

高等学校化学学报 ›› 2024, Vol. 45 ›› Issue (8): 20240196.doi: 10.7503/cjcu20240196
• 综合评述 • 上一篇
收稿日期:2024-04-17
出版日期:2024-08-10
发布日期:2024-05-17
通讯作者:
鲁显福,李宜明
E-mail:luxianfu@ahmu.edu.cn;ymli@hfut.edu.cn
作者简介:第一联系人:共同第一作者.
基金资助:
XU Ling1, YIN Panpan1, LU Xianfu1( ), LI Yiming2(
), LI Yiming2( )
)
Received:2024-04-17
Online:2024-08-10
Published:2024-05-17
Contact:
LU Xianfu, LI Yiming
E-mail:luxianfu@ahmu.edu.cn;ymli@hfut.edu.cn
Supported by:摘要:
蛋白质化学合成是获取特定序列与结构蛋白质分子的关键技术, 为研究蛋白质的结构与功能提供了物质基础. 传统的固相多肽合成受限于分步氨基酸偶合和脱保护反应效率, 难以一次性合成分子量较大的蛋白质. 自然化学连接和酰肼连接技术作为无保护肽片段间连接的策略, 具有高效选择性, 极大地推动了蛋白质化学合成的发展. 然而, 这些连接策略需依托蛋白质中丰度较低的半胱氨酸, 难以适用于无半胱氨酸或者半胱氨酸不合适作为连接位点的蛋白质的合成. 连接-脱硫策略的提出首次将连接位点拓展至丙氨酸, 并促进了硫代氨基酸的发展, 使得蛋白化学合成不再受限于连接位点的选择. 