

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (5): 20220129.doi: 10.7503/cjcu20220129
张宏伟1, 陈雯1, 赵美淇1, 马超2( ), 韩云虎1(
), 韩云虎1( )
)
收稿日期:2022-03-03
出版日期:2022-05-10
发布日期:2022-04-04
通讯作者:
马超,韩云虎
E-mail:machao2016@tsinghua.edu.cn;iamyhhan@nwpu.edu.cn
基金资助:
ZHANG Hongwei1, CHEN Wen1, ZHAO Meiqi1, MA Chao2( ), HAN Yunhu1(
), HAN Yunhu1( )
)
Received:2022-03-03
Online:2022-05-10
Published:2022-04-04
Contact:
MA Chao,HAN Yunhu
E-mail:machao2016@tsinghua.edu.cn;iamyhhan@nwpu.edu.cn
Supported by:摘要:
目前单原子催化剂的研究呈现爆发式增长, 已然成为材料科学和催化领域的明星材料和研究热点. 前期报道的单原子催化剂研究主要针对某一个应用方向进行探讨, 较少研究催化剂的双功能或多功能应用. 近年来, 为了拓展单原子催化剂在更多领域和方向的应用, 具有双功能甚至多功能的单原子催化剂的设计开发备受关注. 本文综合评述了近年来具有双功能活性的单原子催化剂的研究进展, 重点介绍了其在电化学领域中的最新应用研究. 最后, 对具有双功能活性的单原子催化剂发展研究中存在的问题进行了简要分析, 并对未来发展前景进行了展望.
中图分类号:
TrendMD:
张宏伟, 陈雯, 赵美淇, 马超, 韩云虎. 单原子催化剂在电化学中的研究进展. 高等学校化学学报, 2022, 43(5): 20220129.
ZHANG Hongwei, CHEN Wen, ZHAO Meiqi, MA Chao, HAN Yunhu. Research Progress of Single Atom Catalysts in Electrochemistry. Chem. J. Chinese Universities, 2022, 43(5): 20220129.
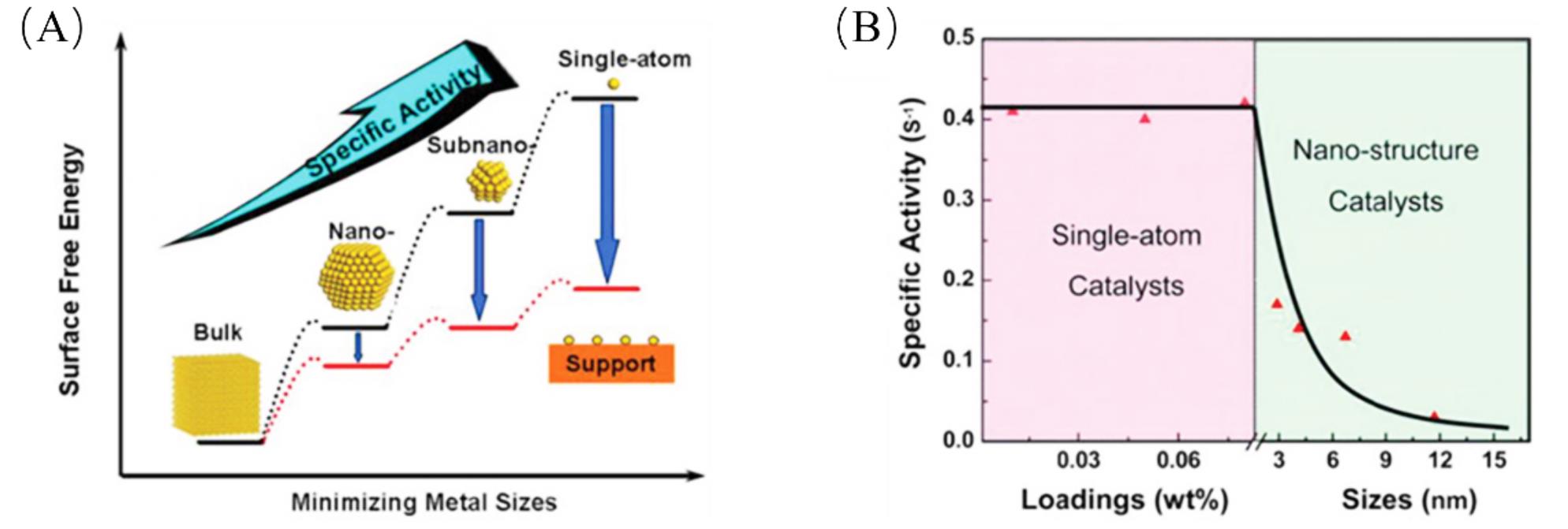
Fig.1 Schematic illustrate of the changes of surface free energy and specific activity per metal atom with metal particle size and the support effects on stabilizing single atoms(A), specific activity as a function of metal loadings/sizes(B)[24]Copyright 2013, American Chemical Society.
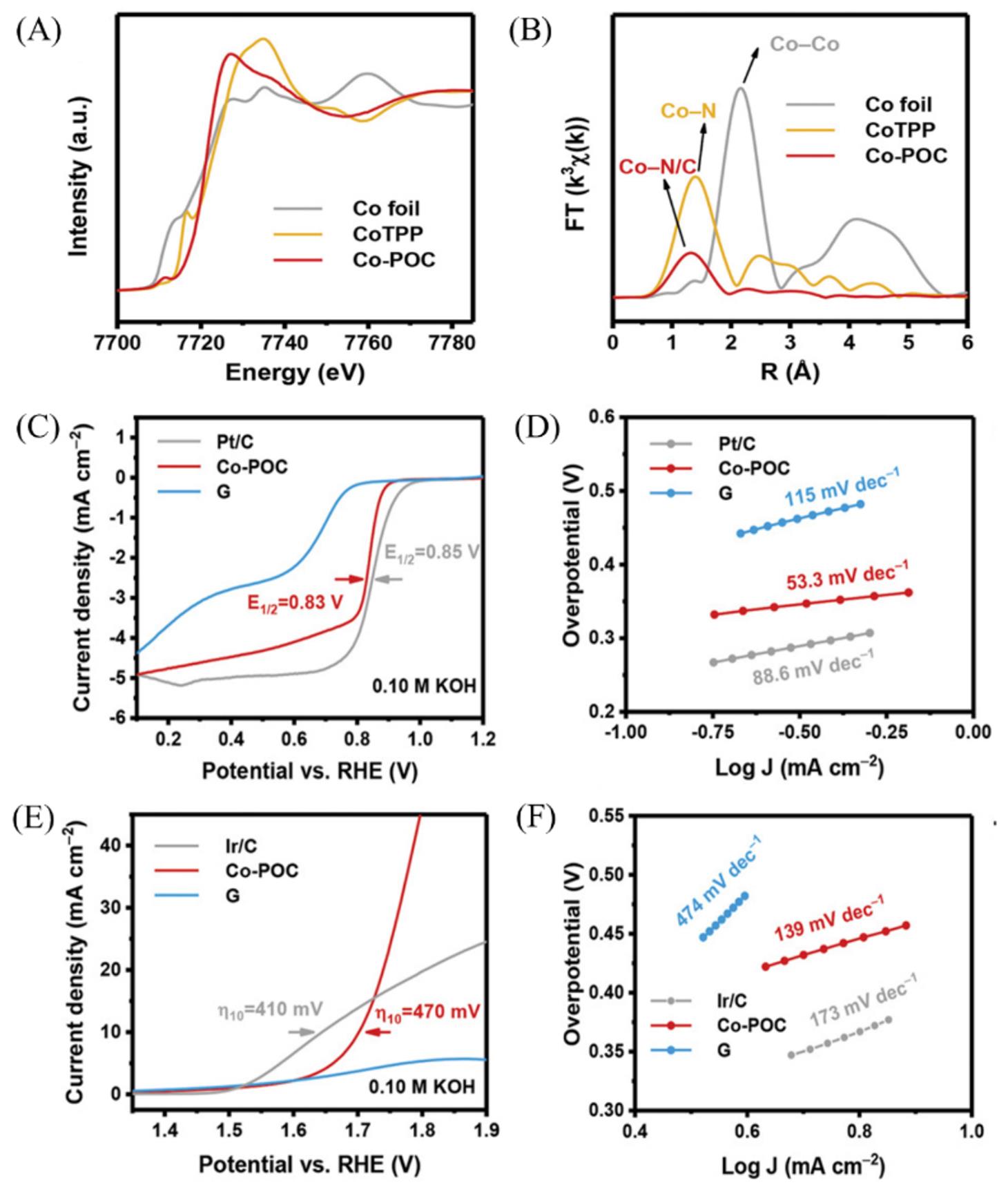
Fig.2 XANES spectra(A) and Fourier?transformed EXAFS spectra(B) at the Co K?edge of cobalt foil, CoTPP, Co?POC, 95% iR?compensated ORR LSV profiles(C), corresponding Tafel plots(D) of Pt/C, Co?POC, G electrocatalysts, 95% iR?compensated OER LSV profiles(E) and corresponding Tafel plots(F) of Ir/C, Co?POC and G electrocatalysts[42]Copyright 2019, Wiley?VCH.
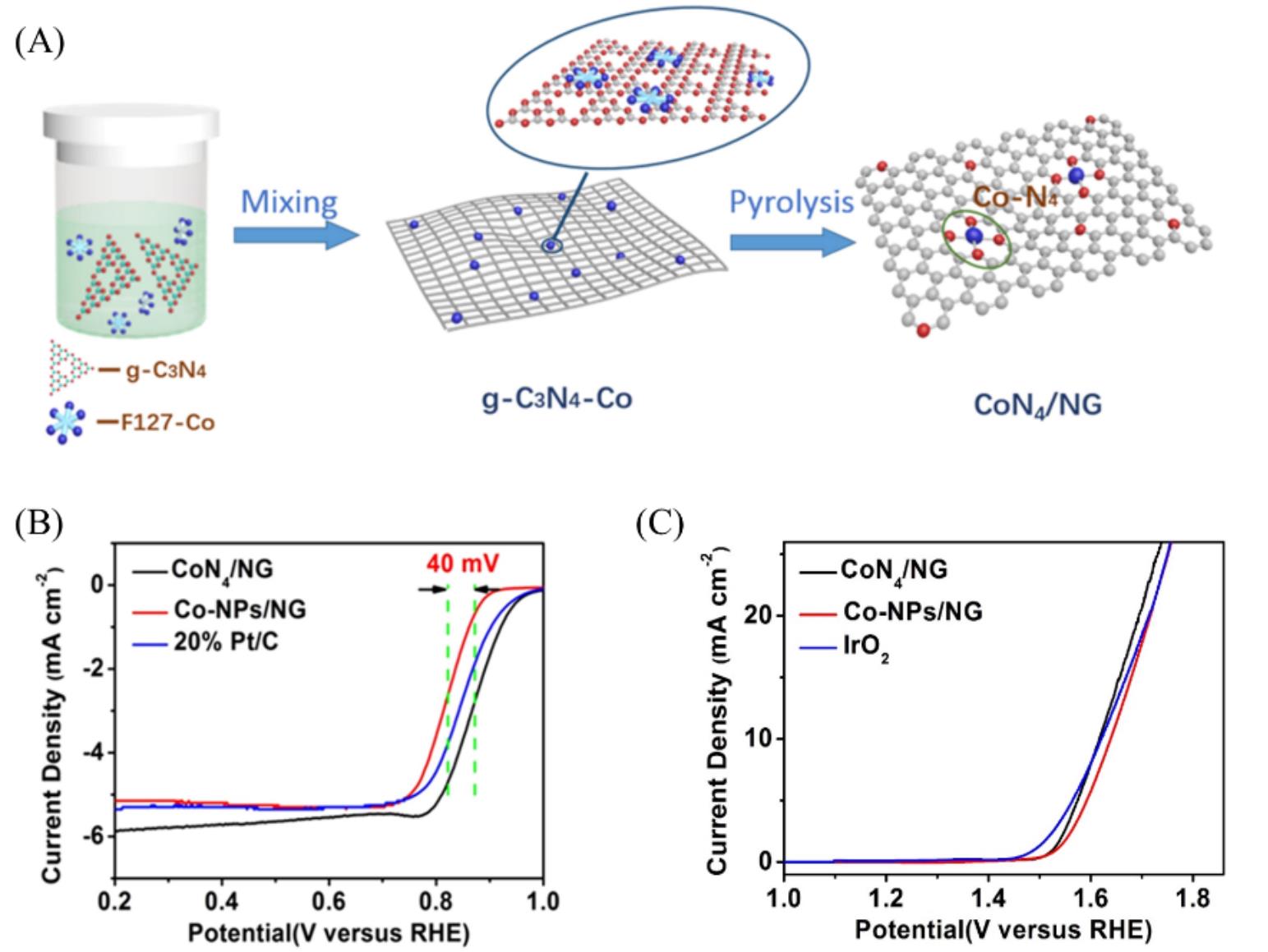
Fig.4 Schematic illustration of the synthesis of single?atom CoN4/NG catalyst(A), RRDE ORR polarization curves of CoN4/NG, Co?NPs/NG and 20% Pt/C in O2?saturated 0.1 mol/L KOH solution at electrode?rotation speed of 1600 r/min(B), OER LSV plots of CoN4/NG, Co?NPs/NG and IrO2 in 0.1 mol/L KOH(C)[47]Copyright 2018, Elsevier.
