

高等学校化学学报 ›› 2024, Vol. 45 ›› Issue (10): 20240369.doi: 10.7503/cjcu20240369
• 有机化学 • 上一篇
王刚, 梁爽, 单忠刚, 英君伍, 吕亮, 李斌, 杨辉斌( )
)
收稿日期:2024-07-28
出版日期:2024-10-10
发布日期:2024-08-21
通讯作者:
杨辉斌
E-mail:yanghuibin@yangnongchem.com
基金资助:
WANG Gang, LIANG Shuang, SHAN Zhonggang, YING Junwu, LYU Liang, LI Bin, YANG Huibin( )
)
Received:2024-07-28
Online:2024-10-10
Published:2024-08-21
Contact:
YANG Huibin
E-mail:yanghuibin@yangnongchem.com
Supported by:摘要:
以取代吡嗪酸和2-甲基-3-硝基苯酚为原料, 经4步反应合成了16个吡嗪酰胺类似物(化合物1~16), 其结构经核磁共振波谱(1H NMR和13C NMR)及高分辨质谱(HRMS)确证. 杀菌活性测试结果表明, 吡嗪酰胺类似物浓度为6.25 mg/L时对玉米锈病具有优异的杀菌活性, 其中化合物4, 5, 7, 8, 15和16对玉米锈病的杀菌活性为100%. 分子对接模拟结果显示, 化合物16通过氢键与琥珀酸脱氢酶(SDH)的TRP-173相互作用, 这可以解释化合物16与目标蛋白之间可能的作用机制. 研究结果表明, 化合物16是一种具有潜在前景的杀菌剂候选物, 为进一步研究提供了参考.
中图分类号:
TrendMD:
王刚, 梁爽, 单忠刚, 英君伍, 吕亮, 李斌, 杨辉斌. 吡嗪酰胺类似物的设计、 合成及杀菌活性. 高等学校化学学报, 2024, 45(10): 20240369.
WANG Gang, LIANG Shuang, SHAN Zhonggang, YING Junwu, LYU Liang, LI Bin, YANG Huibin. Design, Synthesis and Fungicidal Activity of Pyrazinamide Analogs. Chem. J. Chinese Universities, 2024, 45(10): 20240369.
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(ESI) |
|---|---|---|---|---|
| 1 | White solid | 73 | 153—154 | Calcd. for C14H16N3O2 [M+H]+: 258.1164, found: 258.1234 |
| 2 | White solid | 73 | 123—125 | Calcd. for C15H18N3O2 [M+H]+: 272.1321, found: 272.1391 |
| 3 | White solid | 79 | 117—119 | Calcd. for C16H20N3O2 [M+H]+: 286.1477, found: 286.1548 |
| 4 | Yellow solid | 84 | 76—77 | Calcd. for C17H22N3O2 [M+H]+: 300.1634, found: 300.1703 |
| 5 | Brown oil | 78 | 52—54 | Calcd. for C18H24N3O2 [M+H]+: 314.1790, found: 314.1859 |
| 6 | Yellow solid | 75 | 59—61 | Calcd. for C18H24N3O2 [M+H]+: 314.1790, found: 314.1860 |
| 7 | White solid | 76 | 59—60 | Calcd. for C18H24N3O2 [M+H]+: 314.1790, found: 314.1859 |
| 8 | Light yellow oil | 76 | Calcd. for C19H26N3O2 [M+H]+: 328.1947, found: 328.2016 | |
| 9 | Black solid | 84 | 67—69 | Calcd. for C17H22N3O2 [M+H]+: 300.1634, found: 300.1703 |
| 10 | Red oil | 70 | Calcd. for C18H24N3O2 [M+H]+: 314.1790, found: 314.1860 | |
| 11 | Brown solid | 63 | 68—70 | Calcd. for C18H24N3O2 [M+H]+: 314.1790, found: 314.1860 |
| 12 | Red oil | 66 | Calcd. for C19H26N3O2 [M+H]+: 328.1947, found: 328.2015 | |
| 13 | Red solid | 71 | 70—71 | Calcd. for C18H24N3O2 [M+H]+: 314.1790, found: 314.1859 |
| 14 | Red solid | 50 | 43—45 | Calcd. for C19H26N3O2 [M+H]+: 328.1947, found: 328.2018 |
| 15 | White solid | 69 | 109—111 | Calcd. for C18H21F3N3O2 [M+H]+: 368.1508, found: 368.1580 |
| 16 | White solid | 74 | 91—93 | Calcd. for C19H23F3N3O2 [M+H]+: 382.1664, found: 382.1734 |
Table 1 Physical properties of compounds 1—16
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(ESI) |
|---|---|---|---|---|
| 1 | White solid | 73 | 153—154 | Calcd. for C14H16N3O2 [M+H]+: 258.1164, found: 258.1234 |
| 2 | White solid | 73 | 123—125 | Calcd. for C15H18N3O2 [M+H]+: 272.1321, found: 272.1391 |
| 3 | White solid | 79 | 117—119 | Calcd. for C16H20N3O2 [M+H]+: 286.1477, found: 286.1548 |
| 4 | Yellow solid | 84 | 76—77 | Calcd. for C17H22N3O2 [M+H]+: 300.1634, found: 300.1703 |
| 5 | Brown oil | 78 | 52—54 | Calcd. for C18H24N3O2 [M+H]+: 314.1790, found: 314.1859 |
| 6 | Yellow solid | 75 | 59—61 | Calcd. for C18H24N3O2 [M+H]+: 314.1790, found: 314.1860 |
| 7 | White solid | 76 | 59—60 | Calcd. for C18H24N3O2 [M+H]+: 314.1790, found: 314.1859 |
| 8 | Light yellow oil | 76 | Calcd. for C19H26N3O2 [M+H]+: 328.1947, found: 328.2016 | |
