

高等学校化学学报 ›› 2024, Vol. 45 ›› Issue (7): 20240148.doi: 10.7503/cjcu20240148
收稿日期:2024-03-29
出版日期:2024-07-10
发布日期:2024-05-08
通讯作者:
陈俊杰
E-mail:202051000007@jmu.edu.cn
基金资助:
CHEN Junjie1( ), ZHANG Ruidan2, CHEN Yue2
), ZHANG Ruidan2, CHEN Yue2
Received:2024-03-29
Online:2024-07-10
Published:2024-05-08
Contact:
CHEN Junjie
E-mail:202051000007@jmu.edu.cn
Supported by:摘要:
开发具有快速充放电速率和有利于金属离子存储的负极材料对可充电金属离子电池来说意义重大. 本文利用基于密度泛函理论(DFT)的第一性原理方法, 探讨了具有独特锯齿状皱褶层结构的单层碲化锗(GeTe)作为锂/钠/钾离子(Li+/Na+/K+)电池负极材料的应用前景. 计算结果表明, 单层GeTe有利于Li+/Na+/K+的稳定 吸附(-0.636, -0.794和-1.260 eV), 并通过差分电荷密度及分波态密度图证明了两者之间的强相互作用. Li+/Na+/K+ 在单层GeTe上的低扩散势垒(1.103, 0.344和0.483 eV)以及通过分子动力学模拟计算出的扩散系数(3.65×10-12, 2.385×10-10和9.43×10-12 cm2/s)表明, 其在充放电过程中具有优异的扩散能力和快速的充放电速率. 合理的开路电压(0.39, 0.64和0.25 V)和高于商业石墨负极材料的理论比容量(535.4, 669.2和1070.72 mA·h/g)预示单层GeTe可作为一种有前景的锂/钠/钾离子电池负极材料, 同时可为其它类似皱褶层六方结构在能量转换和存储器件中的合理设计提供参考.
中图分类号:
TrendMD:
陈俊杰, 张瑞丹, 陈越. 单层GeTe在锂/钠/钾离子电池中潜在应用的第一性原理研究. 高等学校化学学报, 2024, 45(7): 20240148.
CHEN Junjie, ZHANG Ruidan, CHEN Yue. First-principles Study of Potential Applications of Monolayer GeTe in Lithium/sodium/potassium Ion Batteries. Chem. J. Chinese Universities, 2024, 45(7): 20240148.
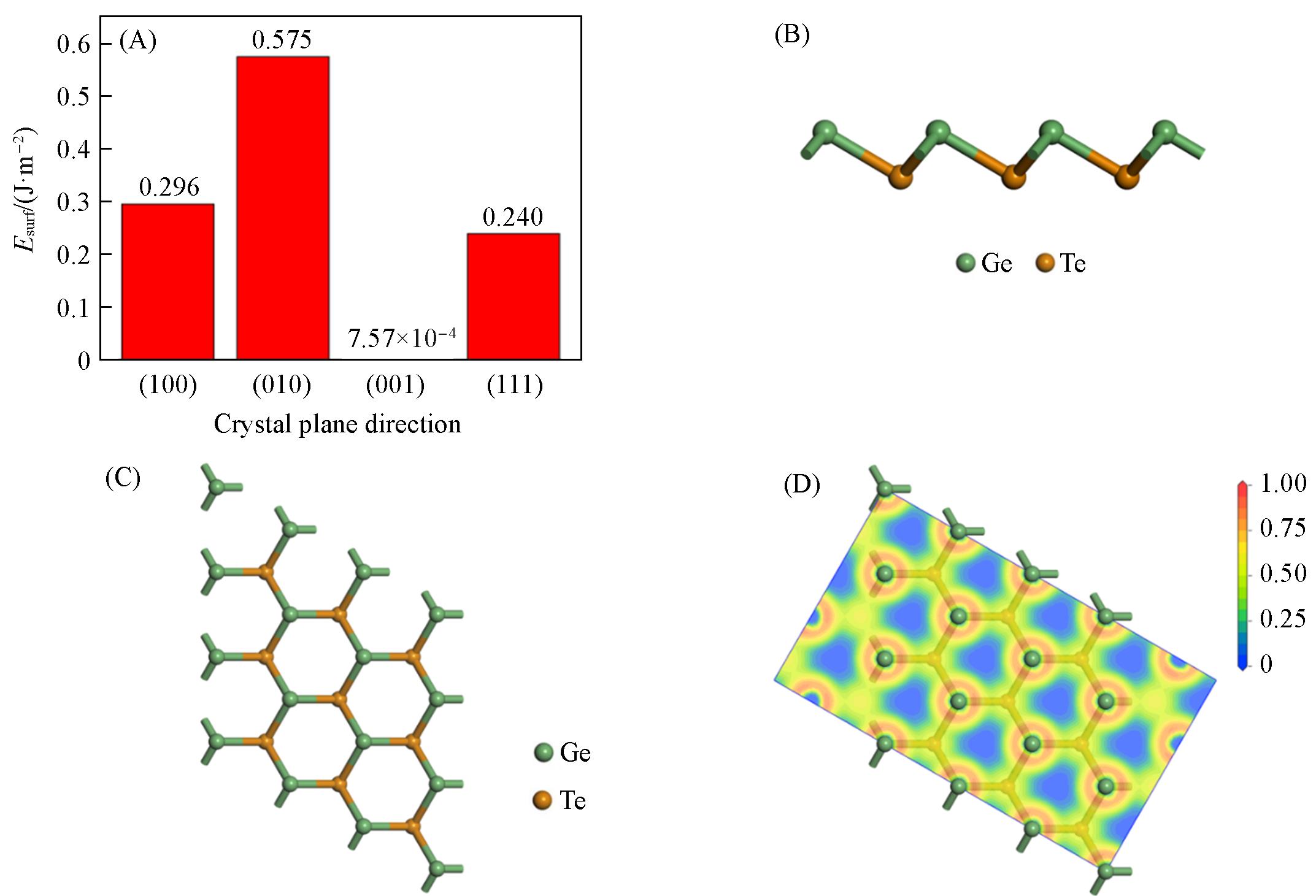
Fig.1 Surface energy of monolayer primary GeTe in different crystal plane directions(A), structure diagram of front view(B) and top view(C) of monolayer GeTe, electron localization function(ELF) of monolayer GeTe(D)
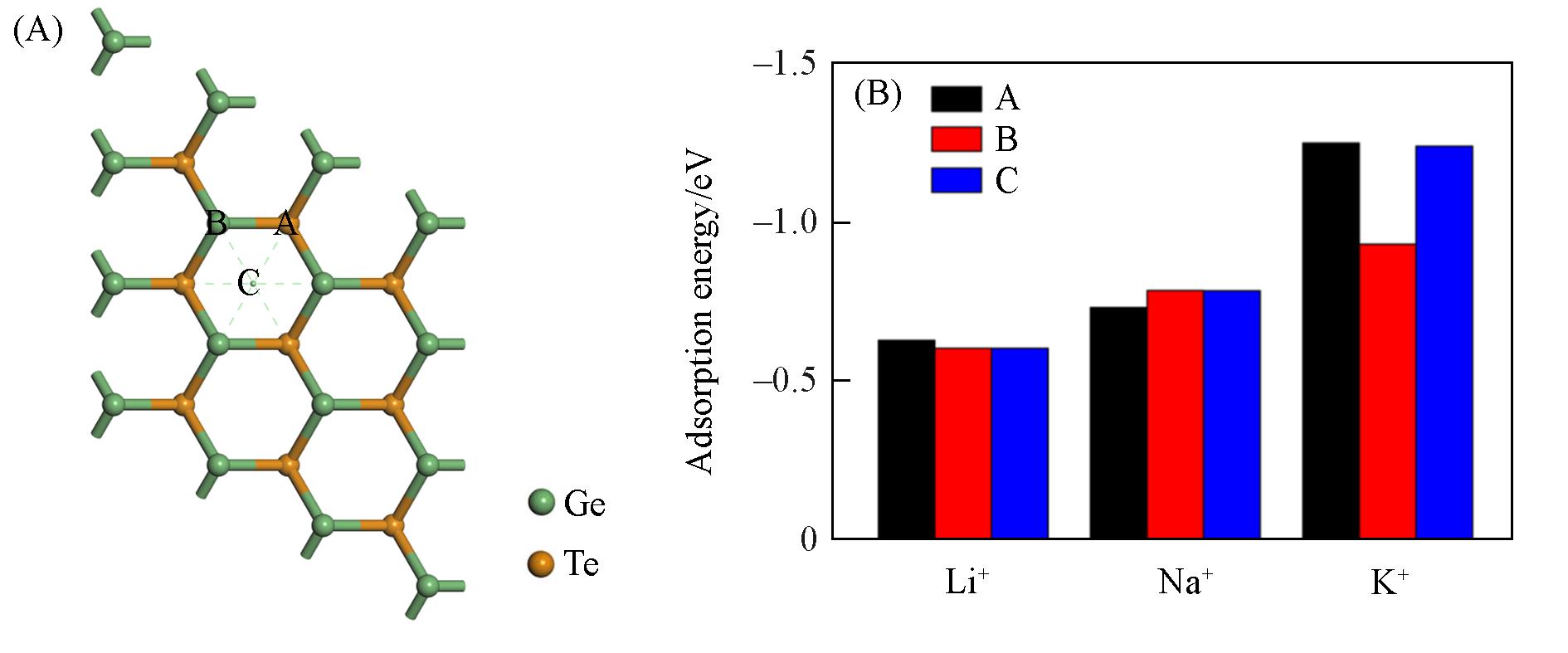
Fig.3 Three adsorption sites for metal ions(A), the adsorption energies of three metal ions at three adsorption sites(B)(A) Site A is located directly above the Te atom, site B is located directly above the Ge atom, and site C is located directly above the center point of the hexagonal structure.