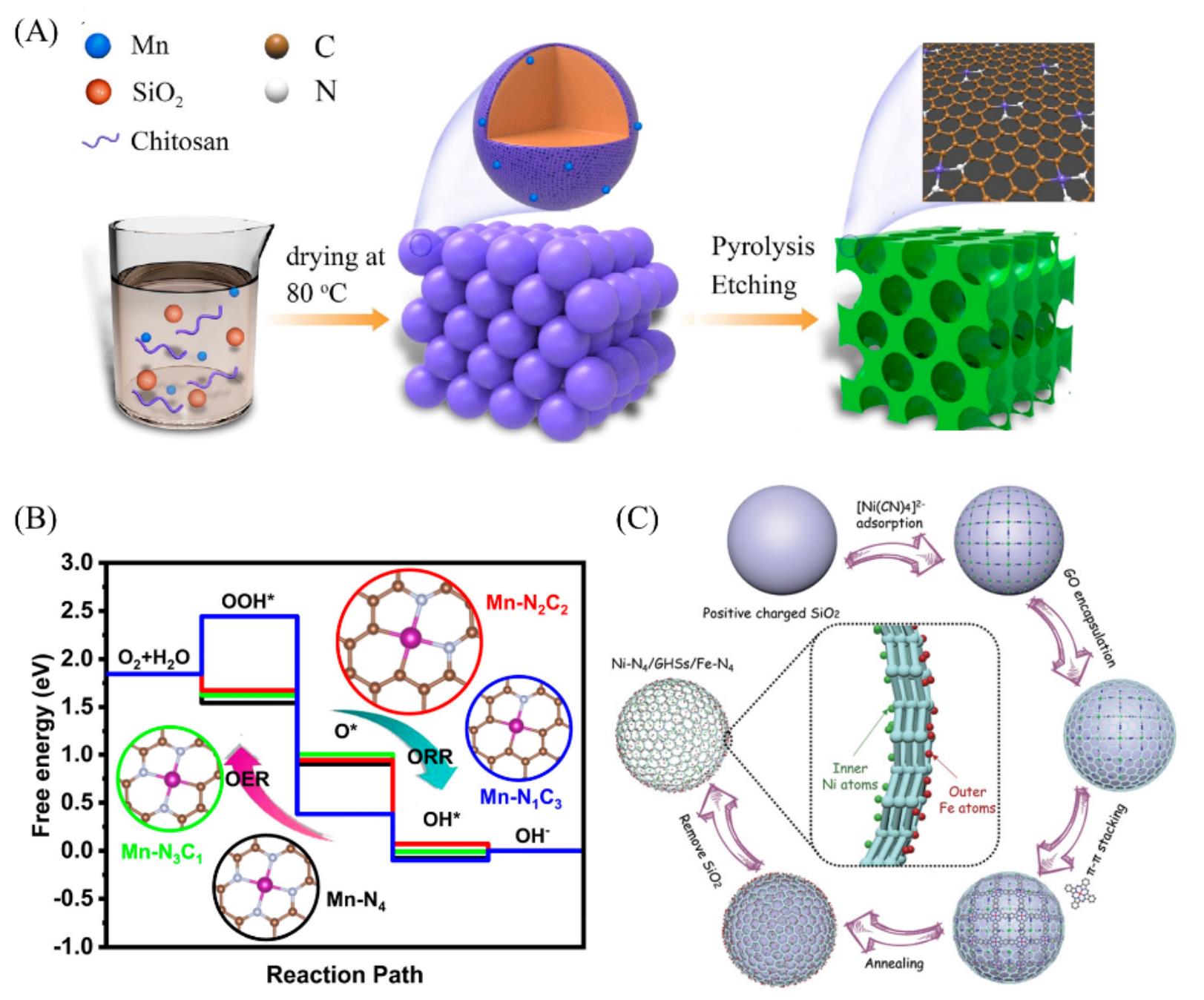
Fig.5 Illustration of the formation of MnSAC(A), free energy diagram for ORR and OER on Mn?N2C2, Mn?N4, Mn?N3C1 and Mn?N1C3(B)[50], synthetic procedure of the Ni?N4/GHSs/Fe?N4 catalyst(C)[51](A,B) Copyright 2020, American Chemical Society; (C) Copyright 2020, Wiley?VCH.
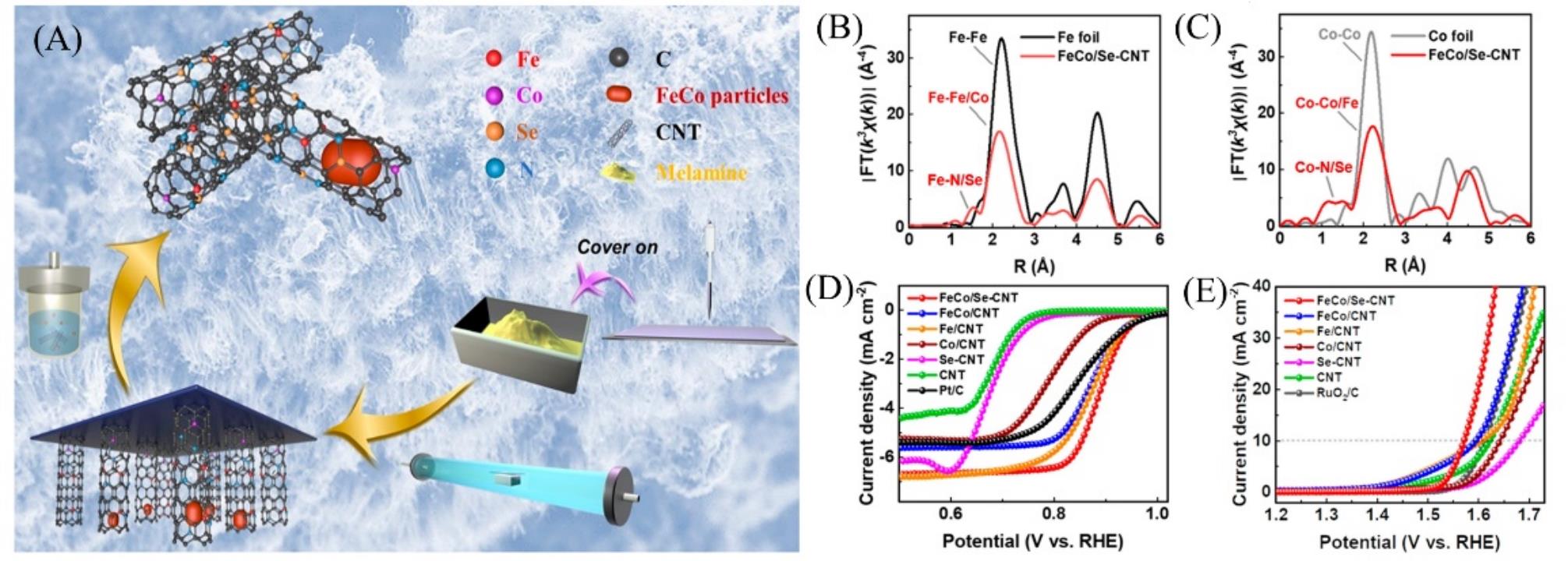
Fig.6 Schematic illustration of the synthesis of the FeCo/Se?CNT catalyst(A), fourier transform EXAFS spectra of the FeCo/Se?CNT catalyst and Fe foil(B), fourier transform EXAFS spectra of the FeCo/Se?CNT catalyst and Co foil(C), LSV polarization curves with iR corrected for ORR in 0.1 mol/L KOH media(D), LSV polarization curves with iR corrected for OER in 1 mol/L KOH media(E)[52]Copyright 2021, American Chemical Society.
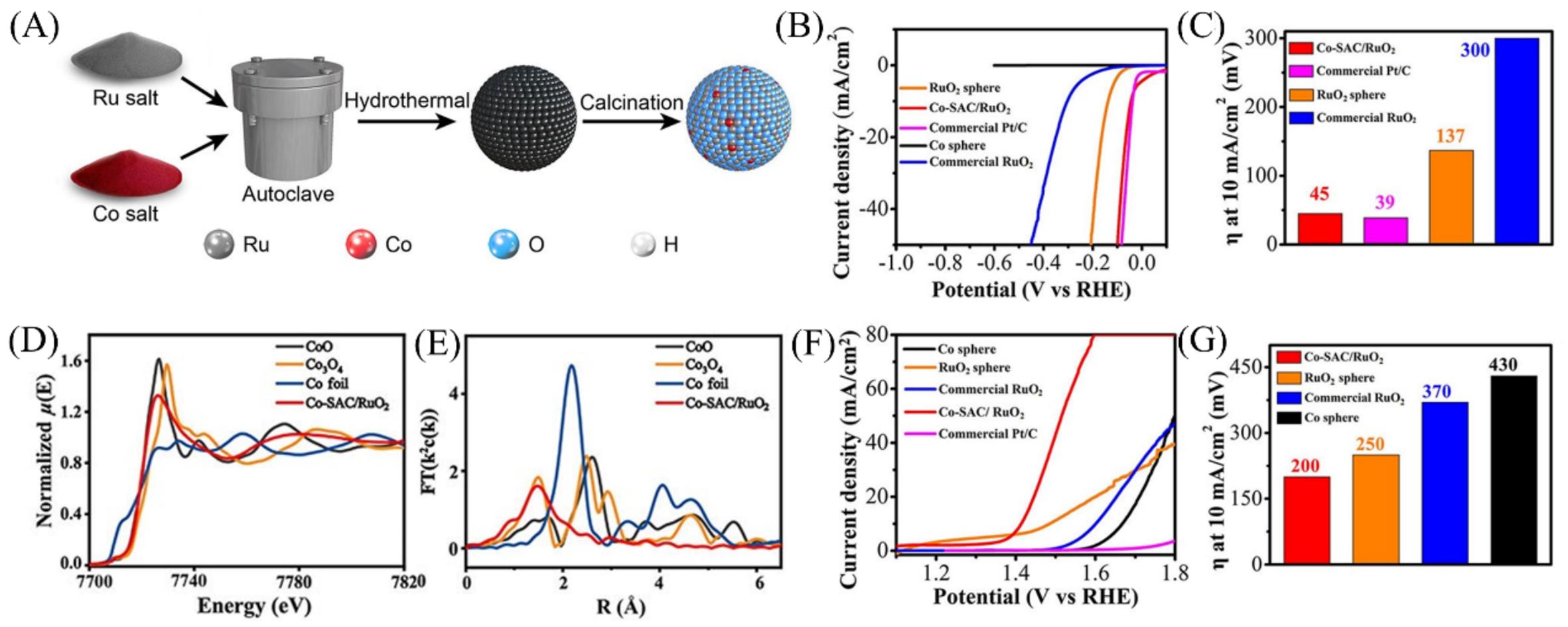
Fig.7 Schematic illustration of the synthesis of the Co single atom incorporated RuO2 sphere(A), LSV curves(B) and overpotentials at 10 mA/cm2(C) of Co?SAC/RuO2, RuO2 sphere, commercial RuO2 and Pt/C of HER in 0.5 mol/L H2SO4, XANES spectra(D) and FT?EXAFS spectra(E) of Co?SAC/RuO2, CoO, Co3O4 and Co foil, LSV curves(F) and overpotentials at 10 mA/cm2(G) of Co?SAC/RuO2, RuO2 sphere, commercial RuO2 and Pt/C of OER in 1 mol/L KOH[59]Copyright 2021, Wiley-VCH.
