

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (6): 1914.doi: 10.7503/cjcu20200789
张仁丽1, 王瑶1( ), 遇治权1,2, 孙志超1,2, 王安杰1,2, 刘颖雅1,2(
), 遇治权1,2, 孙志超1,2, 王安杰1,2, 刘颖雅1,2( )
)
收稿日期:2020-11-02
出版日期:2021-06-10
发布日期:2021-06-08
通讯作者:
王瑶
E-mail:wangyao@dlut.edu.cn;yingya.liu@dlut.edu.cn
作者简介:刘颖雅, 女, 博士, 副教授, 主要从事MOF和COF材料的多相催化及吸附应用研究. E-mail: 基金资助:
ZHANG Renli1, WANG Yao1( ), YU Zhiquan1,2, SUN Zhichao1,2, WANG Anjie1,2, LIU Yingya1,2(
), YU Zhiquan1,2, SUN Zhichao1,2, WANG Anjie1,2, LIU Yingya1,2( )
)
Received:2020-11-02
Online:2021-06-10
Published:2021-06-08
Contact:
WANG Yao
E-mail:wangyao@dlut.edu.cn;yingya.liu@dlut.edu.cn
摘要:
采用预修饰方法对UiO-66进行配体官能团改性, 通过引入—F调控UiO-66的表面亲疏水性质; 其次, 通过引入—NH2在UiO-66骨架上锚定MoO(O2)2. 接触角测试表明, 氟的引入有效地提高了载体表面的疏水性; 热重分析证明, 氟修饰的UiO-66骨架上存在更多配体缺失, 从而有效提高了整体MOF骨架的Lewis酸性. 以二苯并噻吩(DBT)氧化为氧化脱硫模型反应, 过氧化氢异丙苯(CHP)为氧化剂, 采用正交实验考察了反应温度、 氧硫比及催化剂用量对催化性能的影响, 其中氧硫比是影响DBT转化率的决定性因素. 经过氟改性后的催化剂经过5次催化反应循环后其催化活性未见明显变化, 且骨架仍保持稳定.