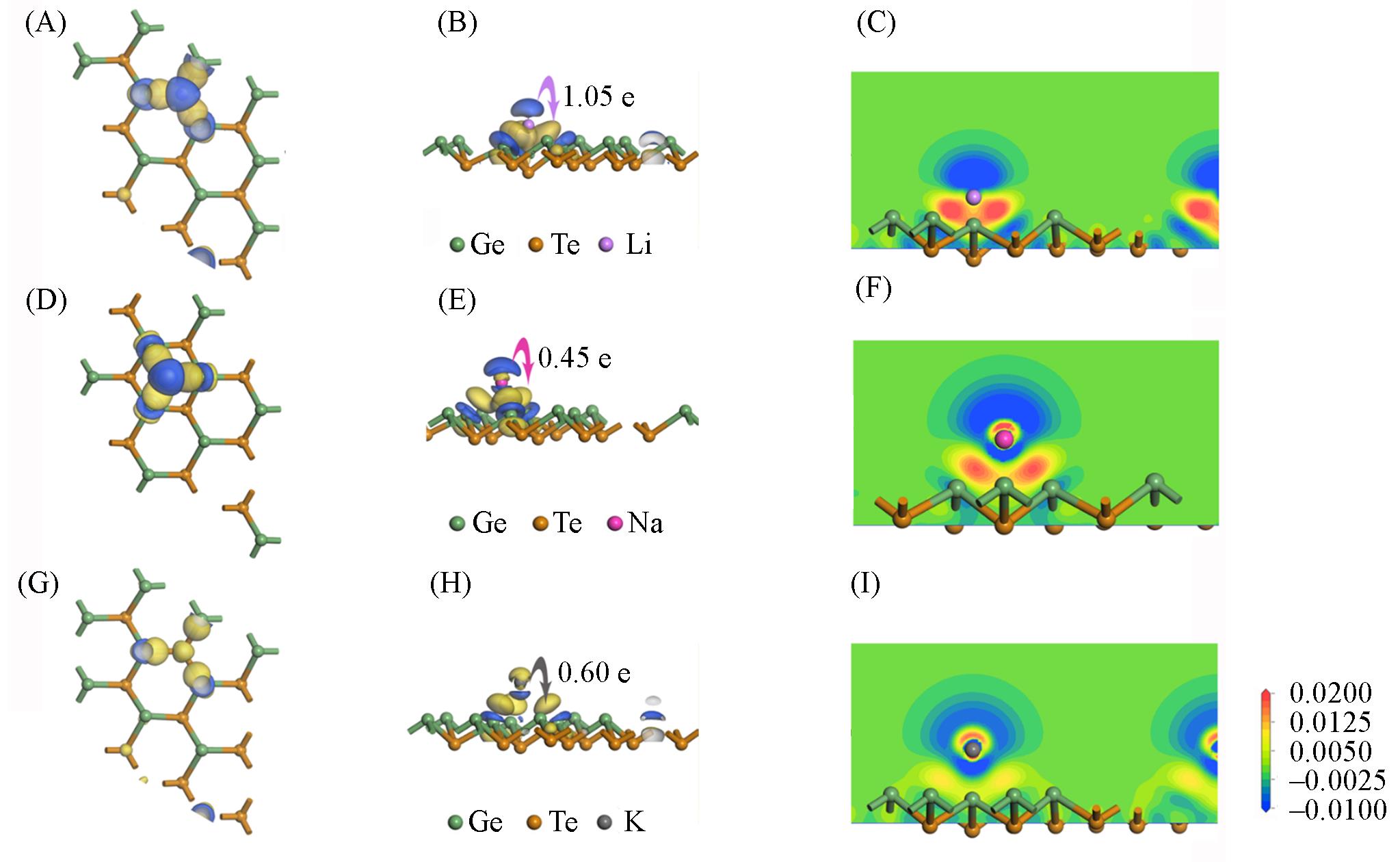
Fig.4 Electron density difference(A, D, G), charge transfer amount(B, E, H) and the electron density difference obtained along the z⁃axis cross⁃section(C, F, I) of Li(A—C), Na(D—F) and K(G—I) adsorbed on the surface of monolayer GeTe
| Ion | Ead/eV | q/e | h/nm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | |
| Li+ | -0.636 | -0.611 | -0.611 | 1.05 | 1.03 | 1.04 | 0.3107 | 0.2519 | 0.2675 |
| Na+ | -0.740 | -0.793 | -0.794 | 0.44 | 0.45 | 0.45 | 0.3745 | 0.2912 | 0.2974 |
| K+ | -1.260 | -0.940 | -1.248 | 0.60 | 0.58 | 0.59 | 0.4120 | 0.3254 | 0.3496 |
Table 1 Adsorption energy(Ead), charge transfer(q) and vertical distance(h) of lithium, sodium and potassium ions at different adsorption sites(A, B, C) on monolayer GeTe
| Ion | Ead/eV | q/e | h/nm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | |
| Li+ | -0.636 | -0.611 | -0.611 | 1.05 | 1.03 | 1.04 | 0.3107 | 0.2519 | 0.2675 |
| Na+ | -0.740 | -0.793 | -0.794 | 0.44 | 0.45 | 0.45 | 0.3745 | 0.2912 | 0.2974 |
| K+ | -1.260 | -0.940 | -1.248 | 0.60 | 0.58 | 0.59 | 0.4120 | 0.3254 | 0.3496 |
| System | ||||
|---|---|---|---|---|
| GeTe | -4.83 | -3.29 | 1.54 | |
| Li+@GeTe | -4.93 | -3.67 | 1.26 | -18.2 |
| Na+@GeTe | -4.84 | -3.78 | 1.06 | -31.2 |
| K+@GeTe | -4.79 | -3.53 | 1.26 | -18.2 |
Table 2 Energies of HOMO, LUMO and LUMO-HOMO gap(Eg )
| System | ||||
|---|---|---|---|---|
| GeTe | -4.83 | -3.29 | 1.54 | |
| Li+@GeTe | -4.93 | -3.67 | 1.26 | -18.2 |
| Na+@GeTe | -4.84 | -3.78 | 1.06 | -31.2 |
| K+@GeTe | -4.79 | -3.53 | 1.26 | -18.2 |
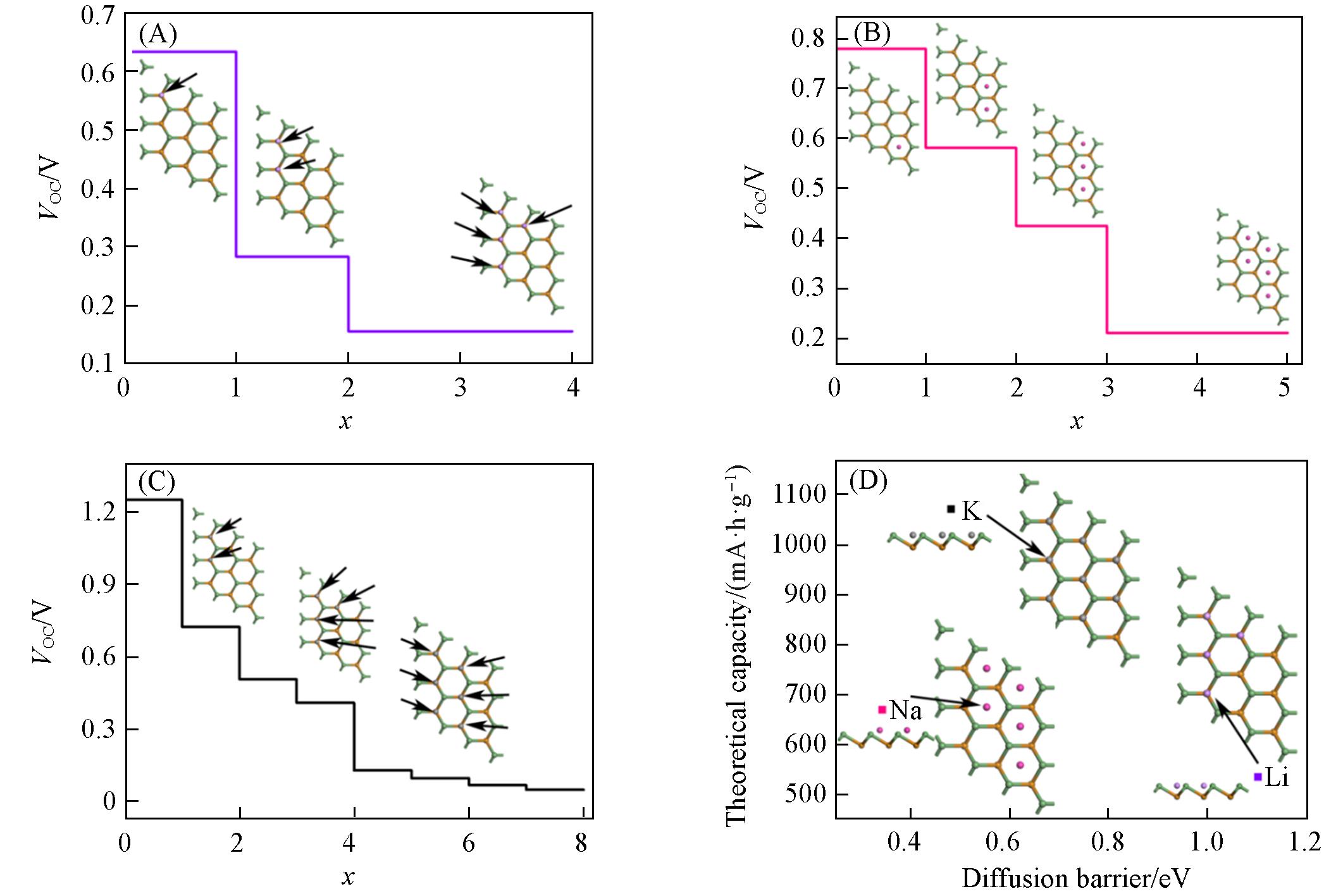