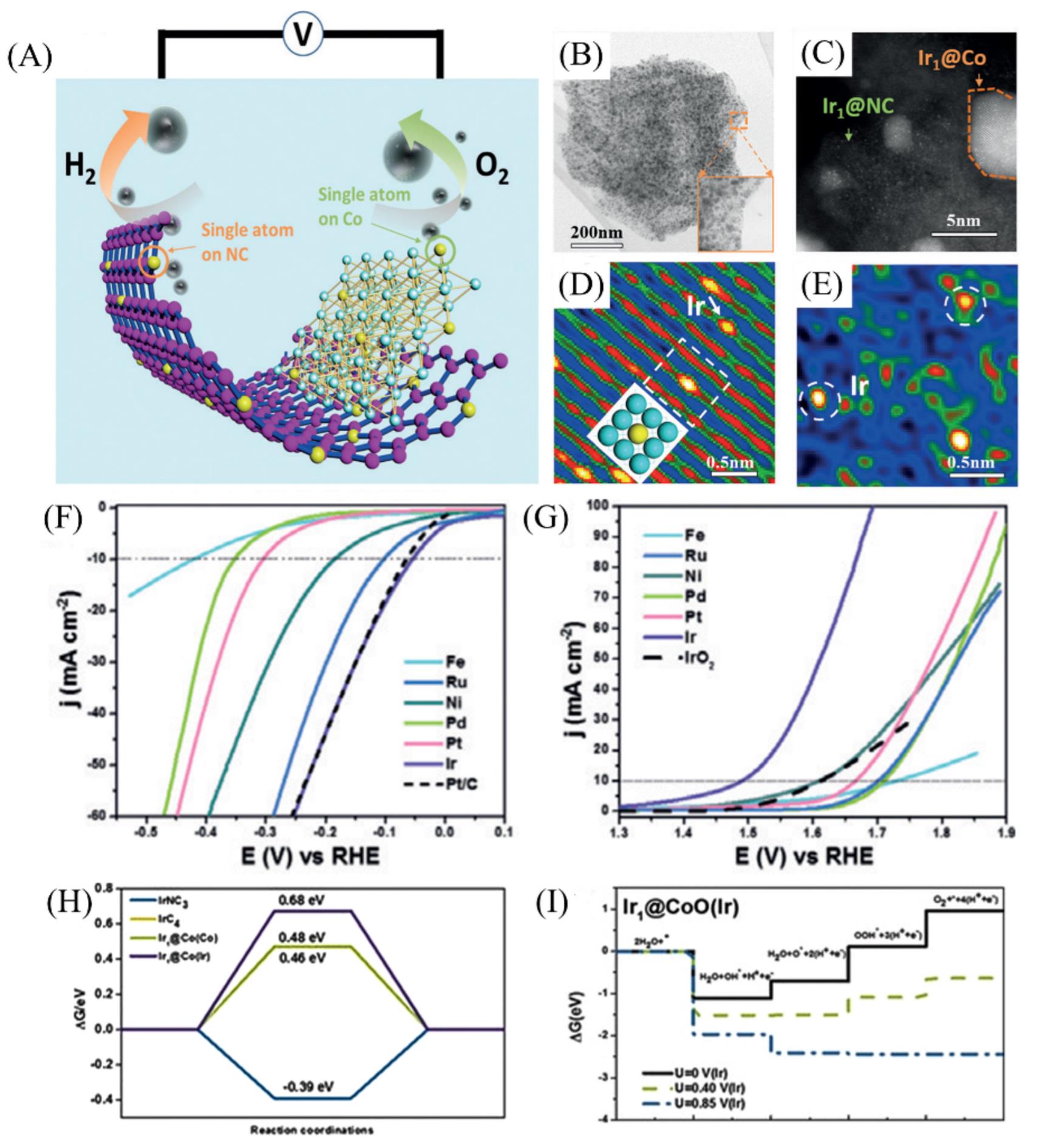
Fig.8 Illustration of the working mechanism of the prepared electrodes(A), Annular bright?field?STEM images of Ir1@Co/NC at low magnification(B), HAADF?STEM image of Ir1@Co/NC at high magnification(C), HAADF?STEM image of Ir1@Co region(D), FFTI?HAADF image of Ir1@NC area(E), polarization curves of the HER and the OER of Ir1@Co/NC, Pt1@Co/NC, Pd1@Co/NC, Ru1@Co/NC, Ni1@Co/NC, Fe1@Co/NC, commercial Pt/C and IrO2 electrodes(F, G), the free?energy diagrams for the HER at pH=14 on IrNC3, IrC4, Ir1@Co(Co) and Ir1@Co(Ir)(H), the reaction free energies of the intermediates on Ir1@CoO(Ir)(I)[60]Copyright 2019, Wiley-VCH.
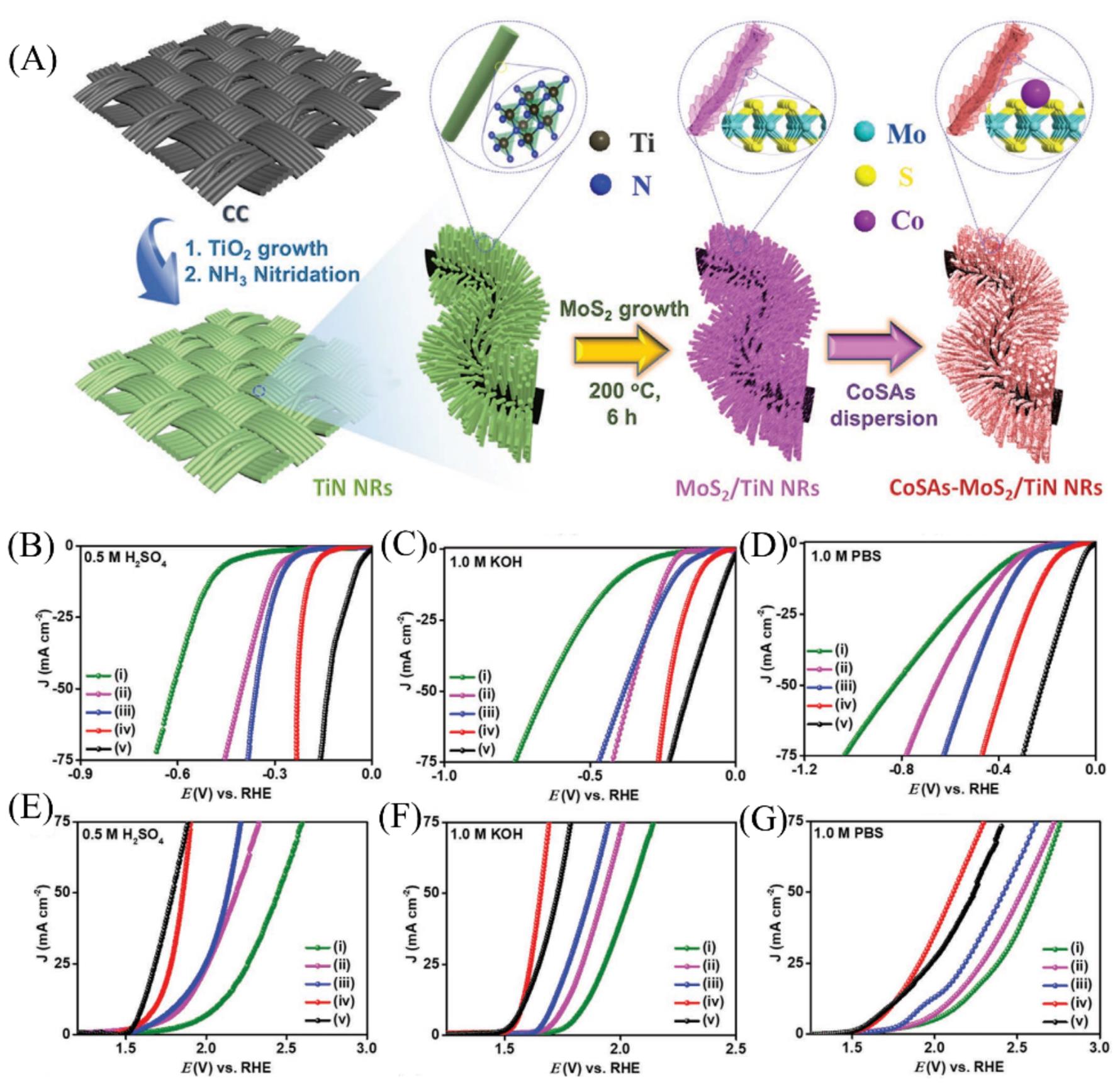
Fig.9 Schematic illustration of the fabrication of the CoSAs?MoS2/TiN hybrid(A), iR?corrected polarization curves of HER activity of the as?prepared samples in 0.5 mol/L H2SO4(B), 1.0 mol/L KOH(C) and 1 mol/L PBS(D), iR?corrected polarization curves of OER activity of the as?prepared samples in 0.5 mol/L H2SO4(E), 1.0 mol/L KOH(F), and 1 mol/L PBS(G)[66](i)TiN NRs; (ii) CoSAs-MoS2 NSs; (iii) MoS2/TiN NRs; (iv) CoSAs-MoS2/TiN NRs;(v) Pt/C of HER and RuO2 of OER. Copyright 2021, Wiley-VCH.
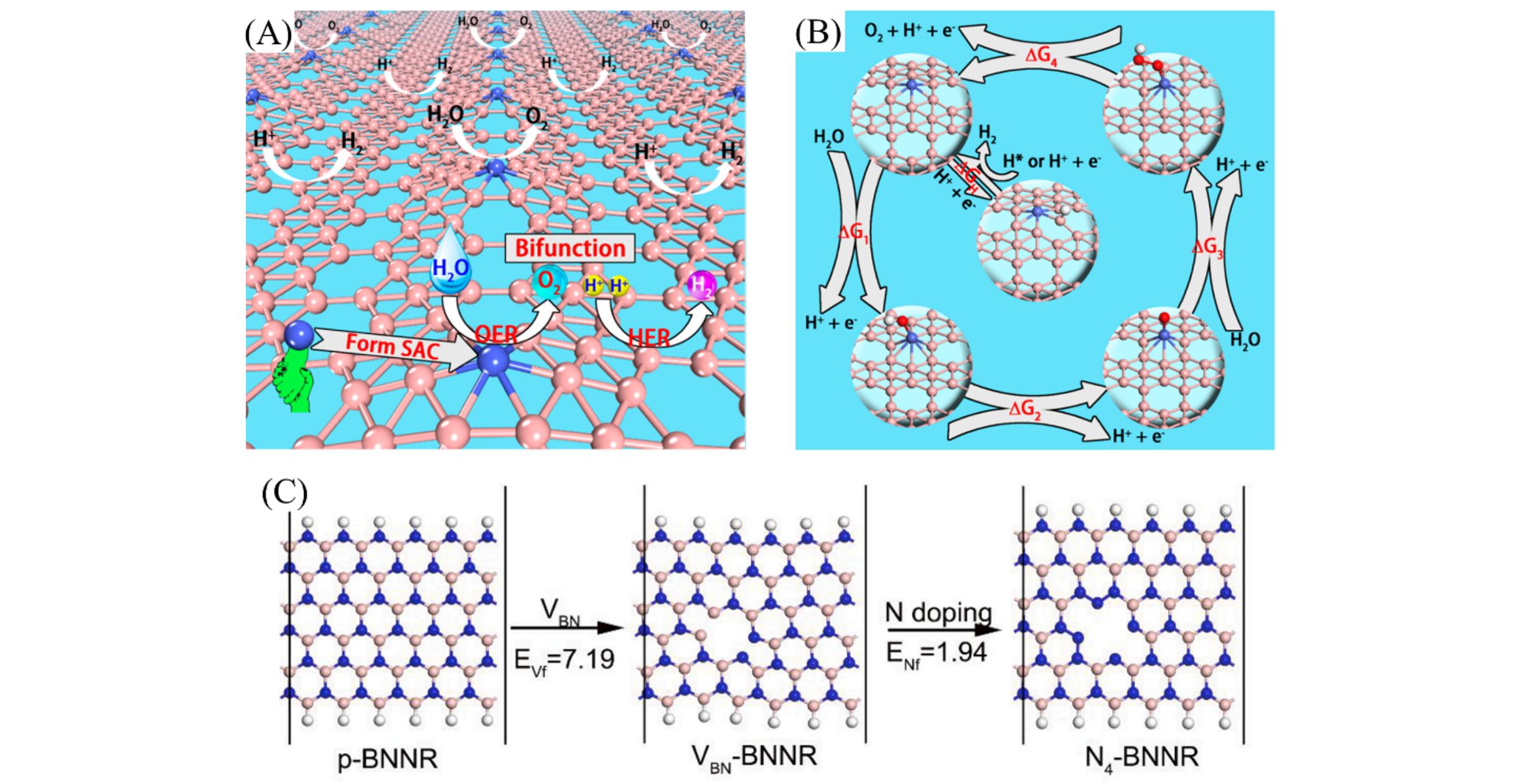
Fig.10 Schematic of the single?atom, bifunctional catalyst for overall water splitting(A), elementary reactions of OER and HER and the structures of the adsorbed states for each species, including *H, *OH, *O, and *OOH(B)[70], the schematic illustration of proposed synthetic route of TMN4?BNNR(C)[73](A, B) Copyright 2017, American Chemical Society; (C) Copyright 2021, Elsevier.
