

高等学校化学学报 ›› 2024, Vol. 45 ›› Issue (10): 20240297.doi: 10.7503/cjcu20240297
• 研究论文: 无机化学 • 上一篇
收稿日期:2024-06-19
出版日期:2024-10-10
发布日期:2024-08-21
通讯作者:
王敏
E-mail:wangmin@qymail.bhu.edu.cn
基金资助:
WANG Xin, QI Jinyang, YANG Ruijie, SONG Zhiguo, WANG Min( )
)
Received:2024-06-19
Online:2024-10-10
Published:2024-08-21
Contact:
WANG Min
E-mail:wangmin@qymail.bhu.edu.cn
Supported by:摘要:
以三水合硝酸铜、 对甲基苯磺酸钠(p-CH3C6H4SO3Na)和咪唑(Im)为原料, 利用溶剂热合成法制备了配合物Cu(Im)4(p-CH3C6H4SO3)2. 通过单晶X射线衍射分析、 红外光谱、 热重分析、 氮气吸附/脱附分析及粉末X射线衍射分析对其进行了表征. 由单晶X射线衍射分析可知, 该配合物的中心Cu2+与2个对甲基苯磺酸根中的O原子和4个咪唑的N原子配位, 通过分子间氢键作用力形成了三维网状结构. 以Knoevenagel缩合反应为探针对配合物的催化性能进行了考察, 结果表明, 该配合物在Knoevenagel缩合反应中具有酸碱协同催化作用, 反应时间短, 产品产率高, 且催化剂可多次重复使用. 最后, 通过密度泛函理论(DFT)对Cu(Im)4(p-CH3C6H4SO3)2、 苯甲醛和丙二腈进行了量化计算, 预测了活性位点和反应位点; 并利用X射线光电子能谱进行了验证, 进而推测出可能的催化作用机理.
中图分类号:
TrendMD:
王鑫, 祁金阳, 杨瑞杰, 宋志国, 王敏. 基于苯磺酸配体构筑的Cu(II)配合物的合成、 表征及催化性能. 高等学校化学学报, 2024, 45(10): 20240297.
WANG Xin, QI Jinyang, YANG Ruijie, SONG Zhiguo, WANG Min. Synthesis, Characterization and Catalytic Property of the Cu(II) Complex Based on Benzene Sulfonic Acid Ligand. Chem. J. Chinese Universities, 2024, 45(10): 20240297.
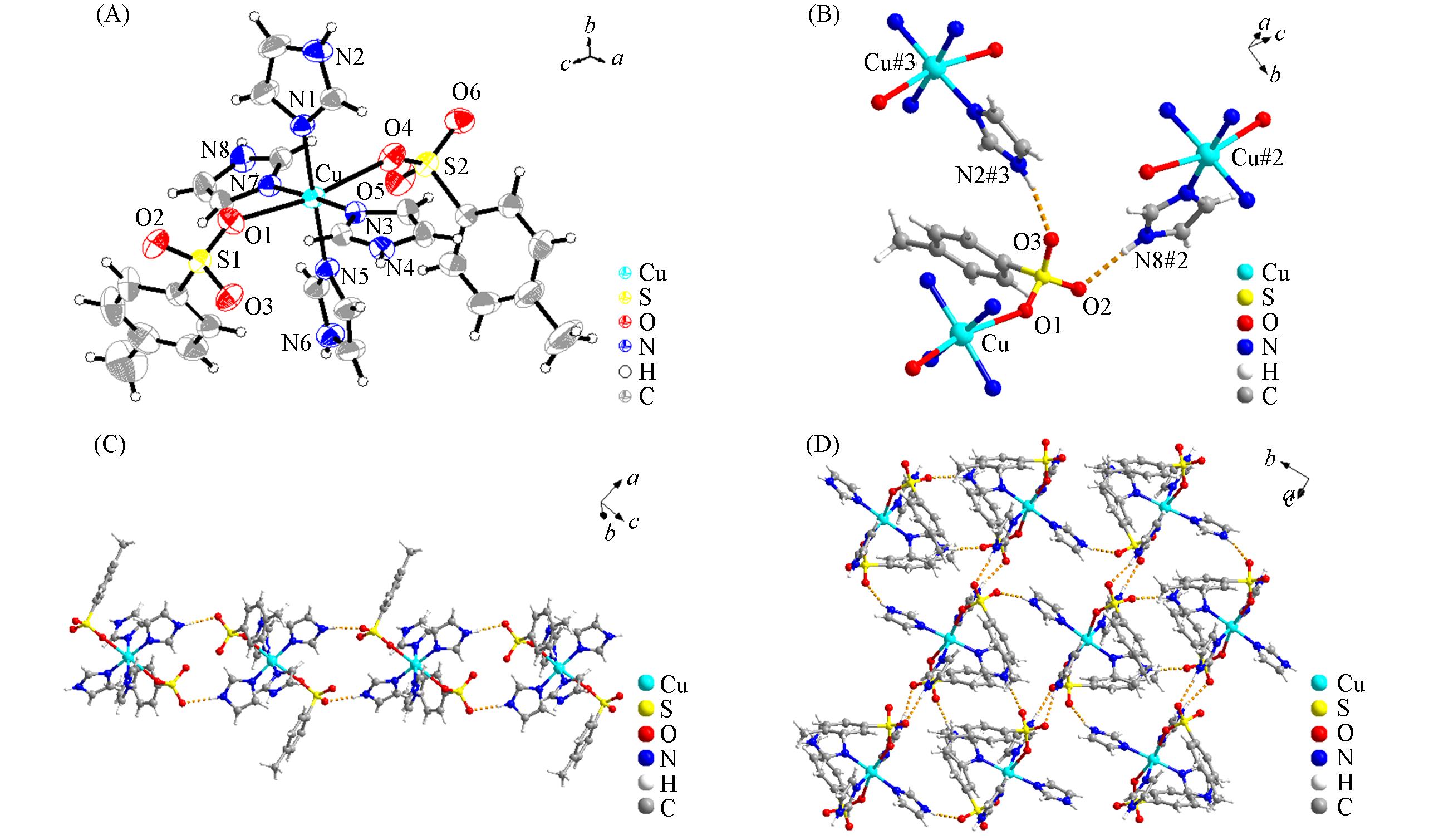
Fig.1 Molecular structure of Cu(Im)4(p⁃CH3C6H4SO3)2 (A), coordination environment of p⁃CH3C6H4SO3- (B), one⁃dimensional chain structure(C) and three⁃dimensional network structure(D) of Cu(Im)4(p⁃CH3C6H4SO3)2
| Formula | C26H30CuN8O6S2 | β/(°) | 95.787(2) |
|---|---|---|---|
| Formula weight | 678.24 | γ/(°) | 90 |
| Crystal system | Monoclinic | V/nm3 | 3.0799(5) |
| Space group | P21/n | Z | 4 |
| a/nm | 1.28841(12) | Dc/(Mg·m-3) | 1.463 |
| b/nm | 1.49801(15) | Goodness⁃of⁃fit F2 | 1.122 |
| c/nm | 1.60396(16) | R1, ωR2 | 0.0380, 0.0947 |
| α/(°) | 90 | R1, ωR2(all data) | 0.0503, 0.1075 |