中图分类号:
TrendMD:
张仁丽, 王瑶, 遇治权, 孙志超, 王安杰, 刘颖雅. 氟改性UiO-66固载钼基过氧化物催化氧化含硫化合物. 高等学校化学学报, 2021, 42(6): 1914.
ZHANG Renli, WANG Yao, YU Zhiquan, SUN Zhichao, WANG Anjie, LIU Yingya. Molybdenum Peroxide Anchored on Fluoronated UiO-66 as Catalyst in the Oxidation of Sulfur Containing Compounds. Chem. J. Chinese Universities, 2021, 42(6): 1914.
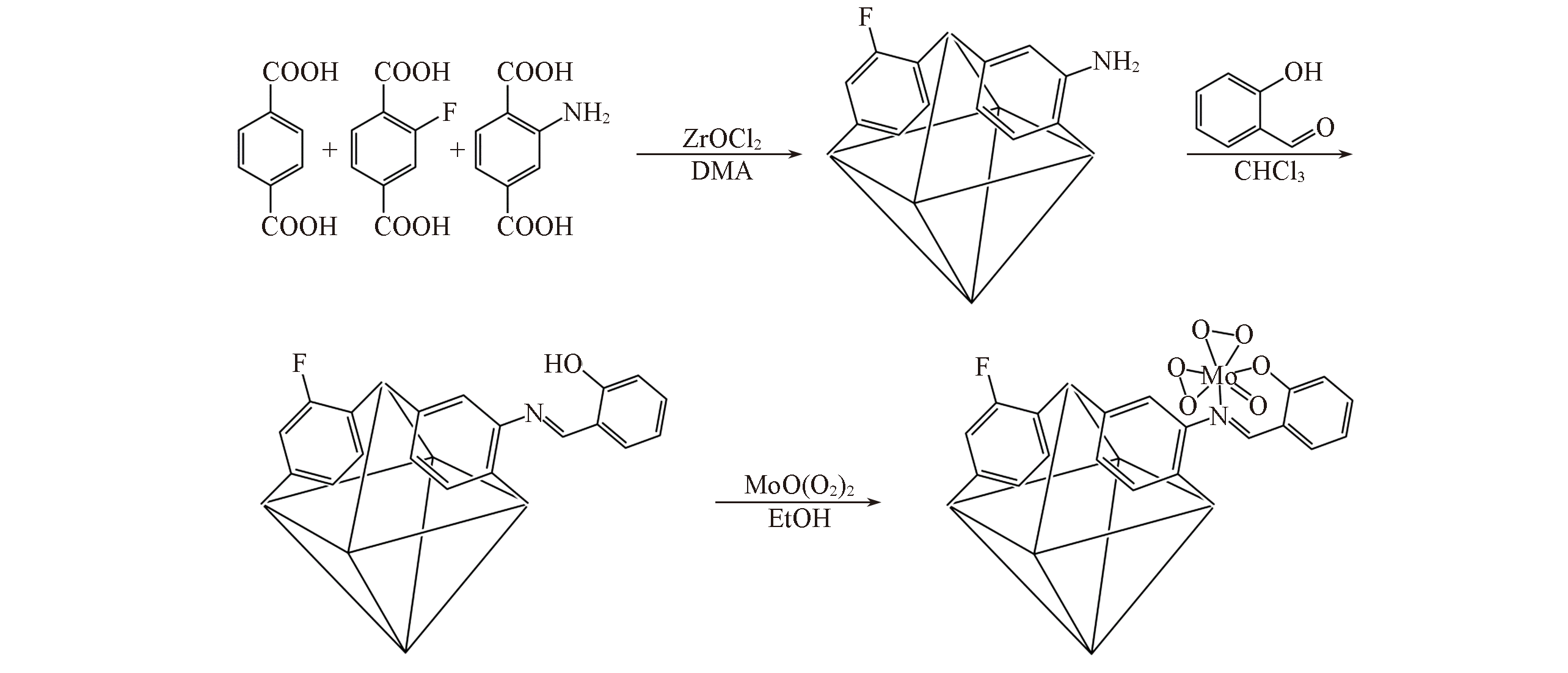
Scheme 1 Schematic diagram of synthetic procedure of UiO?66 modification with amino and fluorine functional groups as well as post?synthetic modification of molybdenum peroxide
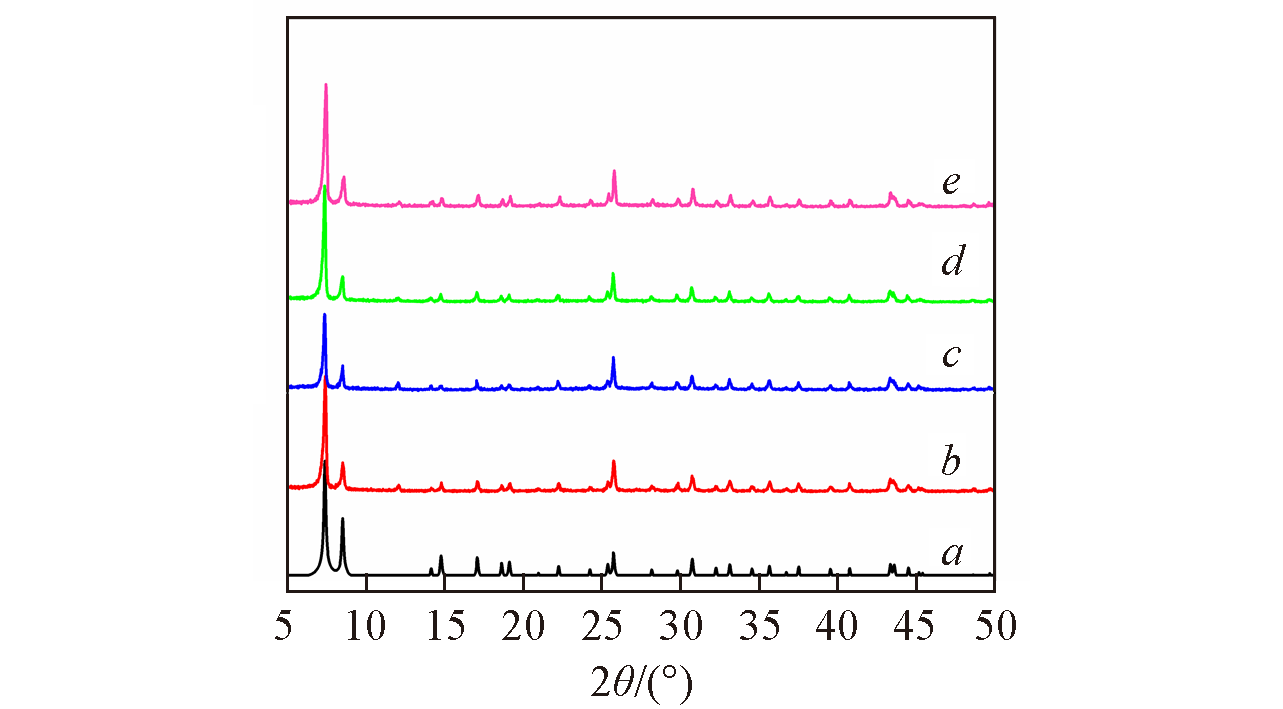
Fig.1 Simulated XRD pattern from single crystal X?ray diffraction of UiO?66(a), XRD patterns of UiO?66?1/4NH2?sal?Mo(b), UiO?66?1/4NH2?1/4F?sal?Mo(c), UiO?66?1/4NH2?1/2F?sal?Mo(d) and UiO?66?1/4NH2?3/4F?sal?Mo(e)