| 9 | Black solid | 84 | 67—69 | Calcd. for C17H22N3O2 [M+H]+: 300.1634, found: 300.1703 |
| 10 | Red oil | 70 | Calcd. for C18H24N3O2 [M+H]+: 314.1790, found: 314.1860 | |
| 11 | Brown solid | 63 | 68—70 | Calcd. for C18H24N3O2 [M+H]+: 314.1790, found: 314.1860 |
| 12 | Red oil | 66 | Calcd. for C19H26N3O2 [M+H]+: 328.1947, found: 328.2015 | |
| 13 | Red solid | 71 | 70—71 | Calcd. for C18H24N3O2 [M+H]+: 314.1790, found: 314.1859 |
| 14 | Red solid | 50 | 43—45 | Calcd. for C19H26N3O2 [M+H]+: 328.1947, found: 328.2018 |
| 15 | White solid | 69 | 109—111 | Calcd. for C18H21F3N3O2 [M+H]+: 368.1508, found: 368.1580 |
| 16 | White solid | 74 | 91—93 | Calcd. for C19H23F3N3O2 [M+H]+: 382.1664, found: 382.1734 |
| Compd. | 1H NMR(600 MHz, CDCl3), δ | 13C NMR(151 MHz, CDCl3), δ |
|---|---|---|
| 1 | 9.87(s, 1H), 8.56(d, J=2.5 Hz, 1H), 8.35(d, J=2.5 Hz, 1H), 7.72(d, J=8.0 Hz, 1H), 7.14(t, J=8.2 Hz, 1H), 6.64(d, J=8.2 Hz, 1H), 3.77(s, 3H), 3.00(s, 3H), 2.18(s, 3H) | 162.22, 157.82, 156.04, 146.17, 142.64, 139.97, 136.52, 126.66, 117.36, 114.57, 107.13, 55.72, 24.05, 9.79 |
| 2 | 9.90(s, 1H), 8.63(d, J=2.5 Hz, 1H), 8.42(d, J=2.2 Hz, 1H), 7.74(d, J=8.1 Hz, 1H), 7.17(t, J=8.3 Hz, 1H), 6.69(d, J=8.3 Hz, 1H), 4.03(q, J=6.9 Hz, 2H), 3.04(s, 3H), 2.23(s, 3H), 1.82(s, 1H), 1.41(t, J=7.0 Hz, 3H) | 162.29, 157.28, 156.17, 146.17, 142.80, 140.00, 136.52, 126.65, 117.77, 114.57, 108.43, 64.10, 24.08, 15.04, 9.89 |
| 3 | 9.86(s, 1H), 8.56(d, J=2.5 Hz, 1H), 8.35(d, J=2.5 Hz, 1H), 7.71(d, J=8.2 Hz, 1H), 7.12(t, J=8.2 Hz, 1H), 6.67(d, J=8.2 Hz, 1H), 4.47(hept, J=6.1 Hz, 1H), 3.00(s, 3H), 2.18(s, 3H), 1.29(d, J=6.2 Hz, 6H) | 162.18, 156.26, 156.03, 146.15, 142.70, 139.97, 136.73, 126.51, 118.80, 114.48, 110.50, 70.85, 24.06, 22.32, 10.09 |
| 4 | 9.87(s, 1H), 8.57(d, J=2.6 Hz, 1H), 8.36(d, J=2.4 Hz, 1H), 7.70(d, J=8.2 Hz, 1H), 7.12(t, J=8.1 Hz, 1H), 6.67(d, J=8.3 Hz, 1H), 4.26(h, J=6.1 Hz, 1H), 3.01(s, 3H), 2.20(s, 3H), 1.72(dp, J=14.3, 7.2 Hz, 1H), 1.61(dq, J=13.8, 7.0 Hz, 1H), 1.25(d, J=6.2 Hz, 3H), 0.95(t, J=7.6 Hz, 3H) | 162.20, 156.47, 156.05, 146.15, 142.73, 139.98, 136.70, 126.51, 118.72, 114.37, 110.18, 75.69, 29.36, 24.06, 19.44, 10.06, 9.85 |
| 5 | 9.89(s, 1H), 8.61(d, J=2.6 Hz, 1H), 8.40(d, J=2.5 Hz, 1H), 7.72(d, J=8.1 Hz, 1H), 7.15(t, J=8.2 Hz, 1H), 6.70(d, J=8.3 Hz, 1H), 4.35(h, J=6.1 Hz, 1H), 3.03(s, 3H), 2.21(s, 3H), 1.78—1.68(m, 1H), 1.59—1.50(m, 1H), 1.52—1.43(m, 1H), 1.45—1.35(m, 1H), 1.27(d, J=6.1 Hz, 3H), 0.92(t, J=7.3 Hz, 3H) | 162.24, 156.52, 156.13, 146.16, 142.79, 139.99, 136.69, 126.54, 118.78, 114.40, 110.18, 74.36, 38.86, 24.09, 19.96, 18.85, 14.21, 10.10 |
| 6 | 9.89(s, 1H), 8.61(d, J=2.6 Hz, 1H), 8.40(d, J=2.5 Hz, 1H), 7.72(d, J=8.5 Hz, 1H), 7.15(t, J=8.2 Hz, 1H), 6.70(d, J=8.3 Hz, 1H), 4.35(h, J=6.1 Hz, 1H), 3.04(s, 3H), 2.21(s, 3H), 1.78—1.69(m, 1H), 1.59—1.35(m, 3H), 1.27(d, J=6.1 Hz, 3H), 0.92(t, J=7.4 Hz, 3H) | 162.24, 156.52, 156.13, 146.16, 142.79, 139.99, 136.69, 126.54, 118.79, 114.41, 110.18, 74.37, 38.86, 24.09, 19.96, 18.85, 14.21, 10.11 |
| 7 | 9.88(s, 1H), 8.58(s, 1H), 8.37(t, J=3.2 Hz, 1H), 7.71(d, J=7.7 Hz, 1H), 7.13(t, J=8.2 Hz, 1H), 6.68(d, J=8.3 Hz, 1H), 4.34(q, J=6.2 Hz, 1H), 3.02(s, 3H), 2.20(s, 3H), 1.72(ddd, J=16.2, 8.7, 5.0 Hz, 1H), 1.53(dq, J=14.5, 5.3 Hz, 1H), 1.45(t, J=6.5 Hz, 1H), 1.43—1.35(m, 1H), 1.26(d, J=6.1 Hz, 3H), 0.91(t, J=7.2 Hz, 3H) | 162.21, 156.50, 156.07, 146.14, 142.75, 139.98, 136.70, 126.52, 118.73, 114.37, 110.14, 74.33, 38.85, 24.06, 19.94, 18.84, 14.20, 10.09 |