Fig.7 Open circuit voltage and corresponding adsorption models(insets) under different Li+(A), Na+(B) and K+(C) ion concentrations, theoretical capacity and diffusion barrier for Li+/Na+/K+ on monolayer GeTe(D)
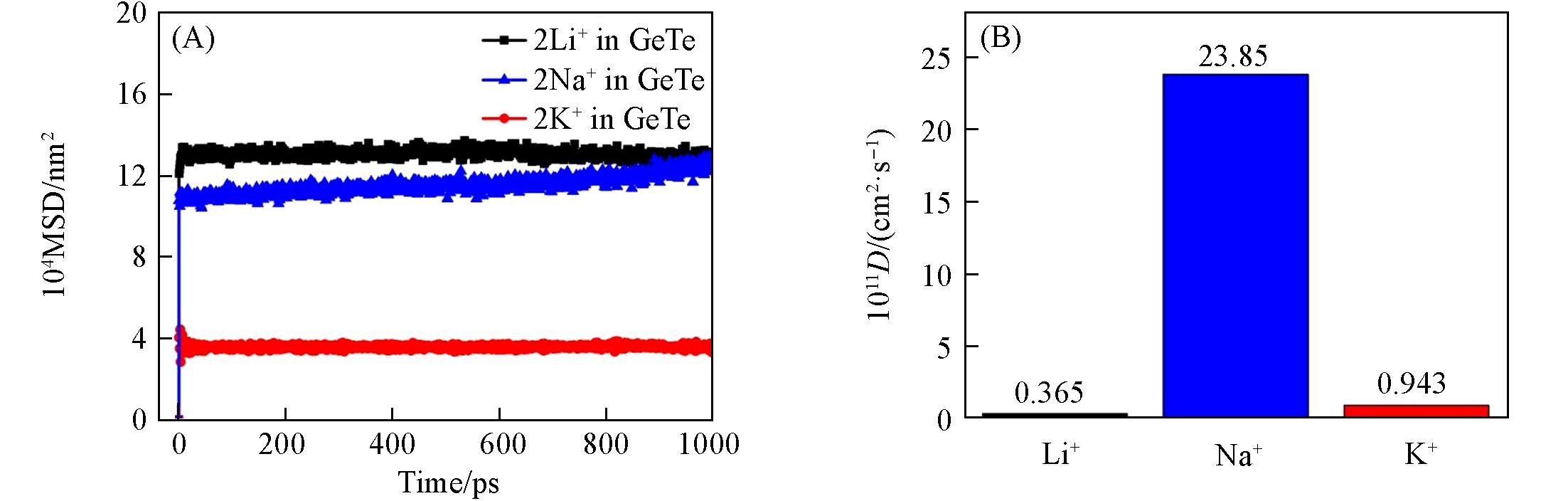
Fig.8 Molecular dynamics simulation for MSD curves of Li+/Na+/K+ at 298 K(A) and corresponding diffusion coefficients of Li+/Na+/K+(B) adsorbed on monolayer GeTe
| 1 | Dincer I., Acar C., Int. J. Energy Res., 2015, 39(5), 585 |
| 2 | Kittner N., Lill F., Kammen D. M., Nat. Energy, 2017, 2, 17125 |
| 3 | Li M., Lu J., Chen Z. W., Amine K., Adv. Mater., 2018, 30(33), 1800561 |
| 4 | Bruce P. G., Scrosati B., Tarascon J. M., Angew. Chem. Int. Ed., 2008, 47(16), 2930—2946 |
| 5 | Goodenough J. B., Park K. S., J. Am. Chem. Soc., 2013, 135(4), 1167—1176 |
| 6 | Tarascon J. M., Nat. Chem., 2010, 2(6), 510 |
| 7 | Wang Q. H., Xu J. T., Zhang W. C., Mao M. L., Wei Z. X., Wang L., Cui C. Y., Zhu Y. X., Ma J. M., J. Mater. Chem. A, 2018, 6(19), 8815—8838 |
| 8 | Hosaka T., Kubota K., Hameed A. S., Komaba S., Chem. Rev., 2020, 120(14), 6358—6466 |
| 9 | Deng J. H., Huang X. L., Gao W., Liu H. D., Xu M. W., Sustain. Energ. Fuels, 2020, 4(9), 4807—4813 |
| 10 | Kim K. H., Choi J., Hong S. H., Chem. Commun., 2019, 55(22), 3207—3210 |
| 11 | Xu Y. F., Ding T. J., Sun D. M., Ji X. L., Zhou X. S., Adv. Funct. Mater., 2023, 33(6), 2211290 |
| 12 | Liu Y. C., Liu X. B., Wang T. S., Fan L. Z., Jiao L. F., Sustain. Energ. Fuels, 2017, 1(5), 986—1006 |
| 13 | Chen K. T., Tuan H. Y., ACS Nano, 2020, 14(9), 11648—11661 |
| 14 | Nobuhara K., Nakayam A H., Nose M., Nakanishi S., Iba H., J. Power Sources, 2013, 243, 585—587 |
| 15 | Novoselov K. S., Geim A. K., Morozov S. V., Jiang D., Zhang Y., Dubonos S. V., Grigorieva I. V., Firsov A. A., Science, 2004, 306(5696), 666—669 |