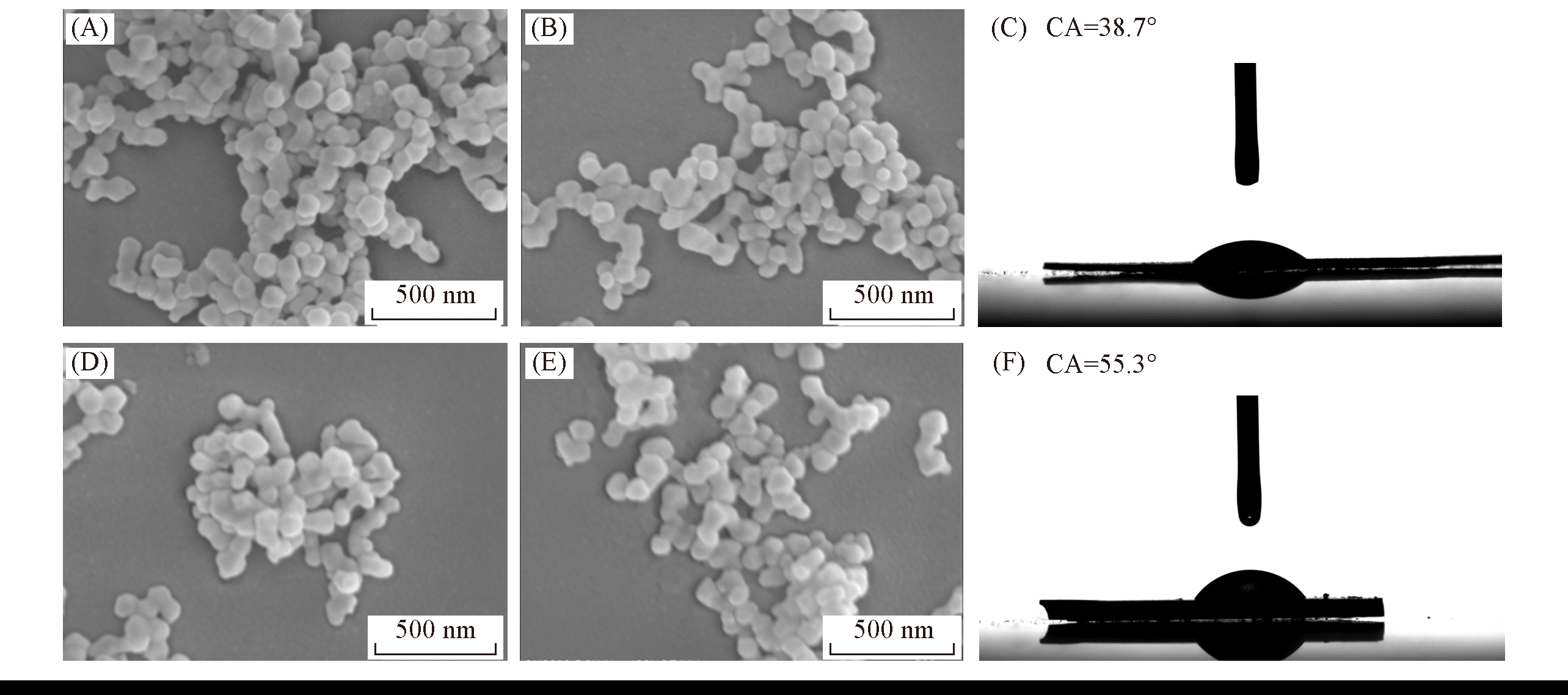
Fig.2 SEM images of UiO?66?1/4NH2(A), UiO?66?1/4NH2?1/4F(B), UiO?66?1/4NH2?sal?Mo(D), UiO?66?1/4NH2?1/4F?sal?Mo(E), and contact angles of water on the pressed pellet of UiO?66?1/4NH2(C), UiO?66?1/4NH2?1/4F(F)
| Sample | SBET/(m2·g-1) | Mo content/(mmol·g-1) |
|---|---|---|
| UiO?66?1/4NH2?sal?Mo | 517 | 1.0 |
| UiO?66?1/4NH2?1/4F?sal?Mo | 581 | 0.8 |
| UiO?66?1/4NH2?1/2F?sal?Mo | 601 | 0.9 |
| UiO?66?1/4NH2?3/4F?sal?Mo | 551 | 1.0 |
Table 1 BET surface area and ICP analysis results
| Sample | SBET/(m2·g-1) | Mo content/(mmol·g-1) |
|---|---|---|
| UiO?66?1/4NH2?sal?Mo | 517 | 1.0 |
| UiO?66?1/4NH2?1/4F?sal?Mo | 581 | 0.8 |
| UiO?66?1/4NH2?1/2F?sal?Mo | 601 | 0.9 |
| UiO?66?1/4NH2?3/4F?sal?Mo | 551 | 1.0 |
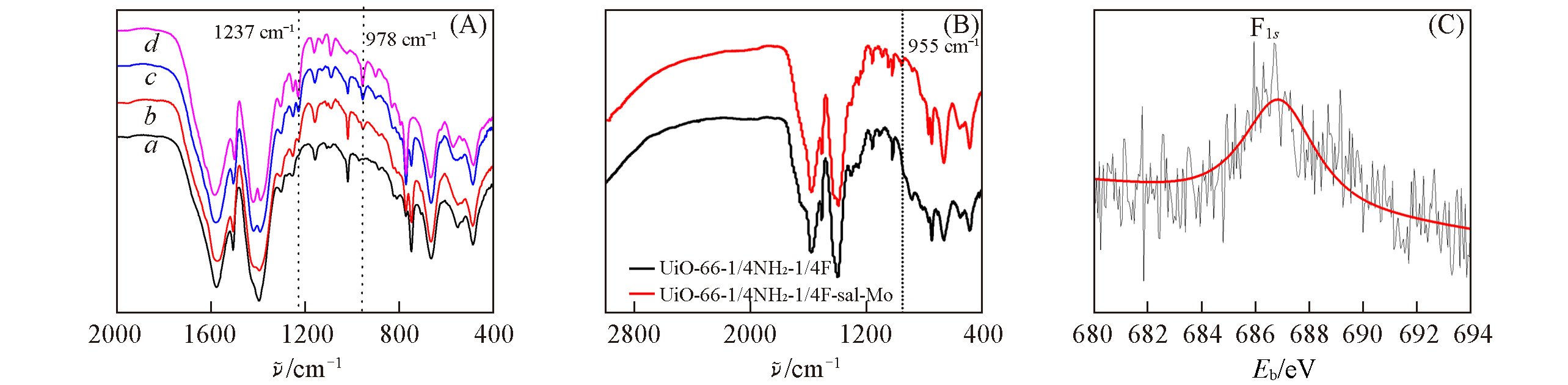