| 8 | 9.90(s, 1H), 8.64(d, J=2.4 Hz, 1H), 8.43(d, J=2.4 Hz, 1H), 7.72(t, J=8.8 Hz, 1H), 7.17(t, J=8.2 Hz, 1H), 6.71(d, J=8.3 Hz, 1H), 4.35(h, J=6.1 Hz, 1H), 3.05(s, 3H), 2.23(d, J=3.2 Hz, 3H), 1.80—1.63(m, 2H), 1.58(ddt, J=14.7, 10.8, 5.2 Hz, 1H), 1.44(ddd, J=16.4, 8.7, 4.2 Hz, 1H), 1.41—1.31(m, 2H), 1.28(d, J=6.1 Hz, 3H), 0.91(dt, J=18.3, 7.4 Hz, 3H) | 162.26, 156.54, 156.20, 146.17, 142.85, 139.99, 136.68, 126.53, 118.83, 114.43, 110.23, 74.67, 36.40, 24.11, 19.99, 18.74, 14.29, 10.12, 9.63 |
| 9 | 9.67(s, 1H), 9.43(s, 1H), 8.69(d, J=2.6 Hz, 1H), 8.50(d, J=2.4 Hz, 1H), 7.73(d, J=8.1 Hz, 1H), 7.11(t, J=8.2 Hz, 1H), 6.66(d, J=8.3 Hz, 1H), 4.32(h, J=6.1 Hz, 1H), 2.19(s, 3H), 1.74—1.65(m, 1H), 1.52(ddd, J=14.3, 10.6, 5.5 Hz, 1H), 1.48—1.32(m, 2H), 1.24(d, J=6.2 Hz, 3H), 0.89(t, J=7.2 Hz, 3H) | 160.63, 156.48, 147.52, 144.75, 144.62, 142.55, 136.31, 126.62, 118.55, 114.29, 110.28, 74.33, 38.82, 19.91, 18.82, 14.19, 9.97 |
| 10 | 9.69(s, 1H), 9.46(s, 1H), 8.74(d, J=2.6 Hz, 1H), 8.54(s, 1H), 7.76(t, J=7.6 Hz, 1H), 7.15(t, J=8.2 Hz, 1H), 6.69(d, J=8.0 Hz, 1H), 4.33(h, J=6.0 Hz, 1H), 2.23(d, J=4.4 Hz, 3H), 1.78—1.61(m, 2H), 1.56(ddt, J=14.6, 10.4, 5.0 Hz, 1H), 1.42(td, J=11.0, 10.5, 5.5 Hz, 1H), 1.40—1.24(m, 5H), 0.88(t, J=6.9 Hz, 3H) | 160.66, 156.50, 147.54, 144.81, 144.70, 142.55, 136.30, 126.66, 118.57, 114.31, 110.34, 74.65, 36.36, 19.96, 18.72, 14.30, 9.99, 9.61 |
| 11 | 9.64(s, 1H), 9.34(s, 1H), 8.41(s, 1H), 7.78(d, J=8.0 Hz, 1H), 7.16(t, J=8.2 Hz, 1H), 6.70(d, J=8.3 Hz, 1H), 4.35(h, J=6.1 Hz, 1H), 2.64(s, 3H), 2.22(s, 3H), 1.78—1.69(m, 1H), 1.59—1.51(m, 1H), 1.51—1.35(m, 2H), 1.27(d, J=6.1 Hz, 3H), 0.92(t, J=7.3 Hz, 3H) | 161.01, 157.41, 156.50, 143.66, 142.32, 142.14, 136.45, 126.64, 118.46, 114.27, 110.22, 74.40, 38.85, 21.93, 19.95, 18.85, 14.20, 9.97 |
| 12 | 9.63(s, 1H), 9.32(s, 1H), 8.39(s, 1H), 7.76(t, J=7.7 Hz, 1H), 7.14(t, J=8.2 Hz, 1H), 6.68(d, J=7.9 Hz, 1H), 4.33(h, J=6.0 Hz, 1H), 2.62(s, 3H), 2.21(s, 2H), 1.78—1.61(m, 2H), 1.56(dp, J=15.0, 5.1 Hz, 1H), 1.42(td, J=11.3, 10.3, 5.9 Hz, 1H), 1.40—1.28(m, 3H), 1.26(d, J=6.0 Hz, 3H), 0.88(q, J=6.9, 5.9 Hz, 3H) | 160.98, 157.40, 156.48, 143.63, 142.31, 142.12, 136.46, 126.62, 118.42, 114.24, 110.20, 74.64, 36.37, 21.90, 19.96, 18.72, 14.29, 9.97 |
| Compd. | 1H NMR(600 MHz, CDCl3), δ | 13C NMR(151 MHz, CDCl3), δ |
| 13 | 9.73(s, 1H), 9.23(s, 1H), 8.58(s, 1H), 7.74(d, J=8.2 Hz, 1H), 7.12(t, J=8.2 Hz, 1H), 6.67(d, J=8.2 Hz, 1H), 4.33(h, J=6.1 Hz, 1H), 2.59(s, 3H), 2.20(s, 3H), 1.75—1.64(m, 1H), 1.57—1.49(m, 1H), 1.49—1.33(m, 2H), 1.25(d, J=6.1 Hz, 3H), 0.90(t, J=7.5 Hz, 3H) | 160.84, 156.49, 152.09, 147.48, 143.62, 141.37, 136.41, 126.60, 118.51, 114.23, 110.25, 74.38, 38.83, 21.54, 19.93, 18.82, 14.19, 9.91 |
| 14 | 9.74(s, 1H), 9.23(s, 1H), 8.59(s, 1H), 7.74(t, J=7.9 Hz, 1H), 7.12(t, J=8.2 Hz, 1H), 6.67(d, J=8.3 Hz, 1H), 4.31(h, J=6.1 Hz, 1H), 2.60(s, 3H), 2.21(d, J=5.6 Hz, 3H), 1.77—1.60(m, 2H), 1.56(ddt, J=14.6, 10.4, 5.1 Hz, 1H), 1.45—1.36(m, 1H), 1.38—1.24(m, 5H), 0.91—0.84(m, 3H) | 160.85, 156.49, 152.09, 147.48, 143.63, 141.38, 136.41, 126.61, 118.52, 114.23, 110.26, 74.64, 36.36, 21.55, 19.95, 18.71, 14.29, 9.92, 9.59 |
| 15 | 9.38(s, 1H), 8.79(s, 1H), 8.76(s, 1H), 7.67(d, J=8.2 Hz, 1H), 7.15(t, J=8.2 Hz, 1H), 6.72(d, J=8.3 Hz, 1H), 4.36(h, J=6.1 Hz, 1H), 2.21(s, 3H), 1.74(ddt, J=15.8, 11.5, 5.7 Hz, 1H), 1.56(ddt, J=14.4, 10.8, 5.6 Hz, 1H), 1.52—1.36(m, 2H), 1.28(d, J=6.2 Hz, 3H), 0.93(t, J=7.5 Hz, 3H) | 159.47, 156.57, 145.30, 145.04, 144.80, 136.00, 126.65, 121.92, 120.10, 119.18, 114.71, 110.72, 74.42, 38.82, 19.88, 18.82, 14.17, 10.04 |