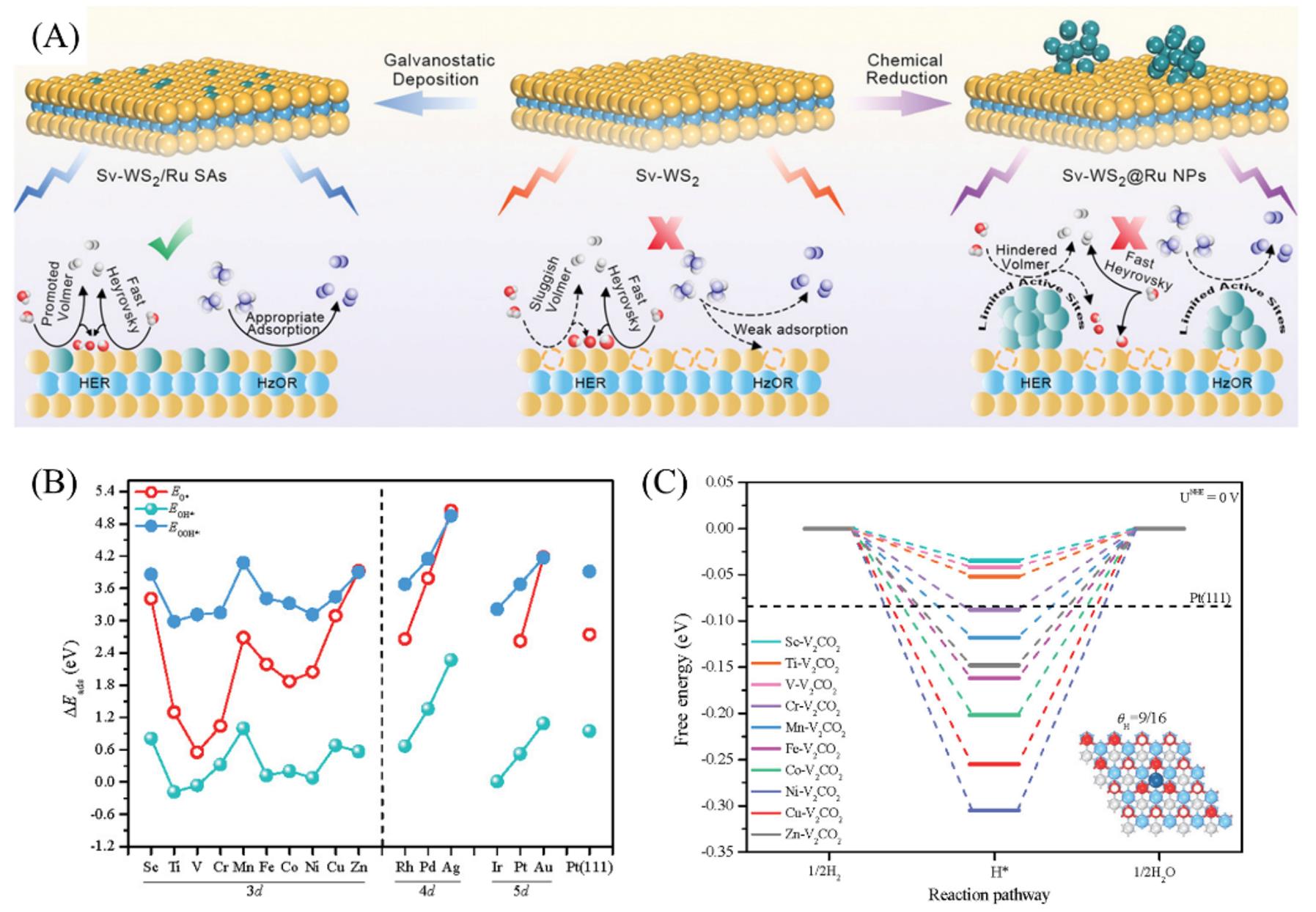
Fig.11 Schematic illustration of the proposed alkaline HER and HzOR mechanism on Sv?WS2, Sv?WS2@Ru NPs, and Sv?WS2/Ru SAs(A)[74], O, OH and OOH adsorption energy(ΔEads) on TM‐V2CO2(B), HOR free energy diagrams on TM‐V2CO2 at θH of 9/16(C)[75](A) Copyright 2022, Wiley-VCH; (B, C) Copyright 2021, Elsevier.
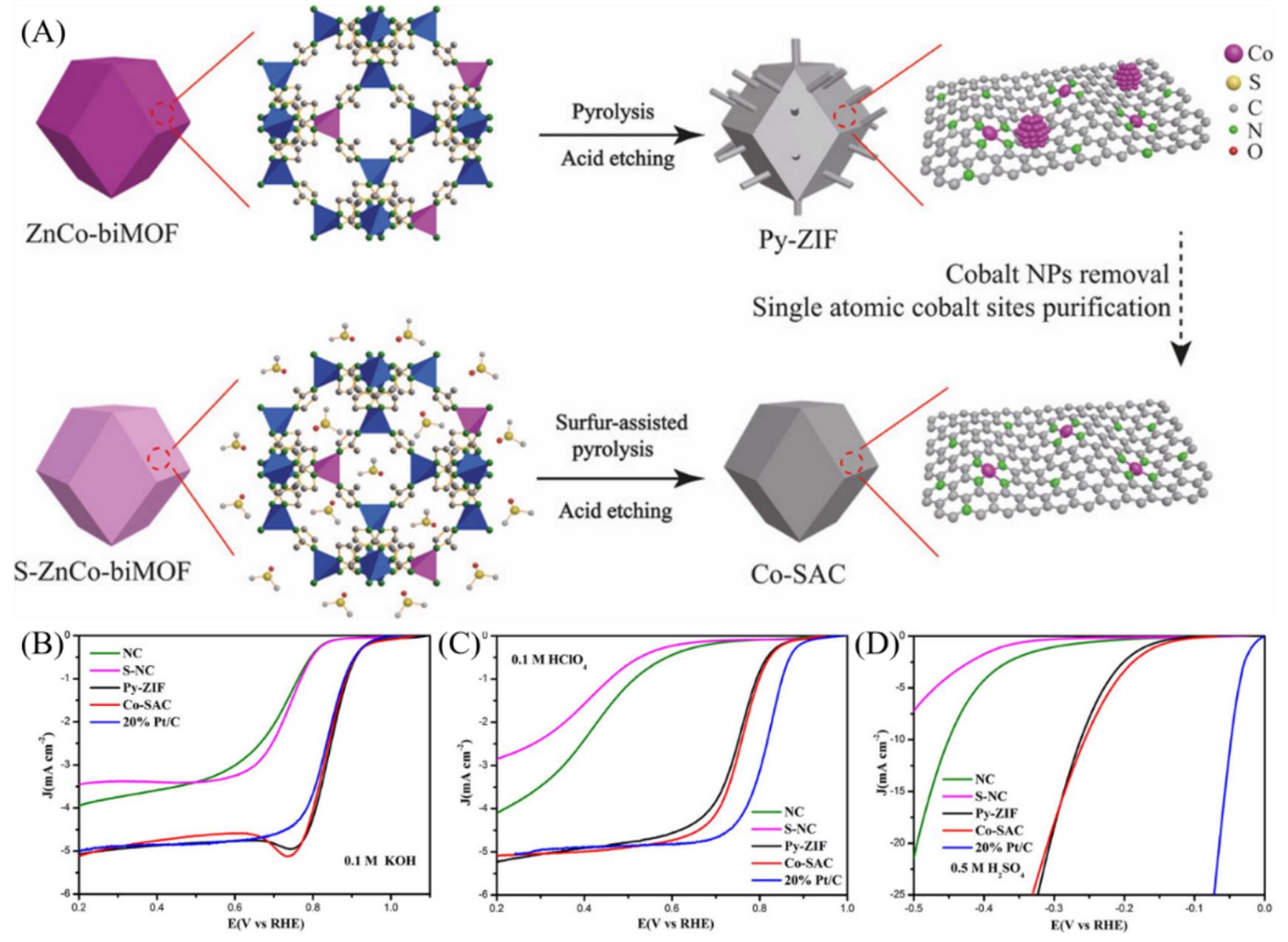
Fig.12 Illustration of the coupled evolution of single?atom cobalt sites and metallic cobalt sites and their decoupling process(A), the LSV curves of catalysts NC, S?NC, Py?ZIF, Co?SAC and 20%Pt/C in oxygen?saturated 0.1 mol/L KOH(B), 0.1 mol/L HClO4(C) and nitrogen?saturated 0.5 mol/L H2SO4(D)[77]Copyright 2018, Chemistry Europe.
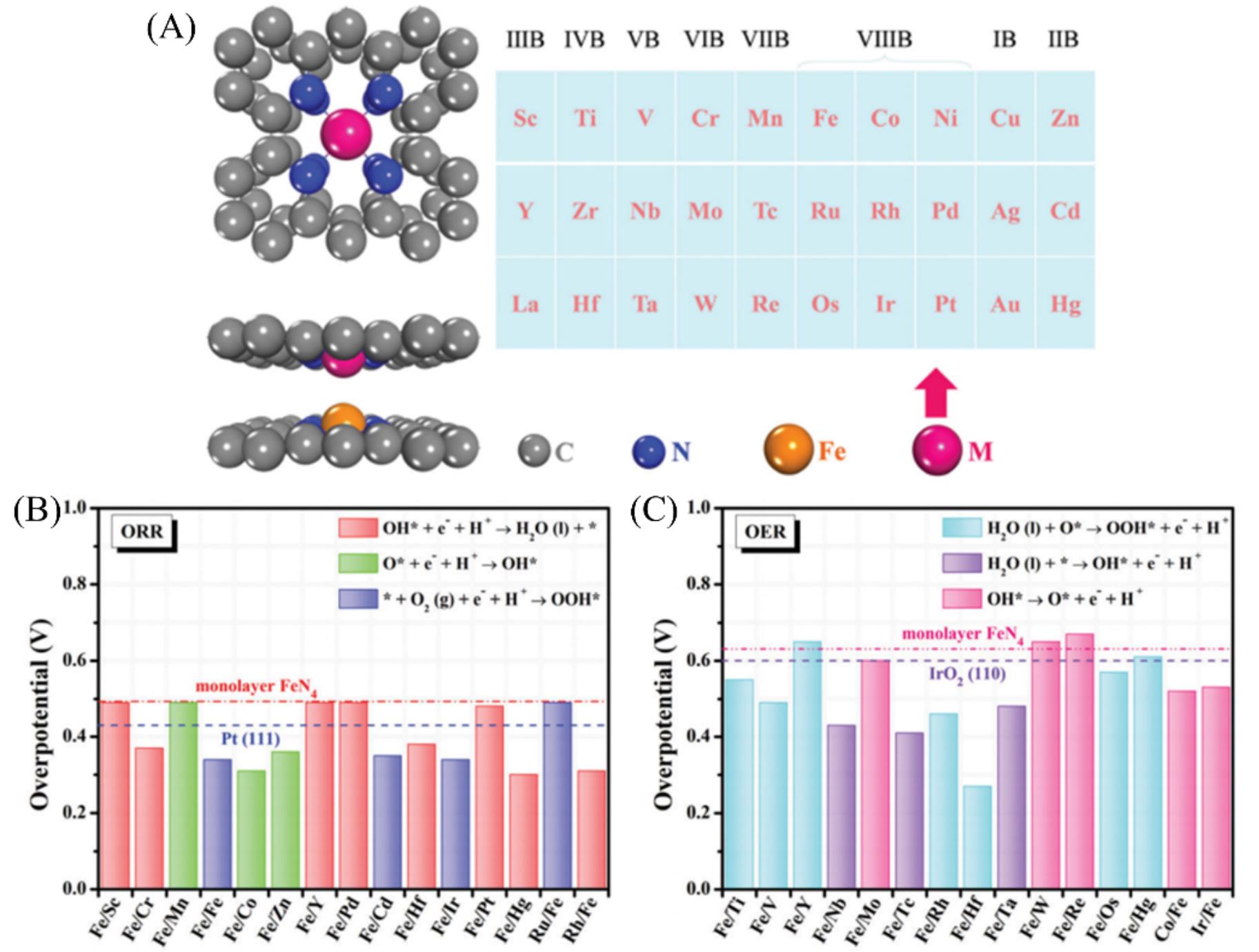
Fig.13 Top and side views of B?SACs(A), calculated theoretical ORR overpotential on highly active heterostructure systems for the ORR under U=0 V(B), calculated theoretical OER overpotential on highly active heterostructure systems for the OER under U=0 V(C)[80]Copyright 2020, Royal Society of Chemistry.