Table 1 Crystallographic data of Cu(Im)4(p-CH3C6H4SO3)2(CCDC No.2111862)*
| Formula | C26H30CuN8O6S2 | β/(°) | 95.787(2) |
|---|---|---|---|
| Formula weight | 678.24 | γ/(°) | 90 |
| Crystal system | Monoclinic | V/nm3 | 3.0799(5) |
| Space group | P21/n | Z | 4 |
| a/nm | 1.28841(12) | Dc/(Mg·m-3) | 1.463 |
| b/nm | 1.49801(15) | Goodness⁃of⁃fit F2 | 1.122 |
| c/nm | 1.60396(16) | R1, ωR2 | 0.0380, 0.0947 |
| α/(°) | 90 | R1, ωR2(all data) | 0.0503, 0.1075 |
| Bond | Bond length/nm | Bond | Bond angle/(°) |
|---|---|---|---|
| Cu(1)—N(1) | 0.20131(17) | N(1)—Cu(1)—N(5) | 177.47(7)° |
| Cu(1)—N(3) | 0.19966(15) | N(3)—Cu(1)—N(7) | 177.48(7)° |
| Cu(1)—N(5) | 0.20396(17) | O(1)—Cu(1)—O(4) | 167.36(5)° |
| Cu(1)—N(7) | 0.19953(16) | N(1)—Cu(1)—N(3) | 92.06(7)° |
| Cu(1)—O(1) | 0.27392(17) | N(1)—Cu(1)—O(1) | 86.18(6)° |
| Cu(1)—O(4) | 0.26029(17) | N(1)—Cu(1)—O(4) | 81.87(6)° |
| S(1)—O(1) | 0.14557(17) | N(3)—Cu(1)—N(5) | 88.25(6)° |
| S(1)—O(2) | 0.14553(16) | N(7)—Cu(1)—N(5) | 89.85(7)° |
| S(1)—O(3) | 0.14602(15) | N(7)—Cu(1)—N(1) | 89.91(7)° |
Table 2 Partial bond lengths(nm) and bond angles(°) of Cu(Im)4(p-CH3C6H4SO3)2
| Bond | Bond length/nm | Bond | Bond angle/(°) |
|---|---|---|---|
| Cu(1)—N(1) | 0.20131(17) | N(1)—Cu(1)—N(5) | 177.47(7)° |
| Cu(1)—N(3) | 0.19966(15) | N(3)—Cu(1)—N(7) | 177.48(7)° |
| Cu(1)—N(5) | 0.20396(17) | O(1)—Cu(1)—O(4) | 167.36(5)° |
| Cu(1)—N(7) | 0.19953(16) | N(1)—Cu(1)—N(3) | 92.06(7)° |
| Cu(1)—O(1) | 0.27392(17) | N(1)—Cu(1)—O(1) | 86.18(6)° |
| Cu(1)—O(4) | 0.26029(17) | N(1)—Cu(1)—O(4) | 81.87(6)° |
| S(1)—O(1) | 0.14557(17) | N(3)—Cu(1)—N(5) | 88.25(6)° |
| S(1)—O(2) | 0.14553(16) | N(7)—Cu(1)—N(5) | 89.85(7)° |
| S(1)—O(3) | 0.14602(15) | N(7)—Cu(1)—N(1) | 89.91(7)° |