Fig.4 FTIR spectra of UiO?66?1/4NH2(a), UiO?66?1/4NH2?1/4F(b), UiO?66?1/4NH2?1/2F(c), UiO?66?1/4NH2?3/4F(d)(A), comparison of FTIR spectra of UiO?66?1/4NH2?1/4F before and after modification with MoO(O2)2(B) and XPS deconvolution of F1s of UiO?66?1/4NH2?1/4F(C)
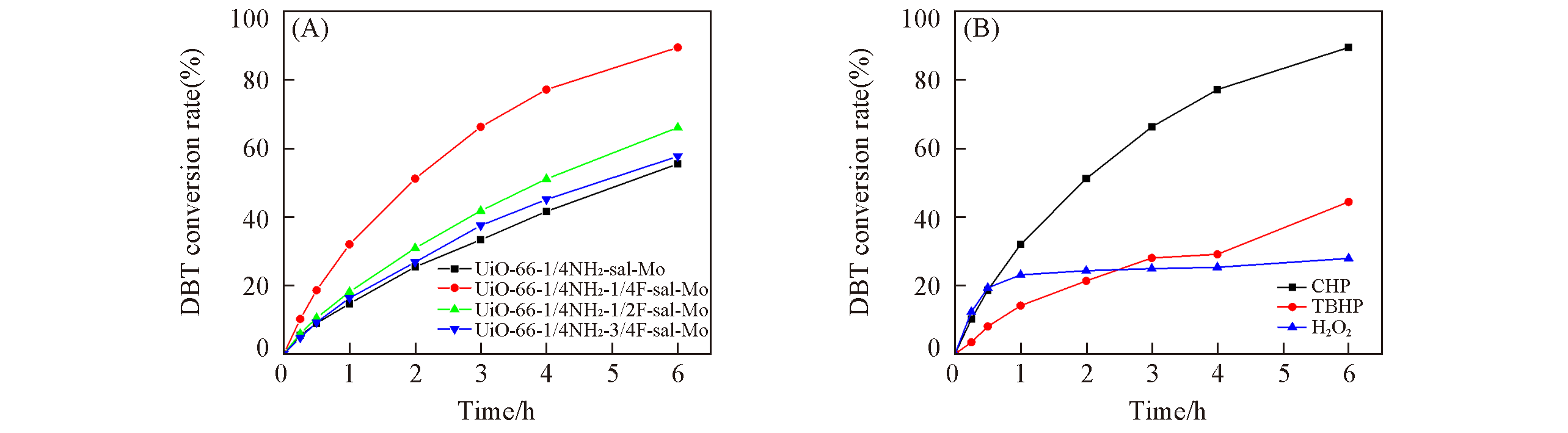
Fig.6 Relationship of time and conversion rate of UiO?66?1/4NH2?x/4F?sal?Mo(A) and UiO?66?1/4NH2? 1/4F?sal?Mo catalyst with different oxidants(B)(A) O/S molar ratio: 4, oxidant: CHP, temperature: 60 ℃, catalyst dosage: 0.08 mmol; (B) O/S molar ratio: 4, temperature: 60 ℃, 0.1 g catalyst.
| Entry | Catalyst dosage/g | O/S molar ratio | Temperature/℃ | DBT conversion*(%) |
|---|---|---|---|---|
| 1 | 0.05 | 3 | 60 | 42.7 |
| 2 | 0.05 | 4 | 70 | 78.7 |
| 3 | 0.05 | 5 | 80 | 99.5 |
| 4 | 0.10 | 3 | 80 | 93.4 |
| 5 | 0.10 | 4 | 60 | 89.5 |
| 6 | 0.10 | 5 | 70 | 97.3 |
| 7 | 0.15 | 3 | 70 | 84.8 |