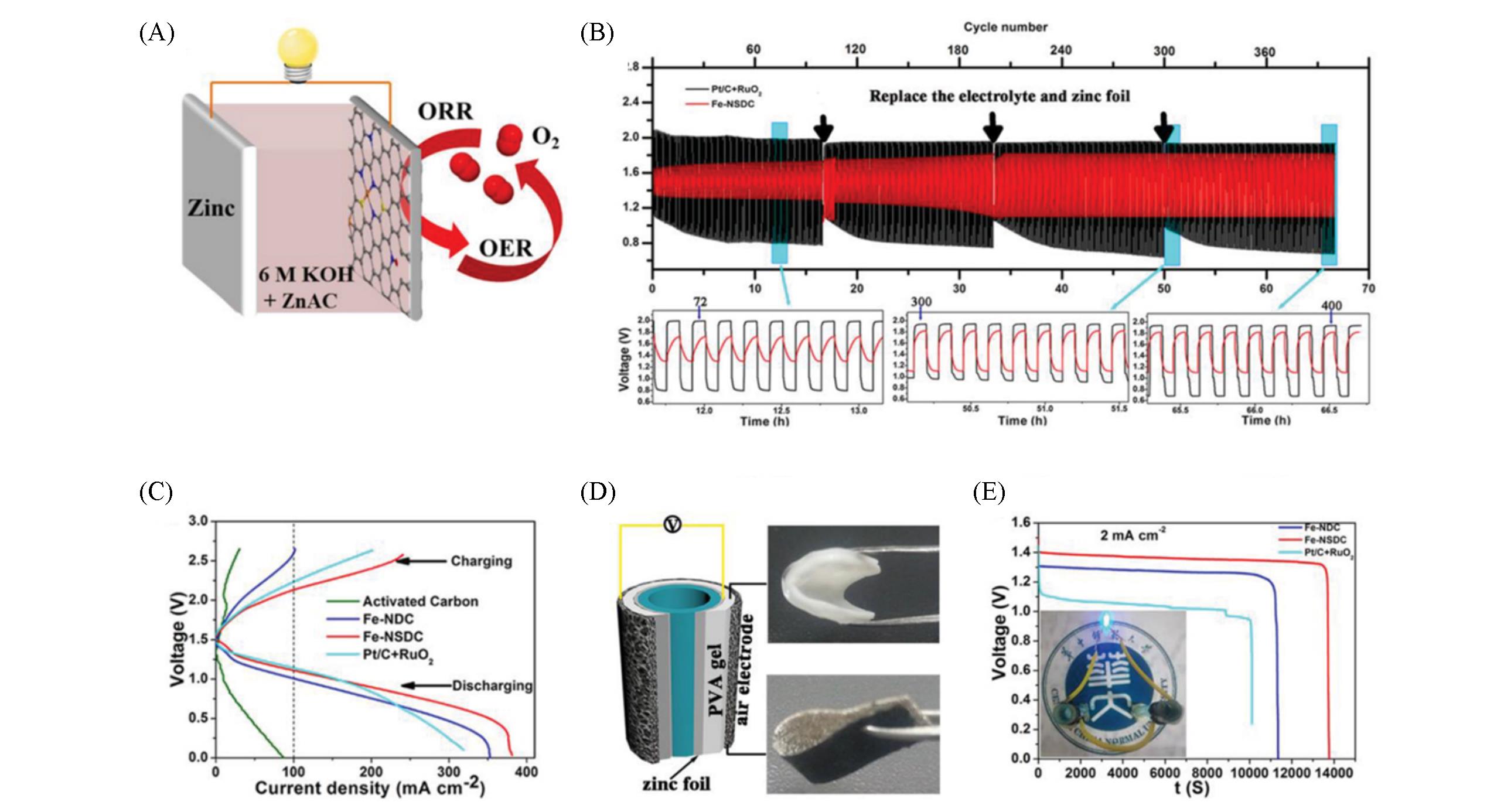
Fig.14 Schematic of the rechargeable Zn?air battery(A), galvanostatic discharge?charge cycling curves using Pt/C+RuO2 and Fe?NSDC catalysts at 4 mA/cm2 with 10 min cycle(B), charge and discharge polarization curves of rechargeable Zn?air batteries using different catalysts as air electrode(C), schematic diagram of the flexible all?solid Zn?air battery(D), the galvanostatic discharge curves of a coiled all?solid Zn?air battery at current density of 2 mA/cm2 and a corresponding image of two batteries in series to power a blue LED light(E)[84]Copyright 2019, Wiley-VCH.
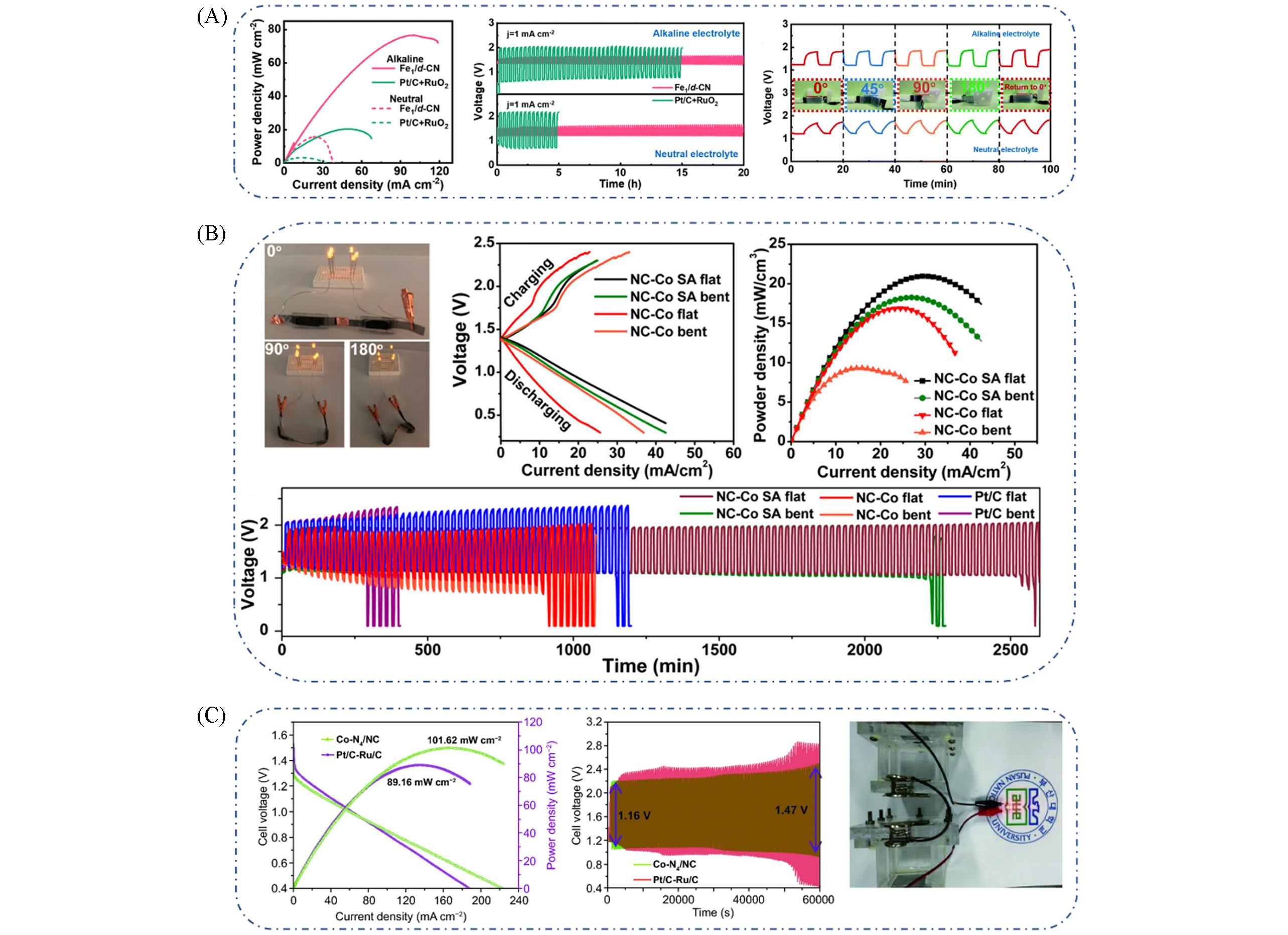
Fig.15 Power density curves of flexible quasi?solid?state alkaline(solid line) and neutral(dotted line) Zn?air batteries, comparison of stability test between the Fe1/d?CN and Pt/C+RuO2 catalysts as the air ca?thode in flexible quasi?solid?state alkaline(top side) and neutral(bottom side) Zn?air batteries, cycling stability of the quasi?solid?state Zn?air battery in alkaline(top side) and neutral(bottom side) electrolytes with different bending angles(A)[87], photograph of 6 LEDs powered by two assembled Zn?air batteries with different bending angles(0°, 90°, 180°), discharge and charge polarization curves, power?current density curves, comparison of the cycling stabilities in both bent and flat states(B)[88], discharging polarization and power density curves based on the Co?N4/NC and Pt/C?Ru/C catalyst, cycling test(100 cycles) at a current density of 10 mA/cm2 with Co?N4/NC and Pt/C?Ru/C catalyst, images of two homemade rechargeable Zn?air batteries in series with a LED(C)[89](A) Copyright 2021, Royal Society of Chemistry; (B) Copyright 2018, American Chemical Society; (C) Copyright 2021, Springer Nature.
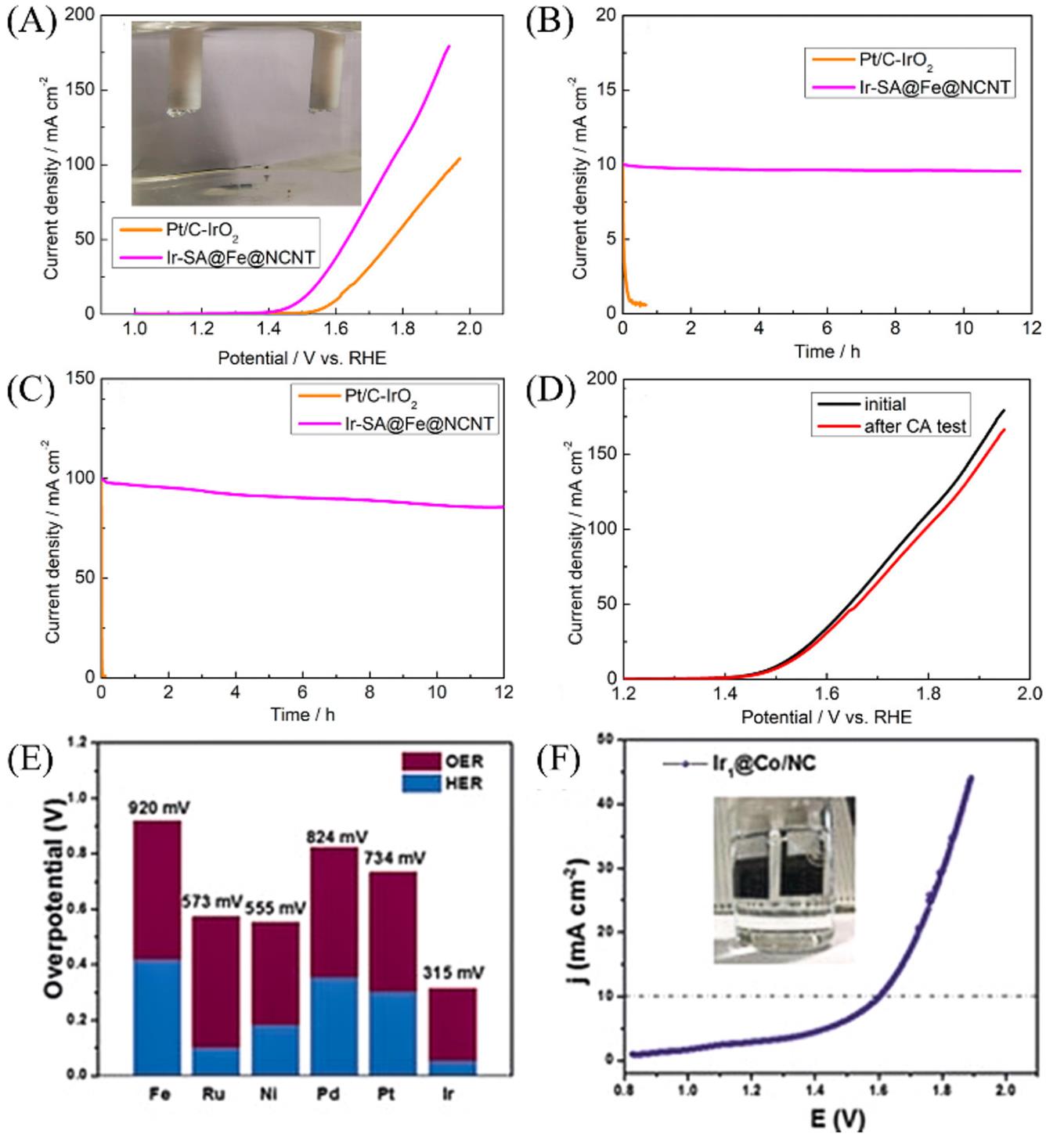
Fig.16 LSV curves of water splitting test in 0.5 mol/L H2SO4 electrolyte of Ir?SA@Fe@NCNT and Pt/C?IrO2(A), chronoamperometric tests of Ir?SA@Fe@NCNT and Pt/C?IrO2 for achieving 10 mA/cm2(B) and 100 mA/cm2(C), LSV curves of water splitting test in 0.5 mol/L H2SO4 electrolyte of Ir?SA@Fe@NCNT before(black line) and after(red line) chronoamperometric test(D)[95], the overall overpotential of the corresponding electrodes obtained at 10 mA/cm2(E), the polarization curves of overall water splitting by the Ir1@Co/NC catalyst(F)[60](A—D) Copyright 2020, American Chemical Society; (E, F) Copyright 2019, Wiley-VCH.