| D—H···A | d(D—H)/nm | d(H…A)/nm | d(D…A)/nm | ∠DHA/(°) |
|---|---|---|---|---|
| N(2)—H(2)···O(3)#3 | 0.086 | 0.196 | 0.2806(2) | 166.9 |
| N(4)—H(4)···O(6)#1 | 0.086 | 0.195 | 0.2795(2) | 168.4 |
| N(6)—H(6)···O(5)#4 | 0.086 | 0.189 | 0.2740(3) | 171.8 |
| N(8)—H(8)···O(2)#2 | 0.086 | 0.196 | 0.2822(2) | 175.9 |
Table 3 Hydrogen bond lengths(nm) and bond angles(°) of Cu(Im)4(p-CH3C6H4SO3)2*
| D—H···A | d(D—H)/nm | d(H…A)/nm | d(D…A)/nm | ∠DHA/(°) |
|---|---|---|---|---|
| N(2)—H(2)···O(3)#3 | 0.086 | 0.196 | 0.2806(2) | 166.9 |
| N(4)—H(4)···O(6)#1 | 0.086 | 0.195 | 0.2795(2) | 168.4 |
| N(6)—H(6)···O(5)#4 | 0.086 | 0.189 | 0.2740(3) | 171.8 |
| N(8)—H(8)···O(2)#2 | 0.086 | 0.196 | 0.2822(2) | 175.9 |
| Entry | R | Product | Time/min | Yield(%) | m. p./℃ | |
|---|---|---|---|---|---|---|
| Found | Reported | |||||
| 4a | H | 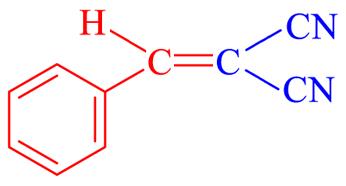 | 10 | 93.6 | 81—83 | 82—84[ |
| 4b | 2⁃Cl | 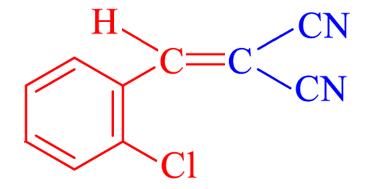 | 7 | 93.4 | 95—96 | 95—96[ |
| 4c | 4⁃Cl | 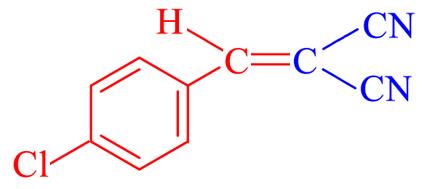 | 8 | 94.3 | 161—163 | 161—163[ |
| 4d | 2,4⁃Cl2 | 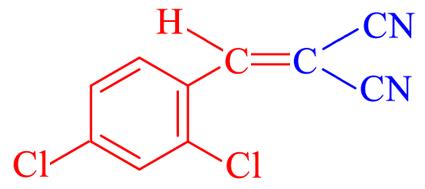 | 9 | 93.8 | 154—156 | 154—155[ |
| 4e | 2⁃NO2 | 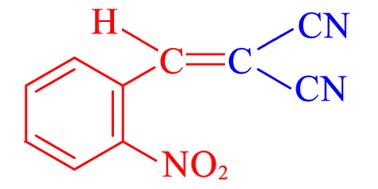 | 2 | 96.4 | 137—138 | 137—138[ |
| 4f | 3⁃NO2 | 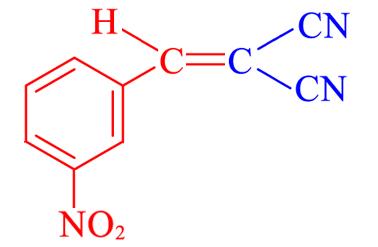 | 10 | 97.9 | 103—105 | 102—104[ |
| 4g | 4⁃NO2 | 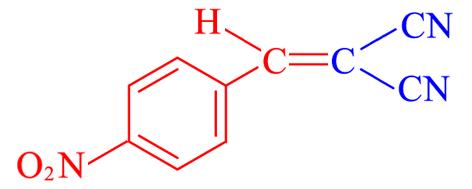 | 2 | 99.2 | 159—161 | 159—160[ |
| 4h | 3⁃OH | 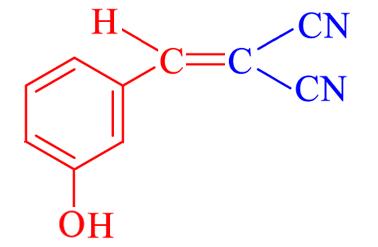 | 23 | 88.8 | 154—155 | — |
| 4i | 4⁃OH | 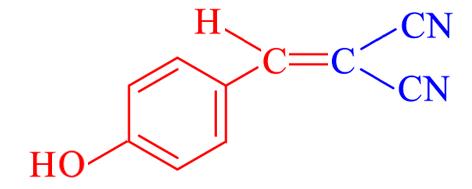 | 10 | 93.9 | 188—189 | 187—188[ |
| 4j | 4⁃CH3O | 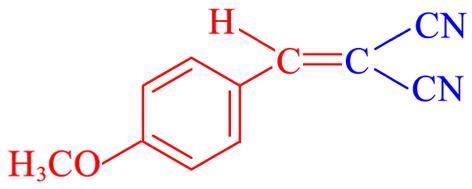 | 19 | 90.6 | 115—116 | 115—116[ |