| 8 | 0.15 | 4 | 80 | 96.2 |
| 9 | 0.15 | 5 | 60 | 92.3 |
Table 2 Orthogonal design and experimental results
| Entry | Catalyst dosage/g | O/S molar ratio | Temperature/℃ | DBT conversion*(%) |
|---|---|---|---|---|
| 1 | 0.05 | 3 | 60 | 42.7 |
| 2 | 0.05 | 4 | 70 | 78.7 |
| 3 | 0.05 | 5 | 80 | 99.5 |
| 4 | 0.10 | 3 | 80 | 93.4 |
| 5 | 0.10 | 4 | 60 | 89.5 |
| 6 | 0.10 | 5 | 70 | 97.3 |
| 7 | 0.15 | 3 | 70 | 84.8 |
| 8 | 0.15 | 4 | 80 | 96.2 |
| 9 | 0.15 | 5 | 60 | 92.3 |
| Factor | Catalyst dosage/g | O/S molar ratio | Temperature/℃ |
|---|---|---|---|
| Mean value 1 | 73.63 | 73.63 | 74.83 |
| Mean value 2 | 93.40 | 88.13 | 86.93 |
| Mean value 3 | 91.10 | 96.37 | 96.37 |
| Range | 19.77 | 22.73 | 21.53 |
Table 3 Range analysis table
| Factor | Catalyst dosage/g | O/S molar ratio | Temperature/℃ |
|---|---|---|---|
| Mean value 1 | 73.63 | 73.63 | 74.83 |