| 16 | Hao K. R., Fang L. C., Yan Q. B., Su G., Phys. Chem. Chem. Phys., 2015, 20(15), 9865 |
| 17 | Souza E. S., Scopel W. L., Miwa R. H., Phys. Chem. Chem. Phys., 2018, 20(26), 17952 |
| 18 | Mak K. F., Sfeir M. Y., Wu Y., Lui C. H., Misewich J. A., Heinz T. F., Phys. Rev. Lett., 2008, 101(19), 196—199 |
| 19 | Deng Y. F., Fang C. C., Chen G. H., J. Power Sources, 2016, 304, 81—101 |
| 20 | Wang S. W., Yang B. C., Chen H. Y., Ruckenstein E., Energy Stor. Mater., 2019, 16, 619—624 |
| 21 | Liu X., Wen Y. W., Chen Z. Z., Shan B., Chen R., Phys. Chem. Chem. Phys., 2015, 17(25), 16398—16404 |
| 22 | Tritsaris G. A., Kaxiras E., Meng S., Wang E. G., Nano Lett., 2013, 13(5), 2258—2263 |
| 23 | Liang P., Cao Y. T., Tai B., Zhang L., Shu H. B., Li F., Chao D. L., Du X. Q., J. Alloys Compd., 2017, 704, 152—159 |
| 24 | Er D. Q., Li J. W., Naguib M., Gogotsi Y., Shenoy V. B., ACS Appl. Mater. Interfaces, 2014, 6(14), 11173—11179 |
| 25 | Ares P., Aguilar⁃Galindo F., Rodriguez⁃San⁃Miguel D., Aldave D. A., Díaz⁃Tendero S., Alcamí M., Martín F., Gómez⁃Herrero J., Zamora F., Adv. Mater., 2016, 28(30), 6332—6336 |
| 26 | Fuller C. S., Severiens J. C., Phys. Rev., 1954, 96, 21—24 |
| 27 | Yue C., Yu Y. J., Wu Z. G., He X., Wang J. Y., Li J. T., Li C., Wu S. T., Li J., Kang J. Y., Nanoscale, 2014, 6, 1817—1822 |
| 28 | Lim L. Y., Liu N., Cui Y., Toney M. F., Chem. Mater., 2014, 26, 3739—3746 |
| 29 | Lu X., Adkins E. R., He Y., Zhong L., Luo L., Mao S. X., Wang C. M., Korgel B. A., Chem. Mater., 2016, 28, 1236—1242 |
| 30 | Xin S., Yin Y. X., Guo Y. G., Wan L. J., Adv. Mater., 2013, 26, 1261—1265 |
| 31 | Zhang Y., Zhou Q., Zhu J., Yan Q., Dou S. X., Sun W., Adv. Funct. Mater., 2017, 27, 1702317 |