| 16 | 9.39(s, 1H), 8.82(d, J=2.5 Hz, 1H), 8.78(d, J=2.5 Hz, 1H), 7.69(d, J=7.8 Hz, 1H), 7.16(t, J=8.2 Hz, 1H), 6.73(d, J=7.8 Hz, 1H), 4.35(h, J=6.1 Hz, 1H), 2.22(d, J=4.7 Hz, 3H), 1.80—1.63(m, 2H), 1.59(ddq, J=14.6, 10.5, 5.4 Hz, 1H), 1.43(tq, J=12.1, 6.6, 6.2 Hz, 1H), 1.40—1.32(m, 2H), 1.34—1.23(m, 3H), 0.91(dt, J=18.6, 7.0 Hz, 3H) | 159.42, 156.56, 145.33, 145.03, 144.74, 136.00, 126.67, 119.10, 114.68, 110.72, 74.70, 36.35, 27.81, 22.78, 19.92, 18.72, 14.27, 10.06, 9.58 |
Table 2 1H NMR and 13C NMR data of compounds 1—16
| Compd. | 1H NMR(600 MHz, CDCl3), δ | 13C NMR(151 MHz, CDCl3), δ |
|---|---|---|
| 1 | 9.87(s, 1H), 8.56(d, J=2.5 Hz, 1H), 8.35(d, J=2.5 Hz, 1H), 7.72(d, J=8.0 Hz, 1H), 7.14(t, J=8.2 Hz, 1H), 6.64(d, J=8.2 Hz, 1H), 3.77(s, 3H), 3.00(s, 3H), 2.18(s, 3H) | 162.22, 157.82, 156.04, 146.17, 142.64, 139.97, 136.52, 126.66, 117.36, 114.57, 107.13, 55.72, 24.05, 9.79 |
| 2 | 9.90(s, 1H), 8.63(d, J=2.5 Hz, 1H), 8.42(d, J=2.2 Hz, 1H), 7.74(d, J=8.1 Hz, 1H), 7.17(t, J=8.3 Hz, 1H), 6.69(d, J=8.3 Hz, 1H), 4.03(q, J=6.9 Hz, 2H), 3.04(s, 3H), 2.23(s, 3H), 1.82(s, 1H), 1.41(t, J=7.0 Hz, 3H) | 162.29, 157.28, 156.17, 146.17, 142.80, 140.00, 136.52, 126.65, 117.77, 114.57, 108.43, 64.10, 24.08, 15.04, 9.89 |
| 3 | 9.86(s, 1H), 8.56(d, J=2.5 Hz, 1H), 8.35(d, J=2.5 Hz, 1H), 7.71(d, J=8.2 Hz, 1H), 7.12(t, J=8.2 Hz, 1H), 6.67(d, J=8.2 Hz, 1H), 4.47(hept, J=6.1 Hz, 1H), 3.00(s, 3H), 2.18(s, 3H), 1.29(d, J=6.2 Hz, 6H) | 162.18, 156.26, 156.03, 146.15, 142.70, 139.97, 136.73, 126.51, 118.80, 114.48, 110.50, 70.85, 24.06, 22.32, 10.09 |
| 4 | 9.87(s, 1H), 8.57(d, J=2.6 Hz, 1H), 8.36(d, J=2.4 Hz, 1H), 7.70(d, J=8.2 Hz, 1H), 7.12(t, J=8.1 Hz, 1H), 6.67(d, J=8.3 Hz, 1H), 4.26(h, J=6.1 Hz, 1H), 3.01(s, 3H), 2.20(s, 3H), 1.72(dp, J=14.3, 7.2 Hz, 1H), 1.61(dq, J=13.8, 7.0 Hz, 1H), 1.25(d, J=6.2 Hz, 3H), 0.95(t, J=7.6 Hz, 3H) | 162.20, 156.47, 156.05, 146.15, 142.73, 139.98, 136.70, 126.51, 118.72, 114.37, 110.18, 75.69, 29.36, 24.06, 19.44, 10.06, 9.85 |
| 5 | 9.89(s, 1H), 8.61(d, J=2.6 Hz, 1H), 8.40(d, J=2.5 Hz, 1H), 7.72(d, J=8.1 Hz, 1H), 7.15(t, J=8.2 Hz, 1H), 6.70(d, J=8.3 Hz, 1H), 4.35(h, J=6.1 Hz, 1H), 3.03(s, 3H), 2.21(s, 3H), 1.78—1.68(m, 1H), 1.59—1.50(m, 1H), 1.52—1.43(m, 1H), 1.45—1.35(m, 1H), 1.27(d, J=6.1 Hz, 3H), 0.92(t, J=7.3 Hz, 3H) | 162.24, 156.52, 156.13, 146.16, 142.79, 139.99, 136.69, 126.54, 118.78, 114.40, 110.18, 74.36, 38.86, 24.09, 19.96, 18.85, 14.21, 10.10 |
| 6 | 9.89(s, 1H), 8.61(d, J=2.6 Hz, 1H), 8.40(d, J=2.5 Hz, 1H), 7.72(d, J=8.5 Hz, 1H), 7.15(t, J=8.2 Hz, 1H), 6.70(d, J=8.3 Hz, 1H), 4.35(h, J=6.1 Hz, 1H), 3.04(s, 3H), 2.21(s, 3H), 1.78—1.69(m, 1H), 1.59—1.35(m, 3H), 1.27(d, J=6.1 Hz, 3H), 0.92(t, J=7.4 Hz, 3H) | 162.24, 156.52, 156.13, 146.16, 142.79, 139.99, 136.69, 126.54, 118.79, 114.41, 110.18, 74.37, 38.86, 24.09, 19.96, 18.85, 14.21, 10.11 |
| 7 | 9.88(s, 1H), 8.58(s, 1H), 8.37(t, J=3.2 Hz, 1H), 7.71(d, J=7.7 Hz, 1H), 7.13(t, J=8.2 Hz, 1H), 6.68(d, J=8.3 Hz, 1H), 4.34(q, J=6.2 Hz, 1H), 3.02(s, 3H), 2.20(s, 3H), 1.72(ddd, J=16.2, 8.7, 5.0 Hz, 1H), 1.53(dq, J=14.5, 5.3 Hz, 1H), 1.45(t, J=6.5 Hz, 1H), 1.43—1.35(m, 1H), 1.26(d, J=6.1 Hz, 3H), 0.91(t, J=7.2 Hz, 3H) | 162.21, 156.50, 156.07, 146.14, 142.75, 139.98, 136.70, 126.52, 118.73, 114.37, 110.14, 74.33, 38.85, 24.06, 19.94, 18.84, 14.20, 10.09 |
