in Ion-pairs of [EPy][BF4] and [EPy][PF6] in Gas and Liquid Phases [J]. Chem. J. Chinese Universities, 2009, 30(8): 1615. |
| [6] | QIU Yun-Feng, CAO Ze-Xing*. Effect of Intramolecular Structural Environment on Bond Dissociation Energies [J]. Chem. J. Chinese Universities, 2008, 29(12): 2489. |
| [7] | ZHOU Yuan1,3,4, MEI Hu2,3, YANG Li3, ZHOU Peng2, YANG Shan-Bin3,4, LI Zhi-Liang2,3,4*. Amino Acid Structural Descriptions Based on Random Sampling of Pseudo Atomic Probe and Its QSAR Study [J]. Chem. J. Chinese Universities, 2007, 28(7): 1263. |
| [8] | HAO Lan, ZHANG Yong, TAN Hong-Wei, CHEN Guang-Ju*. Theoretical Investigation of Interaction Between Unclassical Trinuclear Antitumor Platinum Complex and DNA Duplex [J]. Chem. J. Chinese Universities, 2007, 28(6): 1160. |
| [9] | WANG Zhao-Xu, ZHANG Jing-Chang, CAO Wei-liang. Theoretical Study on Intermolecular Interactions BetweenHCN(HNC) and NH3, H2O, HF [J]. Chem. J. Chinese Universities, 2007, 28(2): 320. |
| [10] | CHEN Zhao-Xing, LI Qin-Yu, XU Xuan, ZENG He-Ping. Quantum Chemistry Studies on Halogen-benzylidene-quinolin-8-ol Alumium Complex [J]. Chem. J. Chinese Universities, 2007, 28(2): 338. |
| [11] | WANG Su-Wen1, LI An-Yong1*, TAN Hong-Wei2. Theoretical Study on Red and Blue Shifting Hydrogen Bonding Between Pyridine and HCl, CHCl3 [J]. Chem. J. Chinese Universities, 2007, 28(10): 1962. |
| [12] | HUANG Su-Lan, HAO Lan, TAN Hong-Wei, CHEN Guang-Ju. Theoretical Investigation of Interaction Between Di-nuclear Platinum Drug and DNA Duplex [J]. Chem. J. Chinese Universities, 2006, 27(3): 531. |
| [13] | LIU Xiang-Wen, BAO Peng, YU Zhong-Heng. Localization Character of LFMO and NBO and Interaction Energy Analysis [J]. Chem. J. Chinese Universities, 2006, 27(1): 96. |
| [14] | FENG Hua-Sheng, BIAN Jiang, LI Le-Min . A Method for Constructing the Reasonable and Most Contracted Localized Orbitals with Less Computational Efforts [J]. Chem. J. Chinese Universities, 2004, 25(7): 1291. |
| [15] | PAN Qing-Jiang, ZHANG Hong-Xing. Studies on Bonding Property and Stability of [C(AuPH3)m]n+ (m=4—6;n=0—2) [J]. Chem. J. Chinese Universities, 2004, 25(6): 1096. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||