| 32 | Zeng L., Zeng W., Jiang Y., Wei X., Li W., Yang C., Zhu Y., Yu Y., Adv. Energy Mater., 2014, 5, 1401377 |
| 33 | Xu J. T., Ma J. M., Fan Q. H., Guo S. J., Dou S. X., Adv. Mater., 2017, 29(28), 1606454 |
| 34 | Nam K. H., Choi J. H., Park C. M., J. Electrochem. Soc., 2017, 164, A2056—A2064 |
| 35 | Seo J. U., Seong G. K., Park C. M., Sci. Rep., 2015, 5, 7969 |
| 36 | Herman F., Kortum R. L., Ortenburger I. B., Dyke J. P. V., J. Phys. Colloq., 1968, 29, c4—c62 |
| 37 | Tung Y. W., Cohen M. L., Phys. Rev., 1969, 180, 823—826 |
| 38 | Lebedev A. I., Sluchinskaya I. A., Demin V. N., Munro I. H., Phase Transit., 1997, 60, 67—77 |
| 39 | Sung G. K., Park C. M., J. Mater. Chem. A, 2017, 5, 5685—5689 |
| 40 | Qu B., Ma C., Ji G., Xu C., Xu J., Meng Y. S., Wang T., Lee J. Y., Adv. Mater., 2014, 26, 3854—3859 |
| 41 | Nam K. H., Sung G. K., Choi J. H., Youn J. S., Jeon K. J., Park C. M., J. Mater. Chem. A, 2019, 7, 3278—3288 |
| 42 | Sung G. K., Nam K. H., Choi J. H., Park C. M., Electrochim. Acta, 2020, 331, 135393 |
| 43 | Zeng T. B., Feng D., Peng Q. M., Liu Q., Xi G. C., Chen G., ACS Appl. Mater. Interfaces, 2021, 13(13), 15178—15189 |
| 44 | Liu X. H., Ye Q. T., Yao R. Z., Chen B., Liang W., Liu Y. S., Liu Y. P., Chen D. M., Wei Y. Q., Li D., Chen Y., Energy Stor. Mater., 2023, 63, 103039 |
| 45 | Singh A. K., Hennig R. G., Appl. Phys. Lett., 2014, 105(4), 042103 |
| 46 | Zhang P. P., Zhao F. L., Long P., Wang Y., Yue Y. C., Liu X. Y., Feng Y. Y., Li R. J., Hu W. P., Li Y., Feng W., Nanoscale, 2018, 10(34), 15989—15997 |
| 47 | Becke A. D., Phys. Rev. A, 1988, 38(6), 3098—3100 |
| 48 | Avramov P. V., Kudin K. N., Scuseria G. E., Chem. Phys. Lett., 2003, 370(5), 597—601 |