| 4k | 2⁃CH3O | 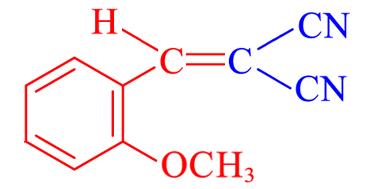 | 10 | 87.6 | 84—86 | 84—86[ |
| 4l | 4⁃CH3 | 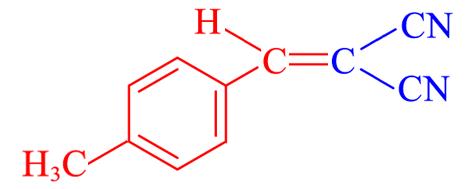 | 10 | 91.1 | 136—138 | 136—138[ |
Table 4 Synthesis of arylidene compounds catalyzed by Cu(Im)4(p⁃CH3C6H4SO3)2
| Entry | R | Product | Time/min | Yield(%) | m. p./℃ | |
|---|---|---|---|---|---|---|
| Found | Reported | |||||
| 4a | H |  | 10 | 93.6 | 81—83 | 82—84[ |
| 4b | 2⁃Cl |  | 7 | 93.4 | 95—96 | 95—96[ |
| 4c | 4⁃Cl |  | 8 | 94.3 | 161—163 | 161—163[ |
| 4d | 2,4⁃Cl2 |  | 9 | 93.8 | 154—156 | 154—155[ |
| 4e | 2⁃NO2 |  | 2 | 96.4 | 137—138 | 137—138[ |
| 4f | 3⁃NO2 |  | 10 | 97.9 | 103—105 | 102—104[ |
| 4g | 4⁃NO2 |  | 2 | 99.2 | 159—161 | 159—160[ |
| 4h | 3⁃OH |  | 23 | 88.8 | 154—155 | — |
| 4i | 4⁃OH |  | 10 | 93.9 | 188—189 | 187—188[ |
| 4j | 4⁃CH3O |  | 19 | 90.6 | 115—116 | 115—116[ |
| 4k | 2⁃CH3O |  | 10 | 87.6 | 84—86 | 84—86[ |
| 4l | 4⁃CH3 |  | 10 | 91.1 | 136—138 | 136—138[ |
| 1 | Yu N. F., Aramini J. M., Germann M. W., Huang Z., Tetrahedron Lett., 2000, 41(36), 6993—6996 |
| 2 | Tietze L. F., Rackelmann N., Pure Appl. Chem., 2004, 76(11), 1967—1983 |
| 3 | Jones G., The Knoevenagel Condensation, John Wiley & Sons, New York, 1967, 204—273 |
| 4 | Prajapati D., Lekhok K. C., Sandhu J. S., Ghosh A. C., J. Chem. Soc. Perk. T. 1, 1996, 9, 959—956 |
| 5 | Rao P. S., Venkataratnam R. V., Tetrahedron Lett., 1991, 32(41), 5821—5822 |
| 6 | Balalaie S., Sheikh⁃Ahmadi M., Bararjanian M., Catal. Commun., 2007, 8(11), 1724—1728 |
| 7 | Liu Y. T., Li R., Xing Y. J., Chin. J. Org. Chem., 2015, 35(7), 1520—1525 |
| 刘玉婷, 李戎, 邢彦军. 有机化学, 2015, 35(7), 1520—1525 | |
| 8 | Li J. T., Wang S. X., Chen G. F., Li T. S., Curr. Org. Synth., 2005, 2(3), 415—436 |