| 8 | 9.90(s, 1H), 8.64(d, J=2.4 Hz, 1H), 8.43(d, J=2.4 Hz, 1H), 7.72(t, J=8.8 Hz, 1H), 7.17(t, J=8.2 Hz, 1H), 6.71(d, J=8.3 Hz, 1H), 4.35(h, J=6.1 Hz, 1H), 3.05(s, 3H), 2.23(d, J=3.2 Hz, 3H), 1.80—1.63(m, 2H), 1.58(ddt, J=14.7, 10.8, 5.2 Hz, 1H), 1.44(ddd, J=16.4, 8.7, 4.2 Hz, 1H), 1.41—1.31(m, 2H), 1.28(d, J=6.1 Hz, 3H), 0.91(dt, J=18.3, 7.4 Hz, 3H) | 162.26, 156.54, 156.20, 146.17, 142.85, 139.99, 136.68, 126.53, 118.83, 114.43, 110.23, 74.67, 36.40, 24.11, 19.99, 18.74, 14.29, 10.12, 9.63 |
| 9 | 9.67(s, 1H), 9.43(s, 1H), 8.69(d, J=2.6 Hz, 1H), 8.50(d, J=2.4 Hz, 1H), 7.73(d, J=8.1 Hz, 1H), 7.11(t, J=8.2 Hz, 1H), 6.66(d, J=8.3 Hz, 1H), 4.32(h, J=6.1 Hz, 1H), 2.19(s, 3H), 1.74—1.65(m, 1H), 1.52(ddd, J=14.3, 10.6, 5.5 Hz, 1H), 1.48—1.32(m, 2H), 1.24(d, J=6.2 Hz, 3H), 0.89(t, J=7.2 Hz, 3H) | 160.63, 156.48, 147.52, 144.75, 144.62, 142.55, 136.31, 126.62, 118.55, 114.29, 110.28, 74.33, 38.82, 19.91, 18.82, 14.19, 9.97 |
| 10 | 9.69(s, 1H), 9.46(s, 1H), 8.74(d, J=2.6 Hz, 1H), 8.54(s, 1H), 7.76(t, J=7.6 Hz, 1H), 7.15(t, J=8.2 Hz, 1H), 6.69(d, J=8.0 Hz, 1H), 4.33(h, J=6.0 Hz, 1H), 2.23(d, J=4.4 Hz, 3H), 1.78—1.61(m, 2H), 1.56(ddt, J=14.6, 10.4, 5.0 Hz, 1H), 1.42(td, J=11.0, 10.5, 5.5 Hz, 1H), 1.40—1.24(m, 5H), 0.88(t, J=6.9 Hz, 3H) | 160.66, 156.50, 147.54, 144.81, 144.70, 142.55, 136.30, 126.66, 118.57, 114.31, 110.34, 74.65, 36.36, 19.96, 18.72, 14.30, 9.99, 9.61 |
| 11 | 9.64(s, 1H), 9.34(s, 1H), 8.41(s, 1H), 7.78(d, J=8.0 Hz, 1H), 7.16(t, J=8.2 Hz, 1H), 6.70(d, J=8.3 Hz, 1H), 4.35(h, J=6.1 Hz, 1H), 2.64(s, 3H), 2.22(s, 3H), 1.78—1.69(m, 1H), 1.59—1.51(m, 1H), 1.51—1.35(m, 2H), 1.27(d, J=6.1 Hz, 3H), 0.92(t, J=7.3 Hz, 3H) | 161.01, 157.41, 156.50, 143.66, 142.32, 142.14, 136.45, 126.64, 118.46, 114.27, 110.22, 74.40, 38.85, 21.93, 19.95, 18.85, 14.20, 9.97 |
| 12 | 9.63(s, 1H), 9.32(s, 1H), 8.39(s, 1H), 7.76(t, J=7.7 Hz, 1H), 7.14(t, J=8.2 Hz, 1H), 6.68(d, J=7.9 Hz, 1H), 4.33(h, J=6.0 Hz, 1H), 2.62(s, 3H), 2.21(s, 2H), 1.78—1.61(m, 2H), 1.56(dp, J=15.0, 5.1 Hz, 1H), 1.42(td, J=11.3, 10.3, 5.9 Hz, 1H), 1.40—1.28(m, 3H), 1.26(d, J=6.0 Hz, 3H), 0.88(q, J=6.9, 5.9 Hz, 3H) | 160.98, 157.40, 156.48, 143.63, 142.31, 142.12, 136.46, 126.62, 118.42, 114.24, 110.20, 74.64, 36.37, 21.90, 19.96, 18.72, 14.29, 9.97 |
| Compd. | 1H NMR(600 MHz, CDCl3), δ | 13C NMR(151 MHz, CDCl3), δ |
| 13 | 9.73(s, 1H), 9.23(s, 1H), 8.58(s, 1H), 7.74(d, J=8.2 Hz, 1H), 7.12(t, J=8.2 Hz, 1H), 6.67(d, J=8.2 Hz, 1H), 4.33(h, J=6.1 Hz, 1H), 2.59(s, 3H), 2.20(s, 3H), 1.75—1.64(m, 1H), 1.57—1.49(m, 1H), 1.49—1.33(m, 2H), 1.25(d, J=6.1 Hz, 3H), 0.90(t, J=7.5 Hz, 3H) | 160.84, 156.49, 152.09, 147.48, 143.62, 141.37, 136.41, 126.60, 118.51, 114.23, 110.25, 74.38, 38.83, 21.54, 19.93, 18.82, 14.19, 9.91 |
| 14 | 9.74(s, 1H), 9.23(s, 1H), 8.59(s, 1H), 7.74(t, J=7.9 Hz, 1H), 7.12(t, J=8.2 Hz, 1H), 6.67(d, J=8.3 Hz, 1H), 4.31(h, J=6.1 Hz, 1H), 2.60(s, 3H), 2.21(d, J=5.6 Hz, 3H), 1.77—1.60(m, 2H), 1.56(ddt, J=14.6, 10.4, 5.1 Hz, 1H), 1.45—1.36(m, 1H), 1.38—1.24(m, 5H), 0.91—0.84(m, 3H) | 160.85, 156.49, 152.09, 147.48, 143.63, 141.38, 136.41, 126.61, 118.52, 114.23, 110.26, 74.64, 36.36, 21.55, 19.95, 18.71, 14.29, 9.92, 9.59 |
| 15 | 9.38(s, 1H), 8.79(s, 1H), 8.76(s, 1H), 7.67(d, J=8.2 Hz, 1H), 7.15(t, J=8.2 Hz, 1H), 6.72(d, J=8.3 Hz, 1H), 4.36(h, J=6.1 Hz, 1H), 2.21(s, 3H), 1.74(ddt, J=15.8, 11.5, 5.7 Hz, 1H), 1.56(ddt, J=14.4, 10.8, 5.6 Hz, 1H), 1.52—1.36(m, 2H), 1.28(d, J=6.2 Hz, 3H), 0.93(t, J=7.5 Hz, 3H) | 159.47, 156.57, 145.30, 145.04, 144.80, 136.00, 126.65, 121.92, 120.10, 119.18, 114.71, 110.72, 74.42, 38.82, 19.88, 18.82, 14.17, 10.04 |