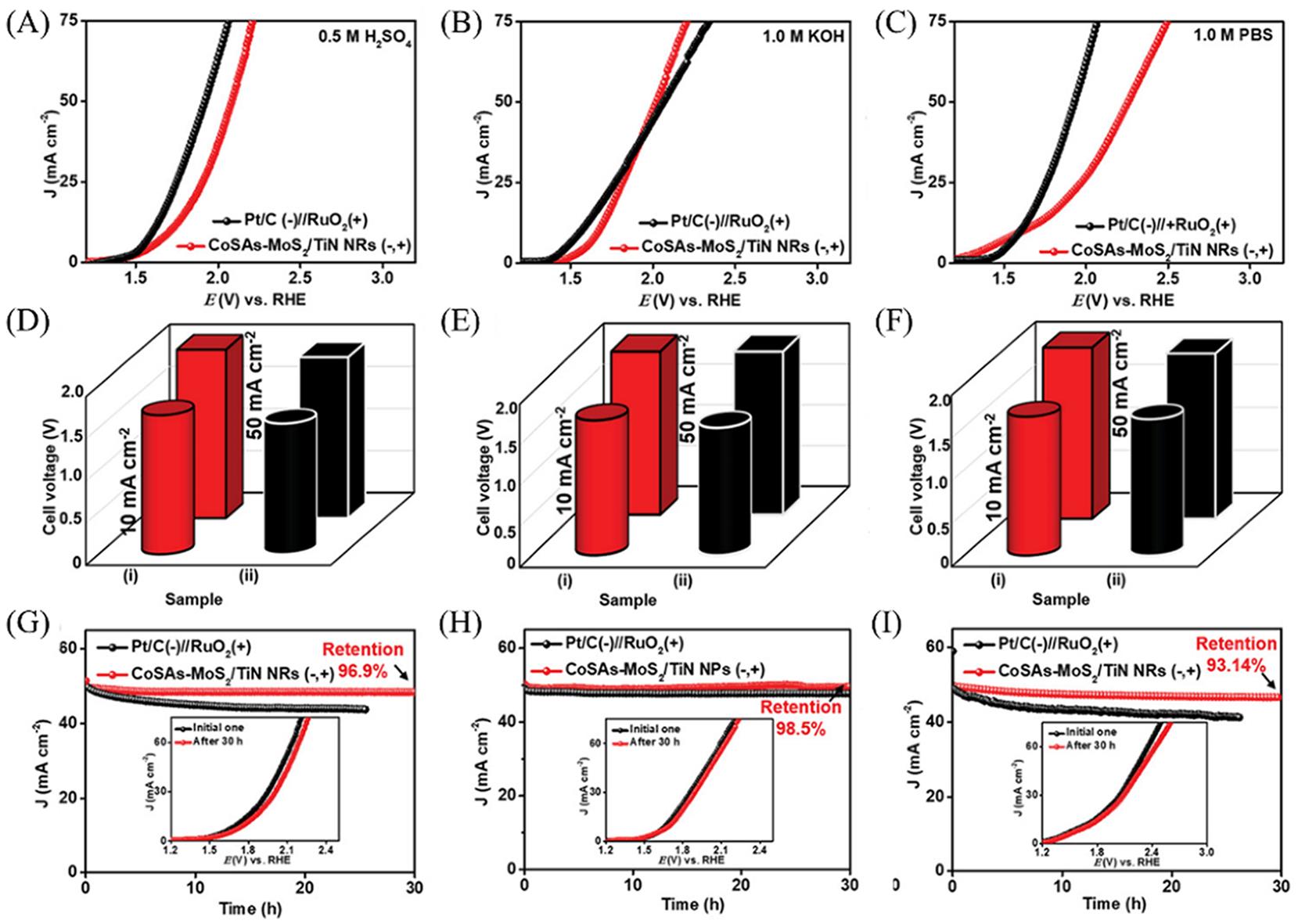
Fig.17 Overall water?splitting measurements with a symmetrical electrode system using CoSAs?MoS2/TiN NRs as both cathode and anode in 0.5 mol/L H2SO4(A), 1.0 mol/L KOH(B) and in 1.0 mol/L PBS(C), in comparison with the Pt/C//RuO2 couple, a comparison of operating voltages at J values of 10 and 50 mA/cm2 between CoSAs?MoS2/TiNNRs and Pt/C//RuO2 in 0.5 mol/L H2SO4(D), 1.0 mol/L KOH(E) and in 1.0 mol/L PBS(F), chronoamperometric curves of the CoSAs?MoS2/TiN NRs in 0.5 mol/L H2SO4(G), 1.0 mol/L KOH(H) and in 1.0 mol/L PBS(I)[66]Inset of (G)—(I): polarization curves recorded before and after the chronoamperometric test. Copyright 2021, Wiley?VCH.
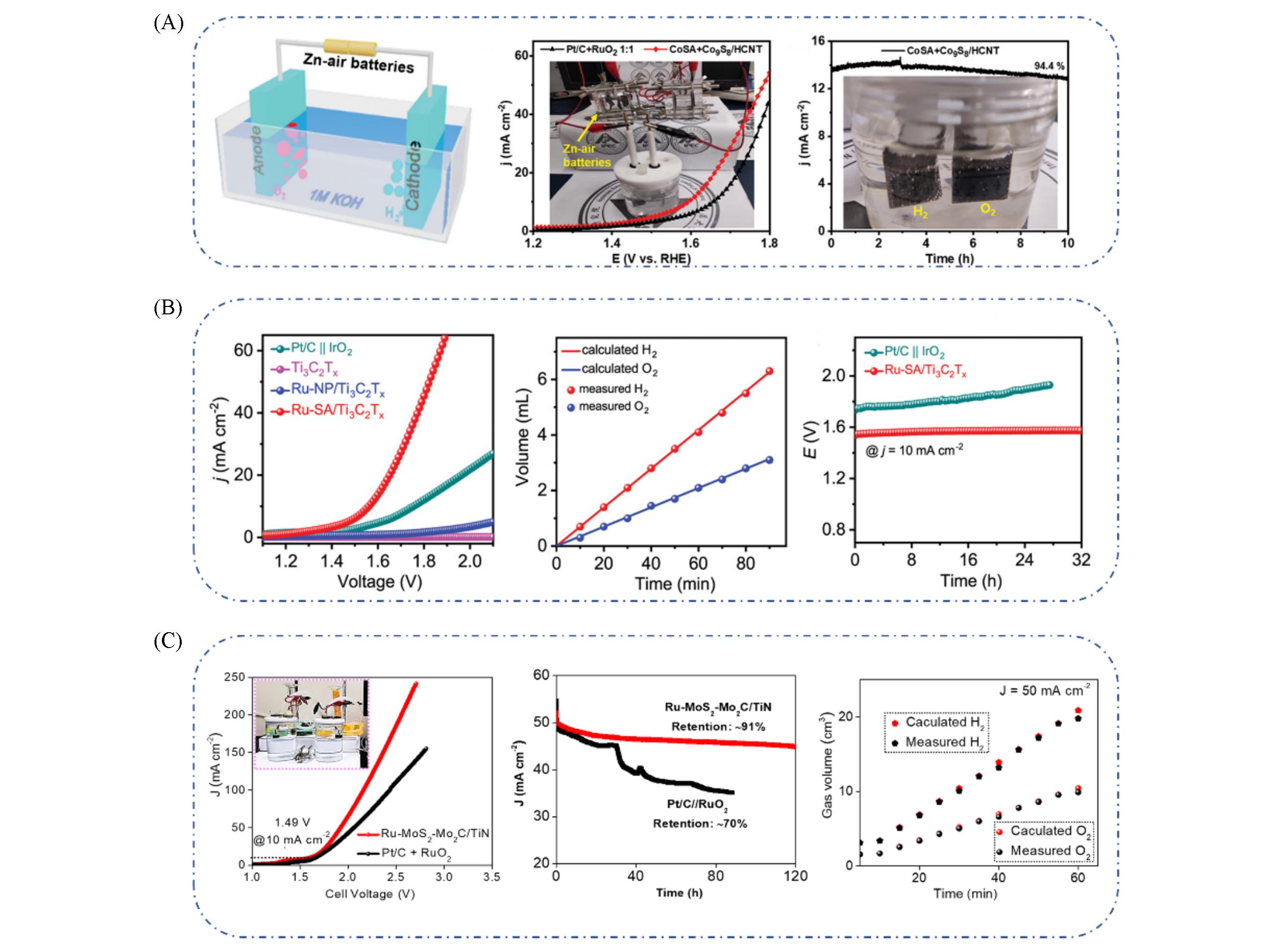
Fig.18 Schematic diagram of self?power water splitting electrolyzer, LSV curve of the CoSA+Co9S8/HCNT electrodes(A)[96], geometric?area?normalized OWS LSV curves without iR compensation, the amount of generated H2 and O2 as a function of the reaction time at j=10 mA/cm2, stability test of Ru?SA/Ti3C2T x and Pt/C||IrO2 for OWS at j=10 mA/cm2(B)[97], LSV measurements of the Ru?MoS2?Mo2C/TiN and Pt?C+RuO2?based devices for overall water splitting in 1.0 mol/L KOH medium, stability of the Ru?MoS2?Mo2C/TiN and Pt?C//RuO2?based devices measured at an initial current response of 50 mA/cm2, faradaic efficiencies of the Ru?MoS2?Mo2C/TiN?based device for water splitting(C)[98](A) Inset is the photograph of self?power water splitting device, chronoamperometric responses of CoSA+Co9S8/HCNT|| CoSA+Co9S8/HCNT cells at 1.6 V and inset is the image of electrodes during the electrolysis of water. (A) Copyright 2020, Wiley-VCH; (B) Copyright 2020, Wiley-VCH; (C) Copyright 2021, Elsevier.