| 49 | Perdew J. P., Burke K., Ernzerhof M., Phys. Rev. Lett., 1996, 77(18), 3865 |
| 50 | Halgren T. A., Lipscomb W. N., Chem. Phys. Lett., 1977, 49(2), 225—232 |
| 51 | Kusumi A., Sako Y., Yamamoto M., Biophys. J., 1993, 65, 2021—2040 |
| 52 | Wei Y. Q., Huang L., Chen J. J., Guo Y. P., Wang S. Q., Li H. Q., Zhai T. Y., ACS Appl. Mater. Interfaces, 2019, 11, 41374—41382 |
| 53 | Ge G. X., Zhang Y. W., Yan H. X., Yang J. M., Zhou L., Sui X. J., Appl. Surf. Sci., 2021, 538, 148009 |
| 54 | Guo L., Qi C. W., Zheng X. W., Zhang R. H., Shen X., Kaya S., RSC Adv., 2017, 7, 29042—29050 |
| 55 | Yu T., Zhao Z. Y., Liu L. L., Zhang S. T., Xu H. Y., Yang G. C., J. Am. Chem. Soc., 2018, 140(18), 5962—5968 |
| 56 | Shen Y. Q., Ma Y. Y., Wu S. Y., Zhou Z. X., Comput. Mater. Sci., 2019, 170, 109200 |
| 57 | Wang Y. W., Tian W., Zhang H. J., Wang Y., Phys. Chem. Chem. Phys., 2021, 23(21), 12288—12295 |
| 58 | Zhang Y., Shi C. J., Brennecke J. F., J. Phys. Chem. B, 2014, 118(23), 6250—6255 |
| 59 | Chen J. H., He L. M., Wang R. L., J. Phys. Chem. A, 2013, 117(24), 5132—5139 |
| 60 | Hosseinian A., Soleimani⁃amiri S., Arshadi S., Vessally E., Edjlali L., Phys. Lett. A, 2017, 381, 2010—2015 |
| 61 | Karimi N., Zarrabeitia M., Mariani A., Gatti D., Varzi A., Passerini S., Adv. Energy Mater., 2021, 11(4), 2003521 |
| 62 | Wu F., Dong R. Q., Bai Y., Li Y., Chen G. H., Wang Z. H., Wu C., ACS Appl. Mater. Interfaces, 2018, 10(25), 21335—21342 |
| 63 | Li F., Qu Y., Zhao M., J. Mater. Chem. A, 2016, 4, 8905—8912 |
| 64 | Zhou Y., Zhao M., Chen Z. W., Shi X. M., Jiang Q., Phys. Chem. Chem. Phys., 2018, 20, 30290—30296 |