| 16 | 9.39(s, 1H), 8.82(d, J=2.5 Hz, 1H), 8.78(d, J=2.5 Hz, 1H), 7.69(d, J=7.8 Hz, 1H), 7.16(t, J=8.2 Hz, 1H), 6.73(d, J=7.8 Hz, 1H), 4.35(h, J=6.1 Hz, 1H), 2.22(d, J=4.7 Hz, 3H), 1.80—1.63(m, 2H), 1.59(ddq, J=14.6, 10.5, 5.4 Hz, 1H), 1.43(tq, J=12.1, 6.6, 6.2 Hz, 1H), 1.40—1.32(m, 2H), 1.34—1.23(m, 3H), 0.91(dt, J=18.6, 7.0 Hz, 3H) | 159.42, 156.56, 145.33, 145.03, 144.74, 136.00, 126.67, 119.10, 114.68, 110.72, 74.70, 36.35, 27.81, 22.78, 19.92, 18.72, 14.27, 10.06, 9.58 |
| Compd. | Antifungal effect(%) | |||||
|---|---|---|---|---|---|---|
| 400 mg/L | 100 mg/L | 25 mg/L | 6.25 mg/L | 1.56 mg/L | 0.04 mg/L | |
| 1 | 100 | 100 | 100 | 50 | — | — |
| 2 | 100 | 100 | 100 | 40 | — | — |
| 3 | 100 | 100 | 80 | 60 | — | — |
| 4 | 100 | 100 | 100 | 100 | 70 | — |
| 5 | 100 | 100 | 100 | 100 | 50 | — |
| 6 | 100 | 100 | 100 | 90 | 20 | — |
| Compd. | Antifungal effect(%) | |||||
| 400 mg/L | 100 mg/L | 25 mg/L | 6.25 mg/L | 1.56 mg/L | 0.04 mg/L | |
| 7 | 100 | 100 | 100 | 100 | 50 | — |
| 8 | 100 | 100 | 100 | 100 | 70 | — |
| 9 | 70 | — | — | — | — | — |
| 10 | 40 | — | — | — | — | — |
| 11 | 100 | 0 | 0 | 0 | — | — |
| 12 | 0 | — | — | — | — | — |
| 13 | 100 | 10 | 0 | 0 | — | — |
| 14 | 0 | — | — | — | — | — |
| 15 | 100 | 100 | 100 | 100 | 90 | 50 |
| 16 | 100 | 100 | 100 | 100 | 100 | 70 |
| Pyraziflumid | 100 | 100 | 100 | 95 | 80 | 20 |
Table 3 In vivo antifungal effects(%) against corn rust(Puccinia sorghi Schw.) of all target compounds*
| Compd. | Antifungal effect(%) | |||||
|---|---|---|---|---|---|---|
| 400 mg/L | 100 mg/L | 25 mg/L | 6.25 mg/L | 1.56 mg/L | 0.04 mg/L | |
| 1 | 100 | 100 | 100 | 50 | — | — |
| 2 | 100 | 100 | 100 | 40 | — | — |
| 3 | 100 | 100 | 80 | 60 | — | — |
| 4 | 100 | 100 | 100 | 100 | 70 | — |
| 5 | 100 | 100 | 100 | 100 | 50 | — |
| 6 | 100 | 100 | 100 | 90 | 20 | — |
| Compd. | Antifungal effect(%) | |||||
| 400 mg/L | 100 mg/L | 25 mg/L | 6.25 mg/L | 1.56 mg/L | 0.04 mg/L | |
| 7 | 100 | 100 | 100 | 100 | 50 | — |
| 8 | 100 | 100 | 100 | 100 | 70 | — |
| 9 | 70 | — | — | — | — | — |
| 10 | 40 | — | — | — | — | — |
| 11 | 100 | 0 | 0 | 0 | — | — |
| 12 | 0 | — | — | — | — | — |
| 13 | 100 | 10 | 0 | 0 | — | — |
| 14 | 0 | — | — | — | — | — |
| 15 | 100 | 100 | 100 | 100 | 90 | 50 |
| 16 | 100 | 100 | 100 | 100 | 100 | 70 |
| Pyraziflumid | 100 | 100 | 100 | 95 | 80 | 20 |
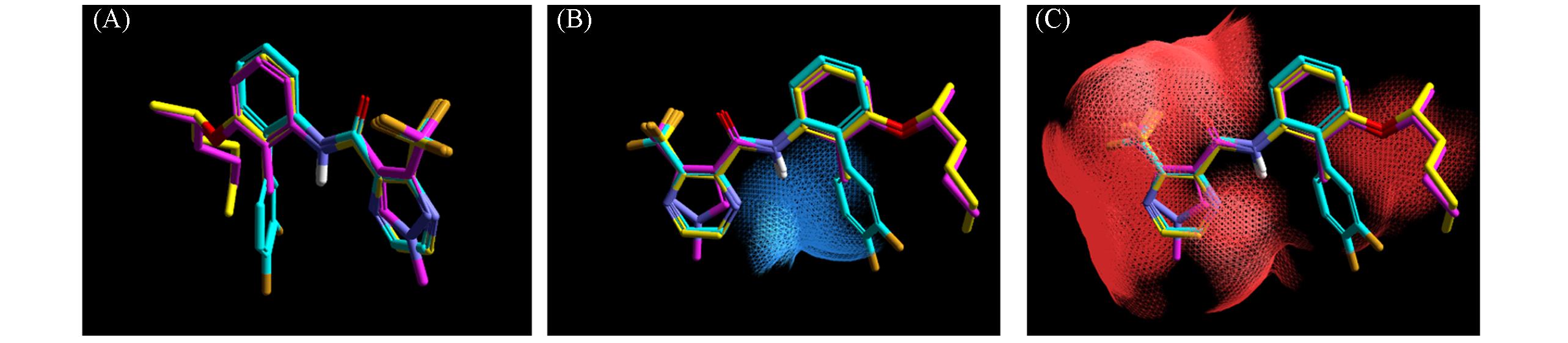
Fig.1 The molecular 3D space superimposition diagram(A), hydrogen bond donor diagram(B) and hydrogen bond acceptor diagram(C) of pyraziflumid, wu jun xian an and compound 16
| 1 | Strange R. N., Scott P. R., Annu. Rev. Phytopathol, 2005, 43, 83—116 |
| 2 | Fisher M. C., Hawkins N. J., Sanglard D., Gurr S. J., Science, 2018, 360(6390), 739—742 |
| 3 | Brown G. D., Denning D. W., Gow N. A. R., Levitz S. M., Netea M. G., White T. C., Sci. Transl. Med., 2012, 4(165), 165rv13 |
| 4 | Xiong Z. Q., Tu X. R., Wei S. J., Huang L., Li X. H., Lu H., Tu G. Q., Biotechnol. Lett., 2013, 35(9), 1475—1479 |
| 5 | Patriarca A., Curr. Opin. Food Sci., 2019, 29, 42—47 |
| 6 | Wang Y. J., Subedi S., de Vries H., Doornenbal P., Vels A., Hensel G., Kumlehn J., Johnston P. A., Qi X. Q., Blilou I., Niks R. E., Krattinger S. G., Nat. Plants, 2019, 5(11), 1129—1135 |