在此基础上, 自由基脱硫、 光化学脱硫、 P-B脱硫和铁催化脱硫等新兴脱硫技术的进步为蛋白质化学合成提供了多样化的选择, 拓展了其应用范围. 连接-脱硫的化学方法不断地演进创新, 丰富了蛋白质化学合成方法库, 为蛋白质工程与化学生物学领域的深入研究提供了重要支持. 本综合评述以时间线的形式, 全面回顾了连接-脱硫化学方法在蛋白质化学合成中的发展历程. 从早期基于半胱氨酸位点的自然化学连接和酰肼连接技术, 到连接-脱硫策略的开创性发展, 再到硫代氨基酸和多样化脱硫策略的探索, 这些技术不仅丰富了多肽合成的策略, 也展示了它们在合成蛋白质中的广阔应用前景和发展潜力. 本文旨在为蛋白质化学合成领域的科研工作者提供深刻的见解和有价值的信息, 激发该领域的进一步探索与创新.
中图分类号:
TrendMD:
许玲, 尹盼盼, 鲁显福, 李宜明. 连接-脱硫策略在蛋白质化学合成中的发展与应用. 高等学校化学学报, 2024, 45(8): 20240196.
XU Ling, YIN Panpan, LU Xianfu, LI Yiming. Development and Applications of Ligation-Desulfurization Strategy in Protein Chemical Synthesis. Chem. J. Chinese Universities, 2024, 45(8): 20240196.
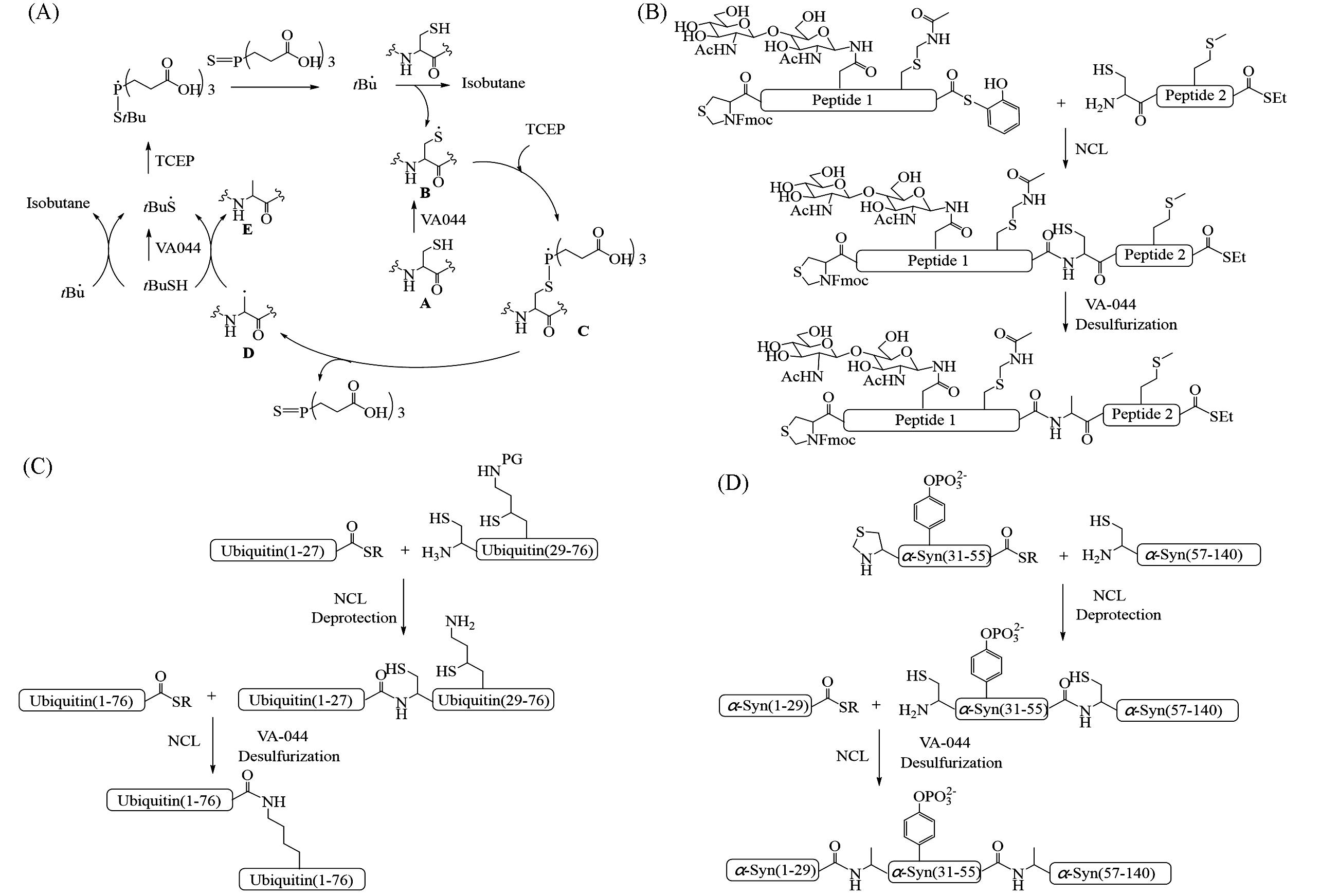