| 9 | Chandrasekhar V., Thirumoorthi R., Metre R. K., Mahanti B., J. Organomet. Chem., 2011, 696(2), 600—606 |
| 10 | Xie Z. L., Xie Y. R., Xu G. H., Du Z. Y., Zhou Z. G., Lai W. L., Inorg. Chim. Acta, 2012, 384, 117—124 |
| 11 | Jianrattanasawat S., Mezei G., Inorg. Chim. Acta, 2012, 384, 318—323 |
| 12 | Zhou Y. H., Huang R. J., Yan J. Y., Li Y. J., Qiu H. H., Yang J. X., Zheng Y. X., Chem. J. Chinese Univerties, 2022, 43(1), 20210415 |
| 周永慧, 黄如军, 严健洋, 李亚军, 邱欢欢, 杨进轩, 郑佑轩. 高等学校化学学报, 2022, 43(1), 20210415 | |
| 13 | Qi J. Y., Wang M., Yang R. J., Zhang Y. C., Song Z. G., Chem. Res. Appl., 2024, 53(2), 300—306 |
| 祁金阳, 王敏, 杨瑞杰, 张迎春, 宋志国. 人工晶体学报, 2024, 53(2), 300—306 | |
| 14 | Guo T. T., Yin S. W., Wang Y., Theor. Chem. Acc., 2017, 136(10), 126 |
| 15 | Sizova O. V., Ivanova N. V., Lyubimova O. O., Nikol’ski A. B., Russ. J. Gen. Chem., 2004, 74(2), 155—163 |
| 16 | Türkyilmaz M., Dönmez M., Altun Ö., Chem. Res. Chinese Universities, 2023, 39(6), 968—975 |
| 17 | He J. Y., Cui L., Qi Y. L., Dai Q. Q., Bai C. X., Chin. J. Polym. Sci., 2019, 37(3), 208—215 |
| 18 | Leng Y., Wang J., Zhu D. R., Wu Y. J., Zhao P. P., J. Mol. Catal. A⁃Chem., 2009, 313(1/2), 1—6 |
| 19 | Burd S. D., Ma S. Q., Perman J. A., Sikora B. J., Snurr R. L. Q., Thallapally P. K., Tian J., Wojtas L., Zaworotko M. J., J. Am. Chem. Soc., 2012, 134(8), 3663—3666 |
| 20 | Yang Y. T., Tu C. Z., Shi J. Y., Zhao T. X., Liu Z. N., Cheng F. X., Luo F., J. Solid State Chem., 2021, 302, 122439 |
| 21 | Dai J. Y., Tao Y. X., Gu X. G., Liu Z., Kong Y., Liu W. J., Ma J. F., Wei Y., J. Appl. Polym. Sci., 2015, 132(17), 41895 |
| 22 | Liao Q. S., Hou H. Y., Duan J. X., Liu S., Yao Y., Dai Z. P., Yu C. Y., Li D. D., J. Appl. Polym. Sci., 2017, 134(24), 44935 |