| 7 | Wang W., Wang J. H., Wu F. R., Zhou H., Xu D., Xu G., J. Agric. Food Chem., 2021, 69(20), 5746—5754 |
| 8 | Yang H. B., Yu H. B., Cong Y. B., Shi X. G., Hu Z. N., Wang J. F., Song Y. Q., Li B., Advanced Agrochem, 2023, 2(4), 371—375 |
| 9 | Yu H. B., Yang H. B., Chen L., Wang G., Zhao G. M., Wang X. L., Wu H. F., Shi X. G., Dong Y., Li B., Org. Process Res. Dev., 2022, 26(12), 3216—3225 |
| 10 | Yu H. B., Cheng Y., Xu M., Song Y. Q., Luo Y. M., Li B., J. Agric. Food Chem., 2016, 64(51), 9586—9591 |
| 11 | Brauer V. S., Rezende C. P., Pessoni A. M., De Paula R. G., Rangappa K. S., Nayaka S. C., Gupta V. K., Almeida F., Biomolecules, 2019, 9(10), 521 |
| 12 | Wang H., Ren S. X., He Z. Y., Wang D. L., Yan X. N., Feng J. T., Zhang X., Int. J. Mol. Sci., 2014, 15(3), 4257—4272 |
| 13 | Ninomiya A., Urayama S. I., Suo R., Itoi S., Fuji S., Moriyama H., Hagiwara D., Front. Microbiol., 2020, 11, 1641 |
| 14 | Ishii H., Zhen F., Hu M. J., Li X. P., Schnabel G., Pest Manag. Sci., 2016, 72(10), 1844—1853 |
| 15 | Umetsu N., Shirai Y, J. Pestic. Sci., 2020, 45(1/2), 54—74 |
| 16 | Wang G., Lv L., Liu J. Y., Shan Z. G., Yang H. B., Li B., Modern Agrochemicals, 2017, 16(4), 12—14 |
| 王刚, 吕亮, 刘吉永, 单中刚, 杨辉斌, 李斌. 现代农药, 2017, 16(4), 12—14 | |
| 17 | Wang G., Hao Z. S., Yang H. B., Shan Z. G., Chen X. M., Lv L., Li B., Modern Agrochemicals, 2017, 16(5), 7—9 |
| 王刚, 郝泽生, 杨辉斌, 单忠刚, 陈宣明, 吕亮, 李斌. 现代农药, 2017, 16(5), 7—9 | |
| 18 | Oporto C. I., Villarroel C. A., Tapia S. M., Garcı´a. V., Cubillos. F. A., Toxins, 2019, 11(7), 400 |
| 19 | Wang Q. Q., Zhang S. G., Jiao J., Dai P., Zhang W. H., Molecules, 2021, 26(2), 372 |
| 20 | Türkyilmaz M., Dönmez M., Altun Ö., Chem. Res. Chinese Universities, 2023, 39(6), 968—975 |
| 21 | Li H., Wang Y. X., Zhu X. L., Yang G. F., J. Agric. Food Chem., 2021, 69(44), 13227—13234 |
| 22 | Xie Q. Y., Zhang S., Zhang Y. H., Zhang B. W., Wan F. X., Li Y., Jiang L., Bioorg. Med. Chem. Lett., 2024, 108, 129813 |
| 23 | Wang X. B., Wang M. Q., Han L., Jin F., Jiao J., Chen M., Yang C. L., Xue W., J. Agric. Food Chem., 2021, 69(33), 9557—9570 |
| 24 | Zhang A. G., Yue Y., Yang J., Shi J. X., Tao K., Jin H., Hou T. P., J. Agric. Food Chem., 2019, 67(17), 5008—5016 |
| 25 | Huang Y. H., Wei G., Wang W. J., Liu Z., Yin M. X., Guo W. M., Zhu X. L., Yang G. F., J. Agric. Food Chem., 2023, 71(47), 18292—18300 |
| 26 | Huang Y. H., Wei G., Liu Z., Lu Q., Jiang J. J., Zhu X. L., Yang G. F., J. Agric. Food Chem., 2022, 70(45), 14480—14487 |
| 27 | Li H., Gao M. Q., Chen Y., Wang Y. X., Zhu X. L., Yang G. F., J. Agric. Food Chem., 2020, 68(47), 14001—14008 |
| 28 | Xiong L., Li H., Jiang L. N., Ge J. M., Yang W. C., Zhu X. L., Yang G. F., J. Agric. Food Chem., 2017, 65(5), 1021—1029 |
| 29 | Xiong L., Zhu X. L., Gao H. W., Fu Y., Hu S. Q., Jiang L. N., Yang W. C., Yang G. F., J. Agric. Food Chem., 2016, 64(24), 4830—4837 |
| 30 | Wei G., Huang M. W., Wang W. J., Wu Y., Mei S. F., Zhou L. M., Mei L. C., Zhu X. L., Yang G. F., J. Agric. Food Chem., 2021, 69(13), 3965—3971 |
| 31 | Zhao Y. T., Zhang A. G., Wang X. G., Tao K., Jin H., Hou T. P., J. Agric. Food Chem., 2022, 70(42), 13464—13472 |
| 32 | Fu W., Shao Z. L., Sun X. J., Zhou C., Xu Z. P., Zhang Y., Cheng J. G., Li Z., Shao X. S., J. Agric. Food Chem., 2022, 70(14), 4279—4290 |
| 33 | Zhao T., Zhang J., Tao Y. C., Liao H., Zhao F. B., Liang R. P., Shi X. Y., Zhang Z. J., Ji J. B., Wu T., Pang J. X., Liu X. Y., Zhan P., J. Med. Chem., 2022, 65(5), 4218—4237 |