| Mean value 2 | 93.40 | 88.13 | 86.93 |
| Mean value 3 | 91.10 | 96.37 | 96.37 |
| Range | 19.77 | 22.73 | 21.53 |
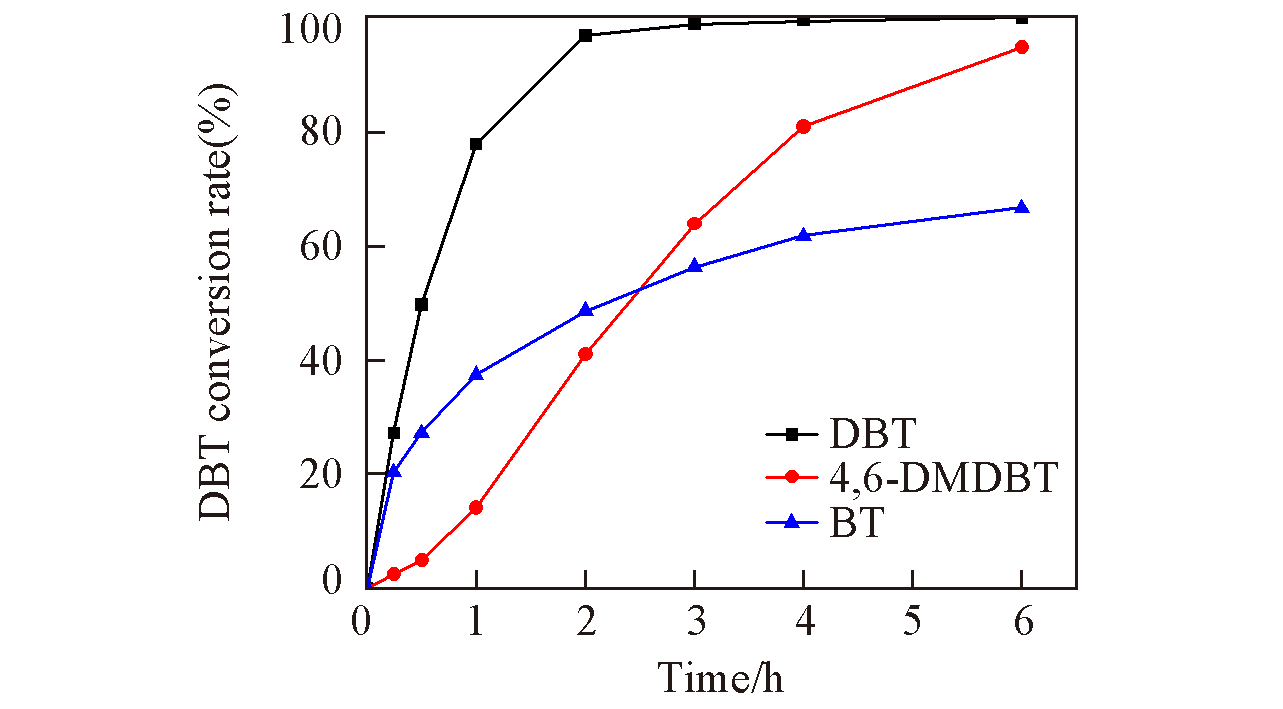
Fig.7 Relationship of time and conversion rate with different reactantsExperimental parameters: 80 ℃, O/S molar ratio: 5,oxidant: CHP, 0.1 g UiO-66-1/4NH2-1/4F-sal-Mo.
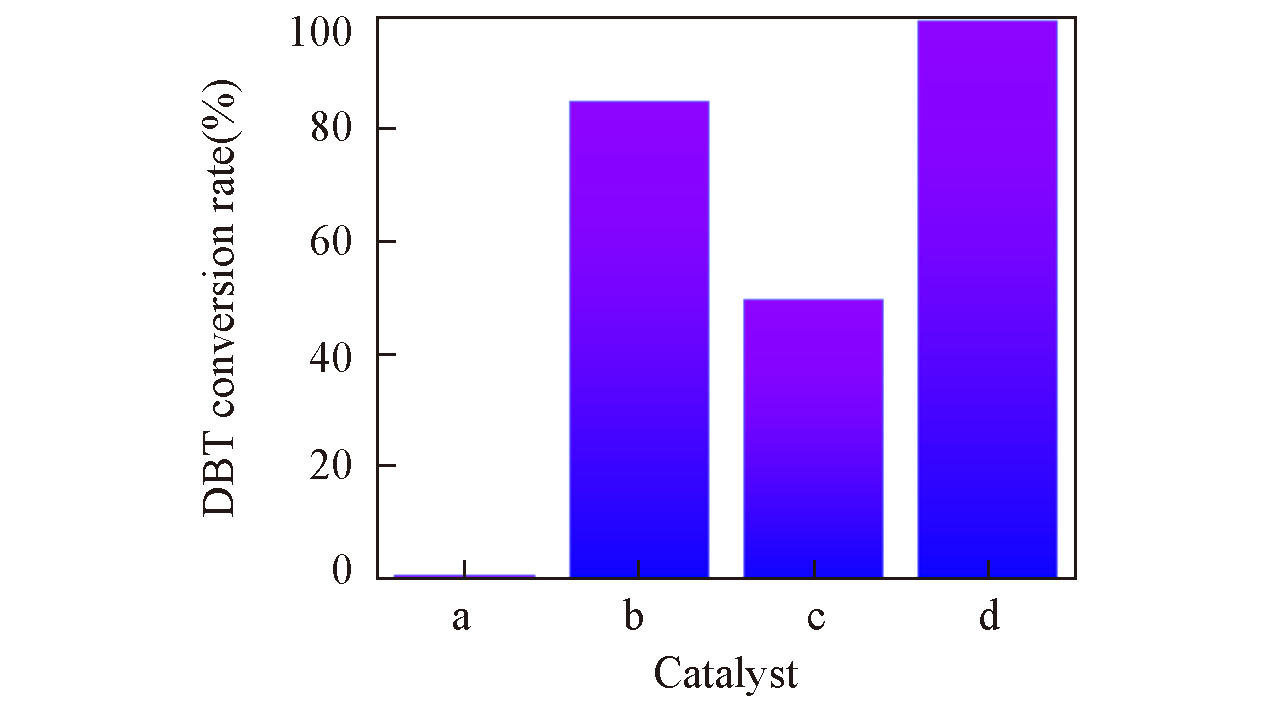
Fig.8 Control experiment of different catalystsa. Blank reaction; b. UiO-66-1/4NH2-1/4F; c. UiO-66- 1/4NH2-1/4F-sal; d. UiO-66-1/4NH2-1/4F-sal-Mo. Experi mental parameters: 80 ℃, 4 h, O/S molar ratio: 5, oxidant: CHP, 0.1 g catalyst.
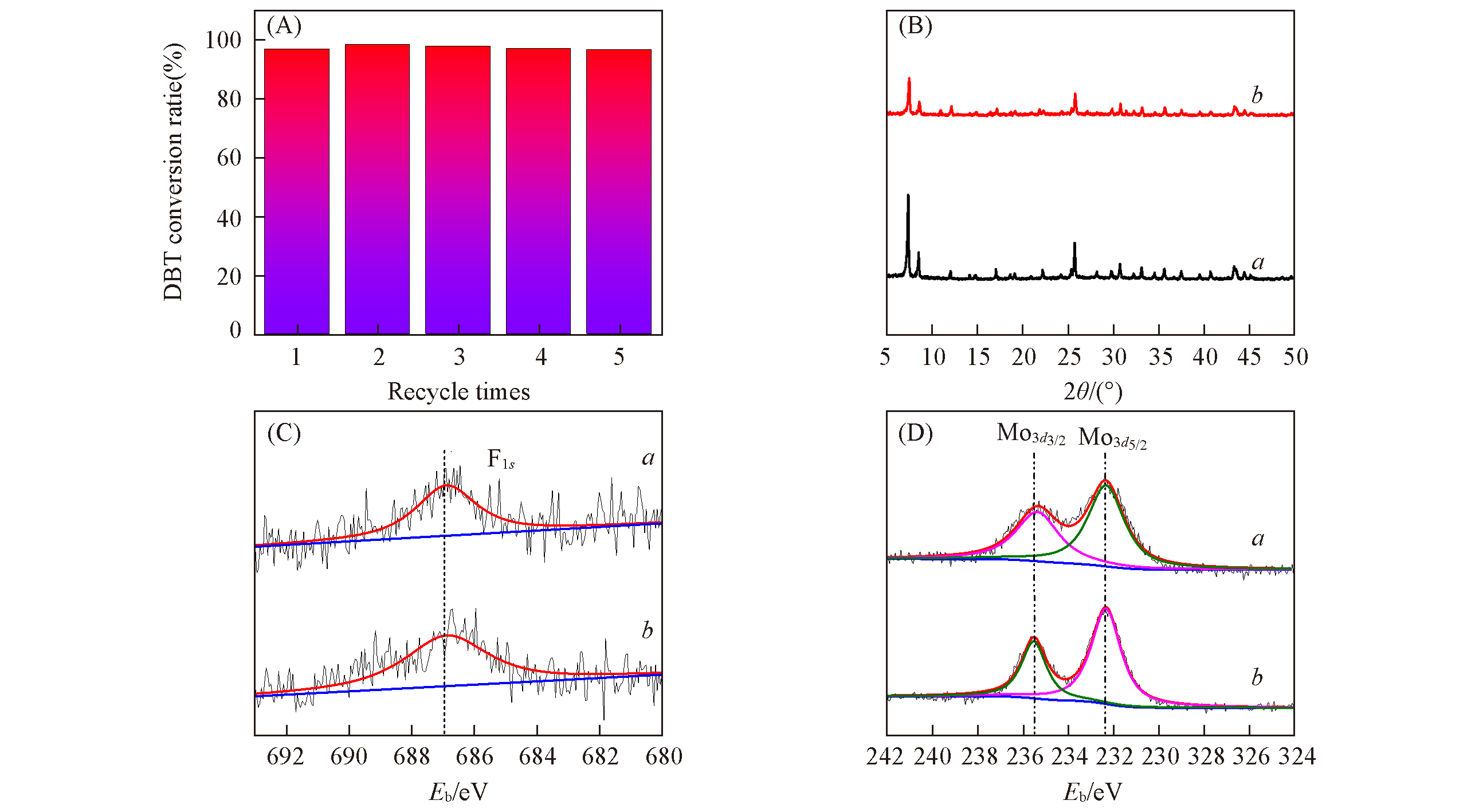
Fig.9 Recycling performance of UiO?66?1/4NH2?1/4F?sal?Mo(A), XRD patterns(B), XPS deconvolution of F1s(C) and Mo3d(D) of fresh(a) and spent(b) catalyst(B) O/S molar ratio: 5, oxidant: CHP, 80 ℃, 0.1 g catalyst.