| 65 | Jiang H., Zhao T., Ren Y., Zhang R., Wu M., Sci. Bull., 2017, 62, 572—578 |
| 66 | Su J., Pei Y., Yang Z., Wang X., RSC Adv., 2014, 4, 43183—43188 |
| 67 | Putungan D. B., Lin S. H., Kuo J. L., ACS Appl. Mater. Interfaces, 2016, 8, 18754—18762 |
| [1] | 何军, 朱傲阳, 魏雨晨, 朱怡全, 蒋莉, 何孝军. 三维氮掺杂分级多孔碳纳米片的制备及储锌性能[J]. 高等学校化学学报, 2024, 45(7): 133. |
| [2] | 王文春, 马天赐, 刘春生. 二维半导体R57-BN作为钠离子电池阳极材料的理论研究[J]. 高等学校化学学报, 2024, 45(6): 20240043. |
| [3] | 张硕, 赵刘洋, 黄昊, 吴爱民, 李爱魁. 基于第一性原理高价元素Mo稳定层状富锂锰基材料的氧框架机制[J]. 高等学校化学学报, 2024, 45(5): 20240035. |
| [4] | 陈荣, 温良英, 岳东, 杨仲卿. Cl2和O2在TiC(100)表面共吸附行为的密度泛函理论分析[J]. 高等学校化学学报, 2024, 45(4): 20230497. |
| [5] | 张晋恺, 李佳莉, 刘晓明, 母瀛. 共价有机骨架在高性能锂离子电池负极材料中的应用[J]. 高等学校化学学报, 2024, 45(3): 20230523. |
| [6] | 陈晴晴, 李江涛, 黄欣蓉, 顾芳, 王海军. 氢键流体中Janus粒子的过量熵[J]. 高等学校化学学报, 2024, 45(2): 20230443. |
| [7] | 童大银, 赵耀林, 王禹齐, 韩子彤, 王杰, 张俊, 喻晨曦. 气态碘在COF-103上吸附的理论研究[J]. 高等学校化学学报, 2024, 45(1): 20230401. |
| [8] | 曹圣哲, 黄欣, 杨志红. 直接Z型In2SSe/Sb范德华异质结光催化水分解的第一性原理研究[J]. 高等学校化学学报, 2023, 44(8): 20230145. |
| [9] | 贺汝涵, 黎浩, 韩方, 陈奥渊, 麦立强, 周亮. 锂离子电池硅基负极界面工程的研究进展[J]. 高等学校化学学报, 2023, 44(5): 20220748. |
| [10] | 富忠恒, 陈翔, 姚楠, 余乐耕, 沈馨, 张睿, 张强. 固态电解质锂离子输运机制研究进展[J]. 高等学校化学学报, 2023, 44(5): 20220703. |
| [11] | 胡平澳, 张琪, 张会茹. 锂硫电池中硒缺陷WSe2催化性能的理论研究[J]. 高等学校化学学报, 2023, 44(2): 20220595. |
| [12] | 胡诗颖, 沈佳艳, 韩峻山, 郝婷婷, 李星. CoO纳米颗粒/石墨烯纳米纤维复合材料的制备及电化学性能[J]. 高等学校化学学报, 2023, 44(2): 20220462. |
| [13] | 彭辛哲, 葛娇阳, 王访丽, 余国静, 冉雪芹, 周栋, 杨磊, 解令海. 基于苯并噻吩平面格的张力与重组能的理论研究[J]. 高等学校化学学报, 2023, 44(2): 20220313. |
| [14] | 张海平, 孔雪, 夏文生, 张庆红, 万惠霖. C18环基过渡金属(Os, Ir)单原子对甲烷C—H的活化[J]. 高等学校化学学报, 2023, 44(11): 20230259. |
| [15] | 周惠, 朱帅波, 王际童, 乔文明, 余子舰, 张寅旭. 氮掺杂碳包覆rGO-纳米硅的制备及电化学性能[J]. 高等学校化学学报, 2023, 44(11): 20230354. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||