| 23 | Martyak N. M., Seefeldt R., Electrochim. Acta, 2004, 49(25), 4303—4311 |
| 24 | Danilov F. I., Sknar I. V., Sknar Y. E., Russ. J. Electrochem., 2014, 50(3), 293—330 |
| 25 | Qian B. H., Ma W. X., Lu L. D., Yang X. J., Wang X., Acta Phys.⁃Chim. Sin., 2010, 26(3), 610—616 |
| 钱保华, 马卫兴, 陆路德, 杨绪杰, 汪信. 物理化学学报, 2010, 26(3), 610—616 | |
| 26 | Bruker S., SMART(Version 5.628), SAINT(Version 6.45), SADABS, Bruker AXS Inc., Madison, WI, 2001 |
| 27 | Dolomanov O. V., Bourhis L. J., Gildea R. J., Howard J. A. K., Puschmann H., J. Appl. Crystallogr., 2009, 42, 339—341 |
| 28 | Sheldrick G. M., SHELXL⁃97, Program for Crystal Structure Solution, University of G9ttingen, G9ttingen, 1997 |
| 29 | Guo X. L., Ding Z. Y., Deng S. M., Wen C. C., Shen X. C., Jiang B. P., Liang H., Carbon, 2018, 134, 519—530 |
| 30 | Liu X. L., Wang C. J., Mao K. L., Zhang Y. J., Zhou X. L., Huang S., Shan L. H., Wang X. Y., J. Inorg. Chem., 2014, 30(8), 1938—1946 |
| 刘晓雷, 王萃娟, 毛凯力, 张亚军, 周先礼, 黄帅, 单连海, 王尧宇. 无机化学学报, 2014, 30(8), 1938—1946 | |
| 31 | Wu G. Z., Wang P. F., Li S. Q., Fang X. L., Inorg. Chem., 2020, 36(2), 226—232 |
| 吴国志, 汪鹏飞, 李善青, 方霄龙. 无机化学学报, 2020, 36(2), 226—232 | |
| 32 | Li C. C., Qiao X. C., Chem. Eng. J., 2016, 302, 388—394 |
| 33 | Li Y. Q., Ye H. O., Chin. J. Org. Chem., 2002, 22(9), 678—680 |
| 李毅群, 叶海鸿. 有机化学, 2002, 22(9), 678—680 | |
| 34 | Wang X. S., Zeng Z. S., Li Y. L., Shi D. Q., Tu S. J., Wei X. Y., Zong Z. M., Synth. Commun., 2005, 35(14), 1915—1920 |
| 35 | Vassileva P., Krastev V., Lakov L., Peshev O., J. Mater. Sci., 2004, 39(9), 3201—3202 |
| 36 | Neuvonen H., Neuvonen K., Koch A., Kleinpeter E., Pasanen P., J. Org. Chem., 2002, 67(20), 6995—7003 |
| 37 | Xiao J., Wu Z. Y., Chen Z. Y., Zhao P. F., Liu C. Y., Chin. J. Org. Chem., 2022, 42(4), 1179—1187 |