| 34 | Ding M. H., Wan S. R., Wu N., Yan Y., Li J. H., Bao X. P., J. Agric. Food Chem., 2021, 69(50), 15084—15096 |
| 35 | Wang C. F., Li J., Qu L. Z., Tang X., Song X. J., Yang F., Chen X. J., Lin Q. M., Lin W. B., Zhou Y., Tu Z. C., Chen Y. H., Zhang Z., Lu X. Y., J. Med. Chem., 2022, 65(22), 15140—15164 |
| 36 | Sardar A., Ansari A., Gupta S., Sinha S., Pandey S., Rai D., Kumar M., Bhatta R. S., Trivedi R., Sashidhara K. V., Eur. J. Med. Chem., 2022, 244, 114813 |
| 37 | Kadagathur M., Patra S., Devabattula G., George J., Phanindranath R., Shaikh A. S., Sigalapalli D. K., Godugu C., Nagesh N., Tangellamudi N. D., Shankaraiah N., Eur. J. Med. Chem., 2022, 238, 114465 |
| 38 | Shi J., Ding M. H., Luo N., Wan S. R., Li P. J., Li J. H., Bao X. P., J. Agric. Food Chem., 2020, 68(36), 9613—9623 |
| 39 | Ivasiv V., Albertini C., Goncalves A. E., Rossi M., Bolognesi M. L., Curr. Top. Med. Chem., 2019, 19(19), 1694—1711 |
| 40 | Hu Y., Stumpfe D., Bajorath J., J. Med. Chem., 2017, 60(4), 1238—1246 |
| 41 | Barber D. M., J. Agric. Food Chem., 2022, 70(36), 11075—11090 |
| 42 | Callis T. B., Garrett T. R., Montgomery A. P., Danon J. J., Kassiou M., J. Med. Chem., 2022, 65(20), 13483—13504 |
| 43 | Cao X. F., Yang H. P., Liu C., Zhang R. F., Maienfisch P., Xu X. Y., J. Agric. Food Chem., 2022, 70(36), 11042—11055 |
| 44 | Lamberth C., J. Agric. Food Chem., 2022, 70(36), 11005—11010 |
| 45 | Lamberth C., J. Agric. Food Chem., 2022, 70(36), 11011—11018 |
| 46 | Maienfisch P., Lamberth C., J. Agric. Food Chem., 2022, 70(36), 10941 |
| 47 | Li P. Y., Feng L., Li G. Y., Bai F. Q., Chem. Res. Chinese Universities, 2023, 39(2), 202—207 |
| 48 | Luo B., Zhao Y. C., Zhang J., Li W., Liu M. X., Yang M. M., Wei L. L., Liu Y. J., Wen B. J., Qu L. L., J. Agric. Food Chem., 2023, 71(24), 9255—9265 |
| 49 | Lv L., Wang G., Wu S. S., Shan Z. G., Li B., Pesticide, 2020, 59(12), 871—872 |
| 吕亮, 王刚, 吴沙沙, 单中刚, 李斌. 农药, 2020, 59(12), 871—872 |
| [1] | 王璐. 离子液体辅助水热合成1T-MoS2及其锌离子储存性能[J]. 高等学校化学学报, 2024, 45(9): 20240145. |
| [2] | 许玲, 尹盼盼, 鲁显福, 李宜明. 连接-脱硫策略在蛋白质化学合成中的发展与应用[J]. 高等学校化学学报, 2024, 45(8): 20240196. |
| [3] | 刘豪, 刘冬梅, 孙浩田, 夏超, 苏贤斌. 利用脂溶性硅基载体连续流动液相合成维洛斯肽[J]. 高等学校化学学报, 2024, 45(7): 20240024. |
| [4] | 孔雪, 张海平, 夏文生, 张庆红, 万惠霖. 石墨烯负载单原子Mo上合成气催化转化机理: 碳组分和Mo-C作用的影响[J]. 高等学校化学学报, 2024, 45(7): 20240038. |
| [5] | 龙磊, 韦伟, 罗运军, 李霄羽. 高效叠氮化方法研究进展[J]. 高等学校化学学报, 2024, 45(5): 20230511. |
| [6] | 赵晓光, 王云龙, 尹海波, 曲亚坤, 苏海伟, 房韡. 不同氮源用于电催化合成氨的研究进展[J]. 高等学校化学学报, 2024, 45(3): 20230527. |
| [7] | 胡文馨, 赵莹, 杜丹阳, 张红丹, 程鹏. ZSM-5封装Pt-La双金属催化剂的制备及对异丁烷裂解反应的催化性能[J]. 高等学校化学学报, 2024, 45(10): 20240244. |
| [8] | 朱佳佳, 芮家亮, 石文文, 甘铠宁, 李宏强, 何孝军. 镍/氮共掺杂自支撑泡沫碳电极的构建及电催化CO2还原性能[J]. 高等学校化学学报, 2024, 45(10): 110. |
| [9] | 张兴红, 耿鹏, 向娟娟, 晏佳莹, 毛妙付, 肖述章. 一锅法合成可印刷室温磷光二氟化硼衍生物[J]. 高等学校化学学报, 2024, 45(1): 20230432. |
| [10] | 杨颖楠, 孙启明. EAB分子筛的合成及在甲醇制烯烃反应中的应用[J]. 高等学校化学学报, 2023, 44(8): 20230119. |
| [11] | 冯瑞沁, 方云, 樊晔, 夏咏梅. 金纳米花的简易合成及催化硼氢化钠还原对硝基酚性能[J]. 高等学校化学学报, 2023, 44(8): 20230027. |
| [12] | 杨威, 刘佳琦, 唐翠曼, 仉昊楠, 王兵兵, 孙钟, 徐小惠. 铜催化醇与氨基吡啶在有氧条件下合成酰胺[J]. 高等学校化学学报, 2023, 44(8): 20230029. |
| [13] | 任毅, 阚媛媛, 孙延娜, 李建丰, 高珂. 石墨炔在光伏领域的研究进展[J]. 高等学校化学学报, 2023, 44(7): 20220752. |
| [14] | 刘金露, 郭嘉禹, 华佳, 李光华, 施展, 冯守华. 一种酰胺基Cu-MOF的构筑与吸附性能研究[J]. 高等学校化学学报, 2023, 44(6): 20220746. |
| [15] | 刘高远, 刘跃含, 赵丽娜, 郭玉鹏. 天冬氨酸调控糖果状碳酸钙的仿生合成及性能[J]. 高等学校化学学报, 2023, 44(6): 20220769. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||