| 1 | Chow J., Kopp R. J., Portney P. R., Science, 2003, 302(5650), 1528—1531 |
| 2 | Bhutto A. W., Abro R., Gao S. R., Abbas T., Chen X. C., Yu G. R., J. Taiwan Inst. Chem. E, 2016, 62, 84—97 |
| 3 | Chen K., Liu N., Zhang M., Wang D., Appl. Catal. B: Environ., 2017, 212, 32—40 |
| 4 | Ganguly T., Das A., Jana M., Inorg. Chem., 2018, 57(18), 11306—11309 |
| 5 | Tripathi D., Negi H., Singh R. K., Singh U. P., Srivastava V. C., J. Coord. Chem., 2019, 72(17),2982—2996 |
| 6 | Chen K., Zhang X. M., Yang X. F., Jiao M. G., Zhou Z., Zhang M. H., Wang D. H., Bu X. H., Appl. Catal. B: Environ., 2018, 238,263—273 |
| 7 | Craven M., Xiao D., Kunstmann-Olsen C., Kozhevnikova E. F., Blanc F., Steiner A., Kozhevnikov I. V., Appl. Catal. B: Environ., 2018, 231, 82—91 |
| 8 | Gu Q. Q., Wen G. D., Ding Y. X., Wu K. H., Chen C. M., Su D. S., Green. Chem., 2017, 19(4), 1175—1181 |
| 9 | Bhadra B. N., Jhung S. H., Appl. Catal. B: Environ., 2019, 259, 1—25 |
| 10 | Jiang H., Jia J., Shkurenko A., Chen Z., Adil K., Belmabkhout Y., Weselinski L. J., Assen A. H., Xue D. X., O'Keeffe M., Eddaoudi M., J. Am. Chem. Soc., 2018, 140(28),8858—8867 |
| 11 | Kirchon A., Feng L., Drake H. F., Joseph E. A., Zhou H. C., Chem. Soc. Rev., 2018, 47(23), 8611—8638 |
| 12 | Liu Y. Y., Leus K., Sun Z. C., Li X., Depauw H., Wang A. J., Zhang J., van der Voort P., Micropor. Mesopor. Mater., 2019, 277, 245—252 |
| 13 | Cavka J. H., Jakobsen S., Olsbye U., Guillou N., Lamberti C., Bordiga S., Lillerud K. P., J. Am. Chem. Soc., 2008, 130(42), 13850—13851 |
| 14 | Ye G., Qi H., Zhou W., Xu W., Sun Y. Y., Inorg. Chem. Front., 2019, 6(5), 1267—1274 |
| 15 | Xiao W. M., Dong Q. L., Wang Y., Li Y., Deng S. J., Zhang N., CrystEngComm, 2018, 20(38),5658—5662 |
| 16 | Ye G., Qi H., Li X., Leng K., Sun Y., Xu W., ChemPysChem, 2017, 18(14), 1903—1908 |
| 17 | Viana A. M., Julião D., Mirante F., Faria R. G., de Castro B., Balula S. S., Cunha-Silva L., Catal. Today, 2021, 362, 28—34 |
| 18 | Afzali N., Kardanpour R., Zadehahmadi F., Tangestaninejad S., Moghadam M., Mirkhani V., Mechler A., Mohammadpoor‐Baltork I., Bahadori M., Appl. Organomet. Chem., 2019, 33(11), 1—11 |
| 19 | Chica A., Corma A., Domine M. E., J. Catal., 2006, 242(2), 299—308 |
| 20 | Goh T. W., Xiao C. X., Maligal⁃Ganesh R. V., Li X. L., Huang W. Y., Chem. Eng. Sci., 2015, 124, 45—51 |
| 21 | Biswas S., van Der Voort P., Eur. J. Inorg. Chem., 2013, 2013(12), 2154—2160 |
| 22 | Tang J., Dong W. J., Wang G., Yao Y. Z., Cai L. M., Liu Y., Zhao X., Xu J. Q., Tan L., Rsc. Adv., 2014, 4(81), 42977—42982 |
| 23 | Pourkhosravani M., Dehghanpour S., Farzaneh F., Sohrabi S., React. Kinet. Mech. Cat., 2017, 122(2), 961—981 |
| 24 | Sheng B. C., Li C., Liu Y. Y., Wang A. J., Wang Y., Zhang J., Liu W. X., Chem. J. Chinese Universities, 2019, 40(7), 1365—1373(盛炳琛, 李从, 刘颖雅, 王安杰, 王瑶, 张箭, 刘伟旭. 高等学校化学学报, 2019, 40(7), 1365—1373) |