| 肖剑, 武志英, 陈姿依, 赵朋飞, 刘春艳. 有机化学, 2022, 42(4), 1179—1187 | |
| 38 | Takakura R., Koyama K., Kuwata M., Yamada T., Sajiki H., Sawama Y., Org. Biomol. Chem., 2020, 18(34), 6594—6597 |
| [1] | 何军, 朱傲阳, 魏雨晨, 朱怡全, 蒋莉, 何孝军. 三维氮掺杂分级多孔碳纳米片的制备及储锌性能[J]. 高等学校化学学报, 2024, 45(7): 20240099. |
| [2] | 陈俊杰, 张瑞丹, 陈越. 单层GeTe在锂/钠/钾离子电池中潜在应用的第一性原理研究[J]. 高等学校化学学报, 2024, 45(7): 20240148. |
| [3] | 张硕, 赵刘洋, 黄昊, 吴爱民, 李爱魁. 基于第一性原理高价元素Mo稳定层状富锂锰基材料的氧框架机制[J]. 高等学校化学学报, 2024, 45(5): 20240035. |
| [4] | 陈荣, 温良英, 岳东, 杨仲卿. Cl2和O2在TiC(100)表面共吸附行为的密度泛函理论分析[J]. 高等学校化学学报, 2024, 45(4): 20230497. |
| [5] | 陈晴晴, 李江涛, 黄欣蓉, 顾芳, 王海军. 氢键流体中Janus粒子的过量熵[J]. 高等学校化学学报, 2024, 45(2): 20230443. |
| [6] | 鲍春竹, 向中华. 非热解共价有机聚合物基氧还原电催化材料[J]. 高等学校化学学报, 2023, 44(5): 20220715. |
| [7] | 富忠恒, 陈翔, 姚楠, 余乐耕, 沈馨, 张睿, 张强. 固态电解质锂离子输运机制研究进展[J]. 高等学校化学学报, 2023, 44(5): 20220703. |
| [8] | 彭辛哲, 葛娇阳, 王访丽, 余国静, 冉雪芹, 周栋, 杨磊, 解令海. 基于苯并噻吩平面格的张力与重组能的理论研究[J]. 高等学校化学学报, 2023, 44(2): 20220313. |
| [9] | 张海平, 孔雪, 夏文生, 张庆红, 万惠霖. C18环基过渡金属(Os, Ir)单原子对甲烷C—H的活化[J]. 高等学校化学学报, 2023, 44(11): 20230259. |
| [10] | 冯林雁, 胡晓波, 闫苗, 苗常青, 陈瑞, 郭谨昌, 王迎进. 平面十二配位MB8C4(M=Ca, Sr, Ba)分子轮团簇的理论研究[J]. 高等学校化学学报, 2023, 44(10): 20230281. |
| [11] | 何鸿锐, 夏文生, 张庆红, 万惠霖. 羟基氧化铟团簇与二氧化碳和甲烷作用的密度泛函理论研究[J]. 高等学校化学学报, 2022, 43(8): 20220196. |
| [12] | 周雷雷, 程海洋, 赵凤玉. Pd基多相催化剂上CO2加氢反应的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220279. |
| [13] | 高文秀, 吕杰琼, 高永平, 孔长剑, 王雪平, 郭胜男, 娄大伟. 富氮多孔有机聚合物催化制备α⁃氰基肉桂酸乙酯[J]. 高等学校化学学报, 2022, 43(6): 20220078. |
| [14] | 黄汉浩, 卢湫阳, 孙明子, 黄勃龙. 石墨炔原子催化剂的崭新道路:基于自验证机器学习方法的筛选策略[J]. 高等学校化学学报, 2022, 43(5): 20220042. |
| [15] | 刘洋, 李旺昌, 张竹霞, 王芳, 杨文静, 郭臻, 崔鹏. Sc3C2@C80与[12]CPP纳米环之间非共价相互作用的理论研究[J]. 高等学校化学学报, 2022, 43(11): 20220457. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||