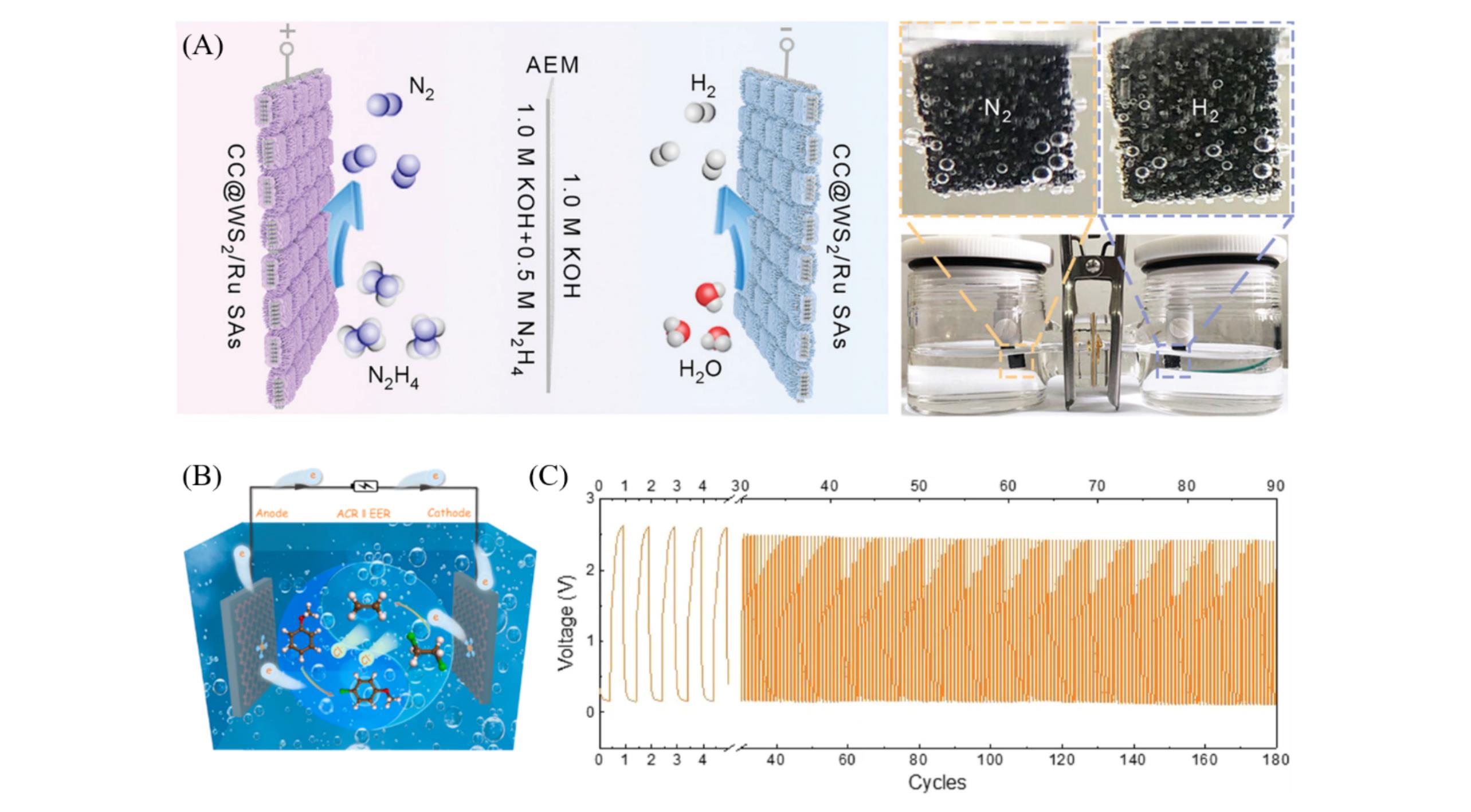
Fig.19 Schematic illustration of the HzOR?assisted OWS mechanism in two?electrode H?type electrolyze using CC@WS2/Ru?450 as both anode and cathode(A)[74], optical image of the two?electrode configuration schematic illustration for EER||ACR(B)[105], galvanostatic discharge?charge cycling curves of rechargeable Zn?CO2 battery cell at the discharge current density of 5 mA/cm2 and charge current density of 2 mA/cm2 for 180 cycles(C)[79](A) Copyright 2022, Wiley-VCH; (B) Copyright 2021, Elsevier; (C) Copyright 2021, Nature.
| 1 | Zang W. J., Kou Z. K., Pennycook S. J., Wang J., Adv. Energy Mater., 2020, 10(9), 1903181 |
| 2 | Chu S., Majumdar A., Nature, 2012, 488, 294—303 |
| 3 | Luo J., Im J. H., Mayer M. T., Schreier M., Nazeeruddin M. K., Park N. G., Tilley S. D., Fan H. J., Science, 2014, 345(6204), 1593—1596 |
| 4 | Zhang Q. Q., Guan J. Q., Adv. Funct. Mater., 2020, 30(31), 2000768 |
| 5 | Suen N. T., Hung S. F., Quan Q., Zhang N., Xu Y. J., Chen H. M., Chem. Soc. Rev., 2017, 46(2), 337—365 |
| 6 | Nie Y., Li L., Wei Z., Chem. Soc. Rev., 2015, 44(8), 2168—2201 |
| 7 | McCrory C. C. L., Jung S., Ferrer I. M., Chatman S. M., Peters J. C., Jaramillo T. F., J. Am. Chem. Soc., 2015, 137(13), 4347—4357 |
| 8 | Wang Y. J., Zhao N., Fang B., Li H., Bi X. T., Wang H., Chem. Rev., 2015, 115(9), 3433—3467 |
| 9 | Higgins D., Zamani P., Yu A., Chen Z., Energy Environ. Sci., 2016, 9(2), 357—390 |
| 10 | Bonaccorso F., Colombo L., Yu G., Stoller M., Tozzini V., Ferrari A. C., Ruoff R. S., Pellegrini V., Science, 2015, 347(6217), 1246501 |
| 11 | Liu L., Su H., Tang F., Zhao X., Liu Q., Nano Energy, 2018, 46, 110—116 |
| 12 | Li X., Yang X., Huang Y., Zhang T., Liu B., Adv. Mater., 2019, 31(50), 1902031 |
| 13 | Liu L., Corma A., Chem. Rev., 2018, 118(10), 4981—5079 |
| 14 | Peng Y., Lu B., Chen S., Adv. Mater., 2018, 30(48), 1801995 |
| 15 | Chen Y., Ji S., Zhao S., Chen W., Dong J., Cheong W. C., Shen R., Wen X., Zheng L., Rykov A. I., Cai S., Tang H., Zhuang Z., Chen C., Peng Q., Wang D., Li Y., Nat. Commun., 2018, 9, 5422 |
| 16 | Zhu C., Shi Q., Feng S., Du D., Lin Y., ACS Energy Lett., 2018, 3(7), 1713—1721 |
| 17 | Black J. R., Yin Q., Rustad J. R., Casey W. H., J. Am. Chem. Soc., 2007, 129(28), 8690—8691 |
| 18 | Nam W., Acc. Chem. Res., 2007, 40(7), 522—531 |
| 19 | Maschmeyer T., Rey F., Sankar G., Thomas J. M., Nature, 1995, 378, 159—162 |
| 20 | Asakura K., Nagahiro H., Ichikuni N., Iwasawa Y., Appl. Catal. A: Gen., 1999, 188(1/2), 313—324 |
| 21 | Fu Q., Saltsburg H., Flytzani⁃Stephanopoulos M., Science, 2003, 301(5635), 935—938 |
| 22 | Hackett S. F. J., Brydson R. M., Gass M. H., Harvey I., Newman A. D., Wilson K., Lee A. F., Angew. Chem. Int. Ed., 2007, 46(45), 8593—8596 |
| 23 | Qiao B., Wang A., Yang X., Allard L. F., Jiang Z., Cui Y., Liu J., Li J., Zhang T., Nat. Chem., 2011, 3, 634—641 |
| 24 | Yang X. F., Wang A., Qiao B., Li J., Liu J., Zhang T., Acc. Chem. Res., 2013, 46(8), 1740—1748 |
| 25 | Zhang X., Shi H., Xu B. Q., Angew. Chem. Int. Ed., 2005, 44(43), 7132—7135 |
| 26 | Lin J., Wang A., Qiao B., Liu X., Yang X., Wang X., Liang J., Li J., Liu J., Zhang T., J. Am. Chem. Soc., 2013, 135(41), 15314—15317 |
| 27 | Zhang Q. Q., Guan J. Q., Adv. Funct. Mater., 2020, 30(31), 2000768 |
| 28 | Mikhaylik Y. V., Akridge J. R., J. Electrochem. Soc., 2004, 151, A1969 |
| 29 | Manthiram A., Fu Y., Su Y. S., Acc. Chem. Res., 2013, 46(5), 1125—1134 |
| 30 | Corma A.,Concepción P., Boronat M., Sabater M. J., Navas J.,Yacaman M. J., Larios E., Posadas A., Arturo López⁃Quintela M., Buceta D., Mendoza E.,Guilera G., Mayoral A., Nat. Chem., 2013, 5, 775—781 |
| 31 | Moliner M., Gabay J. E.,Kliewer C. E.,Robert T. Carr R. T.,Guzman J.,Casty G. L.,Serna P.,Corma A., J. Am. Chem. Soc., 2016, 138(48), 15743—15750 |
| 32 | Peterson E. J., DeLaRiva A. T., Lin S., Johnson R. S., Guo H., Miller J. T., Kwak J. H., Peden C. H. F., Kiefer B., Allard L. F., Ribeiro F. H., Datye A. K., Nat. Commun., 2014, 5, 4885 |
| 33 | Liu L., Corma A., Chem. Rev., 2018, 118(10), 4981—5079 |
| 34 | Zhao C. X., Liu J. N., Wang J., Wang C. D., Guo X., Li X. Y., Chen X., Song L., Li B. Q., Zhang Q., Sci. Adv., 2022, 8(11), eabn5091 |
| 35 | Chen W., Wu B. B., Wang Y. Y., Zhou W., Li Y. Y., Liu T. Y., Xie C., Xu L. T., Du S. Q., Song M. L., Wang D. D., Liu Y. B., Li Y. F., Liu J. L., Zou Y. Q., Chen R., Chen C., Zheng J. Y., Li Y. F., Chen J., Wang S. Y., Energy Environ. Sci., 2021, 14(12), 6428—6440 |
| 36 | Gan W. G., Zhou D. B., Zhou L., Zhang Z. J., Zhao J., Electrochim. Acta, 2015, 182, 430—436 |
| 37 | Hwanga B., Ohb E. S., Kima K., Electrochim. Acta, 2016, 216, 484—489 |
| 38 | Wang J., Ge X., Liu Z., Thia L., Yan Y., Xiao W., Wang X., J. Am. Chem. Soc., 2017, 139(5), 1878—1884 |
| 39 | Zhang Z., Sun J., Wang F., Dai L., Angew. Chem. Int. Ed., 2018, 57(29), 9038—9043 |
| 40 | Zhang M., Wang Y. G., Chen W., Dong J., Zheng L., Luo J., Wan J., Tian S., Cheong W. C., Wang D., Li Y.,J. Am. Chem. Soc., 2017, 139(32), 10976—10979 |
| 41 | Wang J., Gan L., Zhang W., Peng Y., Yu H., Yan Q., Xia X., Wang X., Sci. Adv., 2018, 4(3), eaap7970 |
| 42 | Li B. Q., Zhao C. X., Chen S. M., Liu J. N., Chen X., Song L., Zhang Q., Adv. Mater., 2019, 31(19), 1900592 |
| 43 | Li B. Q., Zhang S. Y., Wang B., Xia Z. J., Tang C., Zhang Q., Energy Environ. Sci., 2018, 11(7), 1723—1729 |
| 44 | Liu W., Zhang L., Yan W., Liu X., Yang X., Miao S., Wang W., Wang A., Zhang T., Chem. Sci., 2016, 7(9), 5758—5764 |
| 45 | Han Y., Wang Y. G., Chen W., Xu R., Zheng L., Zhang J., Luo J., Shen R. A., Zhu Y., Cheong W. C., Chen C., Peng Q., Wang D., Li Y., J. Am. Chem. Soc., 2017, 139(48), 17269—17272 |
| 46 | Han X. P., Ling X. F., Wang Y., Ma T. Y., Zhong C., Hu W. B., Deng Y. D., Angew. Chem. Int. Ed., 2019, 58(16), 5359—5364 |
| 47 | Yang L., Shi L., Wang D., Lv Y. L., Cao D. P., Nano Energy, 2018, 50, 691—698 |
| 48 | Zhang K., Han X., Hu Z., Zhang X., Tao Z., Chen J., Chem. Soc. Rev., 2015, 44(3), 699—728 |
| 49 | Yang Y., Mao K. T., Gao S. Q., Huang H., Xia G. L., Lin Z. Y., Jiang P., Wang C. L., Wang H., Chen, Q. W., Adv. Mater., 2018, 30(28), 1801732 |
| 50 | Shang H. S., Sun W. M., Sui R., Pei J. J., Zheng L. R., Dong J. C., Jiang Z. L., Zhou D. N., Zhuang Z. B., Chen W. X., Zhang J. T., Wang D. S., Li Y. D., Nano Lett., 2020, 20(7), 5443—5450 |
| 51 | Chen J. Y., Li Hao., Fan Chuang, Meng Q. W., Tang Y. W., Qiu X. Y., Fu G. T., Ma T. Y., Adv. Mater., 2020, 32(30), 2003134 |
| 52 | Zhang H. W., Zhao M. Q., Liu H. R., Shi S. R., Wang Z. H., Zhang B., Song L., Shang J. Z., Yang Y., Ma C., Zheng L. R., Han Y. H., Huang W., Nano Lett., 2021, 21(5), 2255—2264 |
| 53 | Daniel M. D., Benjamin J. G., Rob C., Kate M. A., Ana M. F. C., Lisa T., Nikul K., Nathan C., Michael C. N., Matthew W., Andrew I. C., Emily R. D., Alexander J. C., Dave J. A., Adv. Energy Mater., 2020, 10(46), 20022469 |
| 54 | Kasper T. M., Torben R. J., Etsuo A., Hai⁃wen L., Prog. Nat. Sci., 2017, 27(1), 34—40 |
| 55 | Jeff G., Thomas F. J., Jacob B., Ib C., Jens K. N., Nat. Mater., 2006, 5, 909—913 |
| 56 | Gao D. D., Liu R. J., Biskupek J., Kaiser U., Song Y. F., Streb C., Angew. Chem. Int. Ed., 2019, 58(14), 4644—4648 |
| 57 | Sultan S., Ha M., Kim D. Y.., Tiwari J. N., Myung C. W., Meena A., Shin T. J., Chae K. H., Kim K. S., Nat. Commun., 2019, 10, 5195 |
| 58 | Cao L. L., Luo Q. Q., Chen J. J., Wang L., Lin Y., Wang H. J., Liu X. K., Shen X. Y., Zhang W., Liu W., Qi Z. M., Jiang Z., Yang J. L., Yao T., Nat. Commun., 2019, 10, 4849 |