| 25 | Aralikatti N. V., J. Mol. Struct., 2018, 1173, 814—821 |
| 26 | Tressaud A., Moguet F., Flandrois S., Chambon M., Guimon C., Nanse G., Papirer E., Gupta V., Bahl O. P., J. Phys. Chem. Solids, 1996, 57(6—8), 745—751 |
| 27 | Valenzano L., Civalleri B., Chavan S., Bordiga S., Nilsen M. H., Jakobsen S., Lillerud K. P., Lamberti C., Chem. Mater., 2011, 23(7), 1700—1718 |
| 28 | Zhang X. M., Zhang Z. H., Zhang B. H., Yang X. F., Chang X., Zhou Z., Wang D. H., Zhang M. H., Bu X. H., Appl. Catal. B: Environ., 2019, 256, 1—9 |
| 29 | Ghahramaninezhad M., Pakdel F., Shahrak M. N., Polyhedron, 2019, 170, 364—372 |
| 30 | Otsuki S., Nonaka T., Takashima N., Qian W. H., Ishihara A., Imai T., Kabe T., Energ. Fuel., 2000, 14(6), 1232—1239 |
| 31 | Zheng H. Q., Zeng Y. N., Chen J., Lin R. G., Zhuang W. E., Cao R., Lin Z. J., Inorg. Chem., 2019, 58(10), 6983—6992 |
| 32 | Jiang W., Zhu K., Li H., Zhu L., Hua M., Xiao J., Wang C., Yang Z., Chen G., Zhu W., Li H., Dai S., Chem. Eng. J., 2020, 394, 1—8 |
| 33 | Chakarova K., Strauss I., Mihaylov M., Drenchev N., Hadjiivanov K., Micropor. Mesopor. Mater., 2019, 281, 110—122 |
| 34 | Xiao W., Dong Q., Wang Y., Li Y., Deng S., Zhang N., CrystEngComm, 2018, 20(38), 5658—5662 |
| 35 | Zhang X., Huang P., Liu A., Zhu M., Fuel, 2017, 209, 417—423 |
| [1] | 李玉龙, 谢发婷, 管燕, 刘嘉丽, 张贵群, 姚超, 杨通, 杨云慧, 胡蓉. 基于银离子与DNA相互作用的比率型电化学传感器用于银离子的检测[J]. 高等学校化学学报, 2022, 43(8): 20220202. |
| [2] | 丁杨, 王万辉, 包明. 多孔骨架固定分子催化剂催化CO2加氢制备甲酸研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220309. |
| [3] | 鲁聪, 李振华, 刘金露, 华佳, 李光华, 施展, 冯守华. 一种新的镧系金属有机骨架材料的合成、 结构及荧光检测性质[J]. 高等学校化学学报, 2022, 43(6): 20220037. |
| [4] | 邢珮琪, 陆通, 李光华, 王力彦. 两个镉(II)金属有机骨架的可控合成与结构相关性[J]. 高等学校化学学报, 2022, 43(10): 20220218. |
| [5] | 李文, 乔珺一, 刘鑫垚, 刘云凌. 含萘基团的锆金属有机骨架材料对水中硝基芳烃爆炸物的荧光检测性能[J]. 高等学校化学学报, 2022, 43(1): 20210654. |
| [6] | 赵阳洋, 刘启勇, 陈泊鑫, 赵斌, 周海梅, 李昕欣, 郑丹, 冯飞. 以金属有机骨架材料ZIF-8为固定相的硅基微气相色谱柱[J]. 高等学校化学学报, 2021, 42(6): 1736. |
| [7] | 李梅, 夏晓娟, 陈志雄, 杨梦, 李紫滢, 杨通, 孟爽, 杨云慧, 胡蓉. 基于铂纳米颗粒@金属有机骨架纳米模拟酶的无标记电化学赭曲霉毒素适体传感器的构建[J]. 高等学校化学学报, 2021, 42(12): 3615. |
| [8] | 陈晓, 申博渊, 熊昊, 魏飞. 电子束敏感材料的原子尺度结构研究[J]. 高等学校化学学报, 2021, 42(1): 133. |
| [9] | 姜笑天, 尹琦, 刘天赋, 曹荣. 金属有机骨架薄膜用于小分子和离子的高效分离[J]. 高等学校化学学报, 2020, 41(8): 1691. |
| [10] | 谢兴钰, 赵雅香, 赵莉芝, 李日舜, 吴迪昊, 叶卉, 辛清萍, 李泓, 张玉忠. 基于金属卟啉2DMOFs仿酶催化的过氧化氢比色法检测[J]. 高等学校化学学报, 2020, 41(8): 1776. |
| [11] | 高霞,潘会宾,乔成芳,陈凤英,周元,杨文华. 基于多级孔金属有机骨架构筑HRP固定化酶反应器及其染料降解应用[J]. 高等学校化学学报, 2020, 41(7): 1591. |
| [12] | 陆曼,宋春梅,万波. 水性乳液/聚氨酯模型缔合增稠剂的触变性机理[J]. 高等学校化学学报, 2020, 41(5): 1108. |
| [13] | 王瑞, 黄新松, 刘天赋, 曹荣. 金属有机框架用于一氧化碳氧化[J]. 高等学校化学学报, 2020, 41(10): 2174. |
| [14] | 侯俊英, 郝建军, 王雅雅, 刘敬春. Cu3(BTC)2金属有机骨架复合基质膜的制备及流体催化性能[J]. 高等学校化学学报, 2019, 40(9): 1926. |
| [15] | 王鹏程, 单梁, 范勇, 王莉, 徐家宁, 吴淑杰. MIL-53系列金属有机骨架化合物的合成及在Strecker反应中的催化性能[J]. 高等学校化学学报, 2019, 40(8): 1655. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||