| 59 | Shah K., Dai R. Y., Mateen M., Hassan Z., Zhuang Z. W., Liu C. H., Israr M., Cheong W. C., Hu B. T., Tu R. Y., Zhang C., Chen X., Peng Q., Chen C., Li Y. D., Angew. Chem. Int. Ed., 2021, 61(4), e202114951 |
| 60 | Lai W. H., Zhang L. F., Hua W. B., Indris S., Yan Z. C., Hu Z., Zhang B. W., Liu Y. N., Wang L., Liu M., Liu R., Wang Y. X., Wang J. Z., Hu Z. P., Liu H. K., Chou S. L., Dou S. X., Angew. Chem. Int. Ed., 2019, 58(34), 11868—11873 |
| 61 | Naguib M., Mochalin V. N., Barsoum M. W., Gogotsi Y., Adv. Mater., 2014, 26(7), 992—1005 |
| 62 | Tang X., Zhou D., Li P., Guo X., Sun B., Liu H., Yan K., Gogotsi Y., Wang G. X., Adv. Mater., 2020, 32(4), 1906739 |
| 63 | Anand R., Nissimagoudar A. S., Umer M., Ha M. R., Zafari M., Umer S., Lee G., Kim K. S., Adv. Energy Mater., 2021, 11(48), 2102388 |
| 64 | Meng X. Y., Yu L., Ma C., Nan B., Si R., Tu Y. C., Deng J., Deng D. H., Bao X. H., Nano Energy, 2019, 61, 611—616 |
| 65 | Nguyen D. C., Tran D. T., Doan T. L. L., Kim D. H., Kim N. H., Lee J. H., Adv. Energy Mater., 2020, 10(8), 1903289 |
| 66 | Doan T. L. L., Nguyen D. C., Prabhakaran S., Kim D. H., Tran D. T., Kim N. H., Lee J. H., Adv. Funct. Mater., 2021, 31(26), 2100233 |
| 67 | Xu X. P., Xu H. X., Cheng D. J., Nanoscale, 2019, 11(42), 20228—20237 |
| 68 | Mohajeri A., Dashti N. L., J. Phys. Chem. C, 2019, 123(51), 30972—30980 |
| 69 | Kulish, V. V., Phys. Chem. Chem. Phys., 2017, 19(18), 11273—11281 |
| 70 | Ling C. Y., Shi L., Ouyang Y. X., Zeng X. C., Wang J. L., Nano Lett., 2017, 17(8), 5133—5139 |
| 71 | Kumar R., Sahoo S., Joanni E., Singh R.K., Yadav R.M., Verma R.K., Singh D.P., Tan W. K., P´erez del Pino A., Moshkalev S.A., Matsuda A., Nano Res., 2019, 12, 2655—2694 |
| 72 | Golberg D., Bando Y., Huang Y., Terao T., Mitome M., Tang C., Zhi C., ACS Nano, 2010, 4(6), 2979—2993 |
| 73 | Fan G. H., Wang Q., Liu X., Li C. Y., Xu H., Appl. Catal. A: Gen., 2021, 622, 118235 |
| 74 | Li J. C., Li Y., Wang J. A., Zhang C., Ma H. J., Zhu C. H., Fan D. D., Guo Z. Q., Xu M., Wang Y. Y., Ma H. X., Adv. Funct. Mater., 2022, 2109439 |
| 75 | Deng Z. J., Zheng X. Q.,Deng M. M.,Li L., Jing Li., Wei Z. D., Chinese J. Catal., 2021, 42(10), 1659—1666 |
| 76 | Gao G., O’Mullane A. P., Du A., ACS Catal., 2017, 7(1), 494—500 |
| 77 | Zhao W. P., Wan G., Peng C. L., Sheng H. P., Wen J. G., Chen H. R., ChemSusChem, 2018, 11(19), 3473—3479 |
| 78 | Li T. F., Liu J. J., Song Y., Wang F., ACS Catal., 2018, 8(9), 8450—8458 |
| 79 | Zeng Z. P. Gan L. Y., ang H. B., Su X. Z., Gao J. J., Liu W., Matsumoto H., Gong J., Zhang J. M., Cai W. Z., Zhang Z. Y., Yan Y. B., Liu B., Chen P., Nat. Commun., 2021, 12, 4088 |
| 80 | Hu R. M., Li Y. C., Wang F. H., Shang J. X., Nanoscale, 2020, 12(39), 20413—20424 |
| 81 | Wang H. F., Tang C., Zhang Q., Adv. Funct. Mater., 2018, 28(46), 1803329 |
| 82 | Fu J., Cano Z. P., Park M. G., Yu A. P., Fowler M., Chen Z. W., Adv. Mater., 2017, 29(7), 1604685 |
| 83 | Gu P., Xu Y. X., Zhao Y. F., Liu W., Xue H. G., Pang H., Adv. Mater. Interfaces, 2017, 4(19), 1700589 |
| 84 | Zhang J. T., Zhang M., Zeng Y., Chen J. S., Qiu L. X., Zhou H., Sun C. J., Yu Y., Zhu C. Z., Zhu Z. H., Small, 2019, 15(24), 1900307 |
| 85 | Amiinu I. S., Pu Z., Liu X., Owusu K. A., Monestel H. G. R., Boakye F. O., Zhang H., Mu S., Adv. Funct. Mater., 2017, 27(44), 1702300 |
| 86 | Fu G., Cui Z., Chen Y., Li Y., Tang Y., Goodenough J. B., Adv. Energy Mater., 2017, 7(1), 1601172 |
| 87 | Zhao M. Q., Liu H. R., Zhang H. W., Chen W., Sun H. Q., Wang Z. H., Zhang B., Song L., Yang Y., Ma C., Han Y. H., Huang W., Energy Environ. Sci., 2021,14(12), 6455—6463 |
| 88 | Zang W. J., Sumboja A., Ma Y. Y., Zhang H., Wu Y., Wu S. S., Wu H. J., Liu Z. L., Guan C., Wang J., Pennycook S. J., ACS Catal., 2018, 8(10), 8961—8969 |
| 89 | Chen K., Kim S., Je M., Choi H., Shi Z. C., Vladimir N., Kim K. H., Li L., Nano⁃Micro Lett., 2021, 13, 60 |
| 90 | Roger I., Shipman M. A., Symes M. D., Nat. Rev. Chem., 2017, 1, 0003 |
| 91 | Martindale Benjamin C. M., Reisner E., Adv. Energy Mater., 2016, 6(6), 1502095 |
| 92 | Vij V., Sultan S., Harzandi A. M., Meena A., Tiwari J. N., Lee W. G., Yoon T., Kim K. S., ACS Catal., 2017, 7(10), 7196 |
| 93 | Li J., Zheng G., Adv. Sci., 2017, 4(3), 1600380 |
| 94 | Fang M., Dong G., Wei R., Ho Johnny C., Adv. Energy Mater., 2017, 7(23), 1700559 |
| 95 | Luo F., Hu H., Zhao X., Yang Z. H., Zhang Q., Xu J. X., Kaneko T., Zhu C. Z., Cai W. W., Nano Lett., 2020, 20(3), 2120—2128 |
| 96 | Li Y. Z., Cao R., Li L. B., Tang X. N., Chu T. L., Huang B. Y., Yuan K., Chen Y. W., Small, 2020, 16(10), 1906735 |
| 97 | Peng X. Y., Zhao S. Z., Mi Y. Y., Han L. L., Liu X. J., Qi D. F., Sun J. Q., Liu Y. F., Bao H. H., Zhuo L. C., Xin H. L., Luo J., Sun X. M., Small, 2020, 16(33), 2002888 |
| 98 | Hoa V. H., Tran D. T.,Prabhakaran S., Kim D. H., Hameed N.,Wang H., Kim N. H., Lee J. H., Nano Energy, 2021, 88, 106277 |
| 99 | Zhao B., Liu J. W., Xu C. Y., Feng R. F., Sui P. F., Wang L., Zhang J. J., Luo J. L., Fu X. Z., Adv. Funct. Mater., 2021, 31(8), 2008812 |
| 100 | Xu X. J., Guo T., Xia J. Y., Zhao B. L., Su G., Wang H. L., Huang M. H., Toghan A., Chem. Eng. J., 2021, 425, 130514 |
| 101 | Hausmann J. N., Beltrán⁃Suito R., Mebs S., Hlukhyy V., Fässler T. F., Dau H., Driess M., Menezes P. W., Adv. Mater., 2021, 33(27), 2008823 |
| 102 | Zheng J., Chen X. L., Zhong X., Li S. Q., Liu T. Z., Zhuang G. L., Li X. N., Deng S. W., Mei D. H., Wang J. G., Adv. Funct. Mater., 2017, 27(46), 1704169 |
| 103 | Li Y., Zhang J., Liu Y., Qian Q., Li Z., Zhu Y., Zhang G., Sci. Adv., 2020, 6, 4197 |
| 104 | Liu Y., Zhang J. H., Li Y. P., Qian Q. Z., Li Z. Y., Zhang G. Q., Adv. Funct. Mater., 2021, 31(35), 2103673 |
| 105 | Gan G. Q., Li X. Y., Fan S. Y., Yin Z. F., Wang L., Chen G. H., Nano Energy, 2021, 80, 105532 |
| 106 | Hu C. G., Gong L. L., Xiao Y., Yuan Y. F., Bedford N. M., Xia Z. H., Ma L., Wu T. P., Lin Y., Connell J. W., Shahbazian⁃Yassar R., Lu J., Amine K., Dai L. M., Adv. Mater., 2020, 32(16), 1907436 |
| [1] | 滕镇远, 张启涛, 苏陈良. 聚合物单原子光催化剂的载流子分离和表面反应机制[J]. 高等学校化学学报, 2022, 43(9): 20220325. |
| [2] | 王茹玥, 魏呵呵, 黄凯, 伍晖. 单原子材料的冷冻合成[J]. 高等学校化学学报, 2022, 43(9): 20220428. |
| [3] | 杨静怡, 李庆贺, 乔波涛. 铱单原子和纳米粒子在N2O分解反应中的协同催化[J]. 高等学校化学学报, 2022, 43(9): 20220388. |
| [4] | 林高鑫, 王家成. 单原子掺杂二硫化钼析氢催化的进展和展望[J]. 高等学校化学学报, 2022, 43(9): 20220321. |
| [5] | 任诗杰, 谯思聪, 刘崇静, 张文华, 宋礼. 铂单原子催化剂同步辐射X射线吸收谱的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220466. |
| [6] | 汪思聪, 庞贝贝, 刘潇康, 丁韬, 姚涛. XAFS技术在单原子电催化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220487. |
| [7] | 楚宇逸, 兰畅, 罗二桂, 刘长鹏, 葛君杰, 邢巍. 单原子铈对弱芬顿效应活性位点氧还原稳定性的提升[J]. 高等学校化学学报, 2022, 43(9): 20220294. |
| [8] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [9] | 姚青, 俞志勇, 黄小青. 单原子催化剂的合成及其能源电催化应用的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220323. |
| [10] | 范建玲, 唐灏, 秦凤娟, 许文静, 谷鸿飞, 裴加景, 陈文星. 氮掺杂超薄碳纳米片复合铂钌单原子合金催化剂的电化学析氢性能[J]. 高等学校化学学报, 2022, 43(9): 20220366. |
| [11] | 林治, 彭志明, 贺韦清, 沈少华. 单原子与团簇光催化: 竞争与协同[J]. 高等学校化学学报, 2022, 43(9): 20220312. |
| [12] | 韩付超, 李福进, 陈良, 贺磊义, 姜玉南, 徐守冬, 张鼎, 其鲁. CoSe2/C复合电催化材料修饰隔膜对高载量锂硫电池性能的影响[J]. 高等学校化学学报, 2022, 43(8): 20220163. |
| [13] | 赵润瑶, 纪桂鹏, 刘志敏. 吡咯氮配位单原子铜催化剂的电催化二氧化碳还原性能[J]. 高等学校化学学报, 2022, 43(7): 20220272. |
| [14] | 王茹涵, 贾顺涵, 吴丽敏, 孙晓甫, 韩布兴. CO2参与电化学构筑C—N键制备重要化学品[J]. 高等学校化学学报, 2022, 43(7): 20220395. |
| [15] | 彭奎霖, 李桂林, 江重阳, 曾少娟, 张香平. 电解液调控CO2电催化还原性能微观机制的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220238. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||