Fig.2 VA⁃044⁃based free radical desulfurization reaction mechanism(A), VA⁃044⁃based free radical desulfurization strategy used for the synthesis of peptides containing multiple functional groups(B), diubiquitin(C) and phosphorylation⁃modified α⁃synuclein(D)
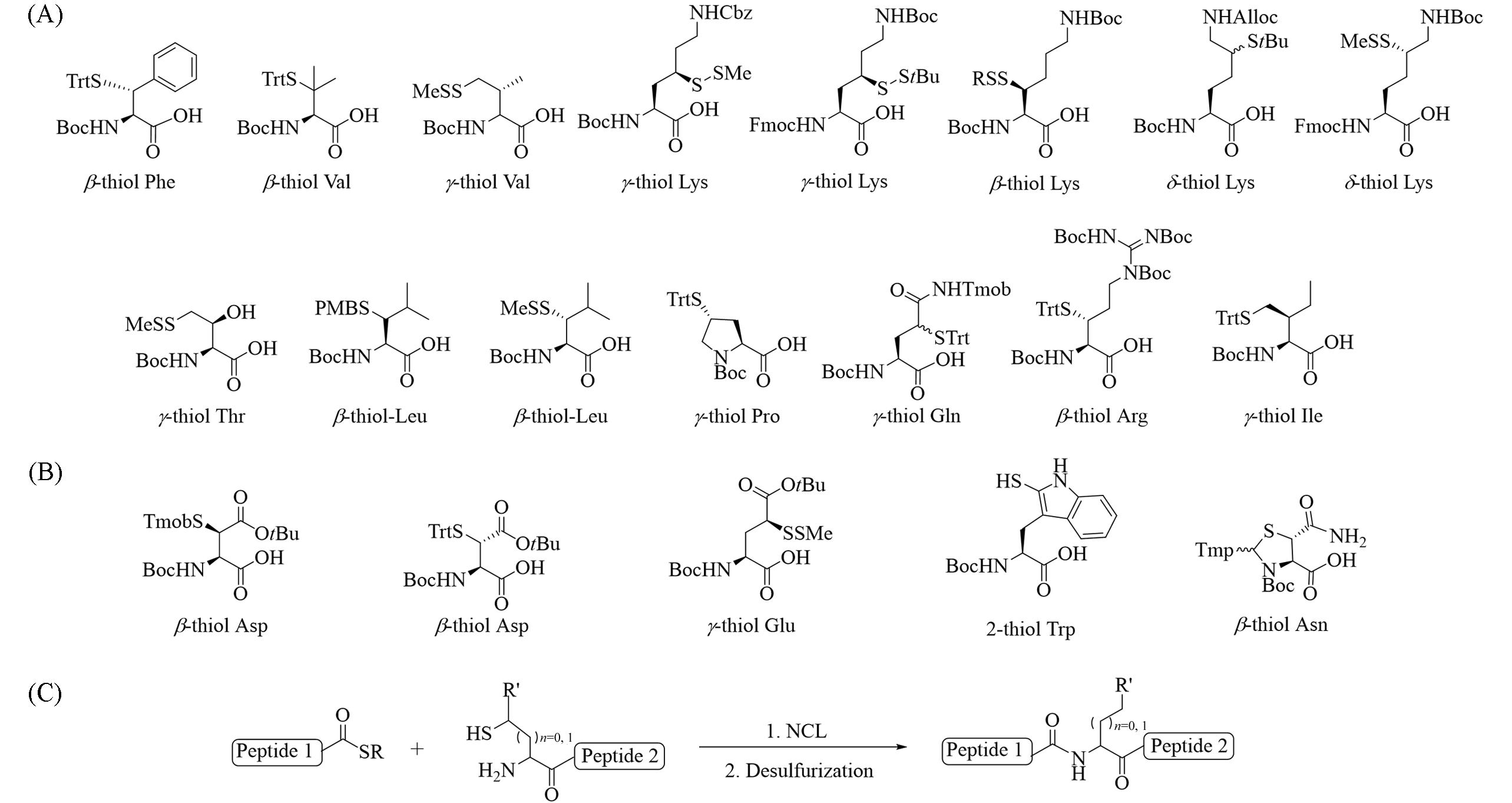
Fig.3 Thioamino acids synthesized by nucleophilic substitution strategy(A) and electrophilic substitution strategy(B), β⁃/γ⁃/δ⁃thioamino acids used for NCL⁃desulfurization chemistry(C)
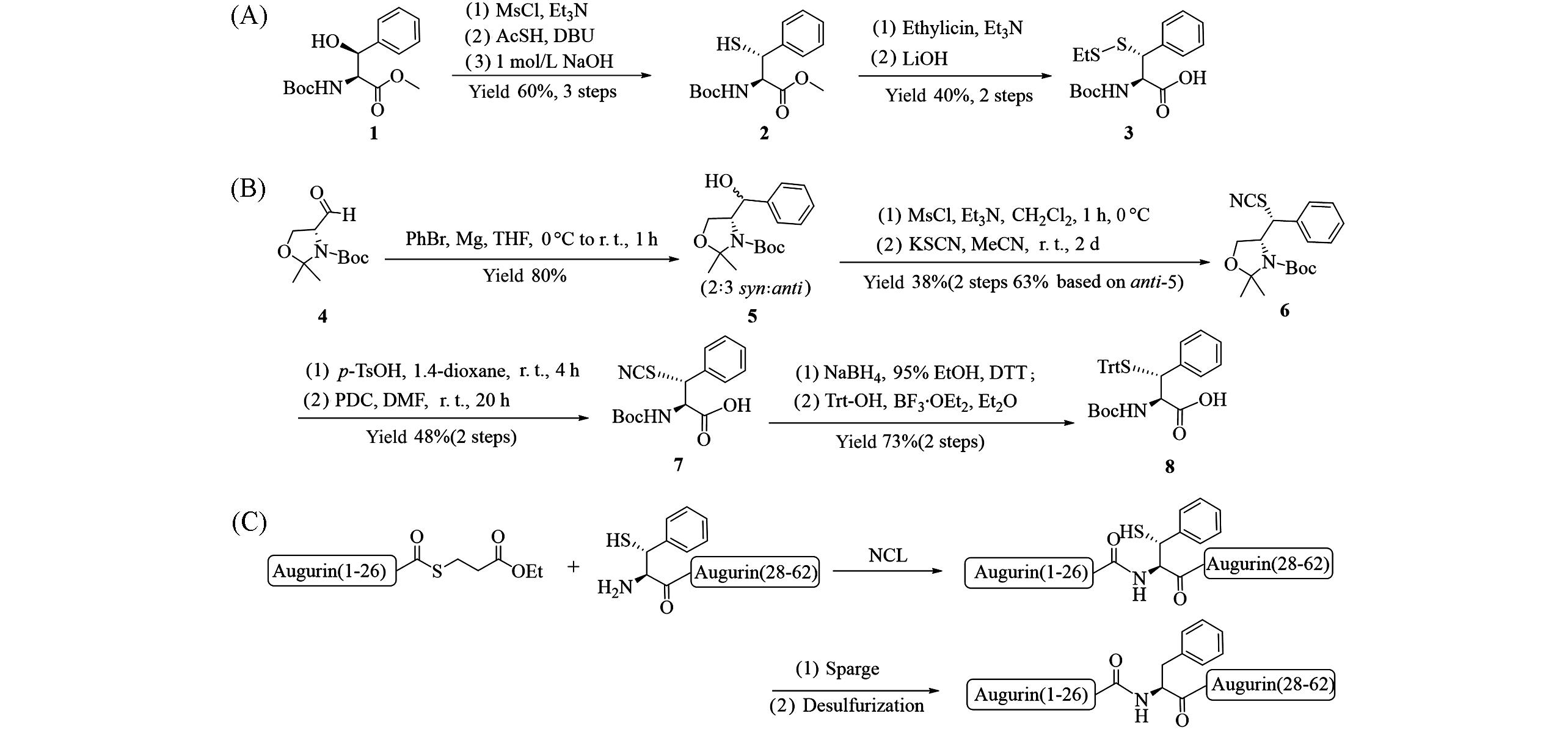
Fig.4 Synthesis of β⁃thiophenylalanine 3(A) and 8(B), β⁃thiophenylalanine used for one⁃pot ligation⁃desulfurization to synthesize of the hormone Augurin(C)
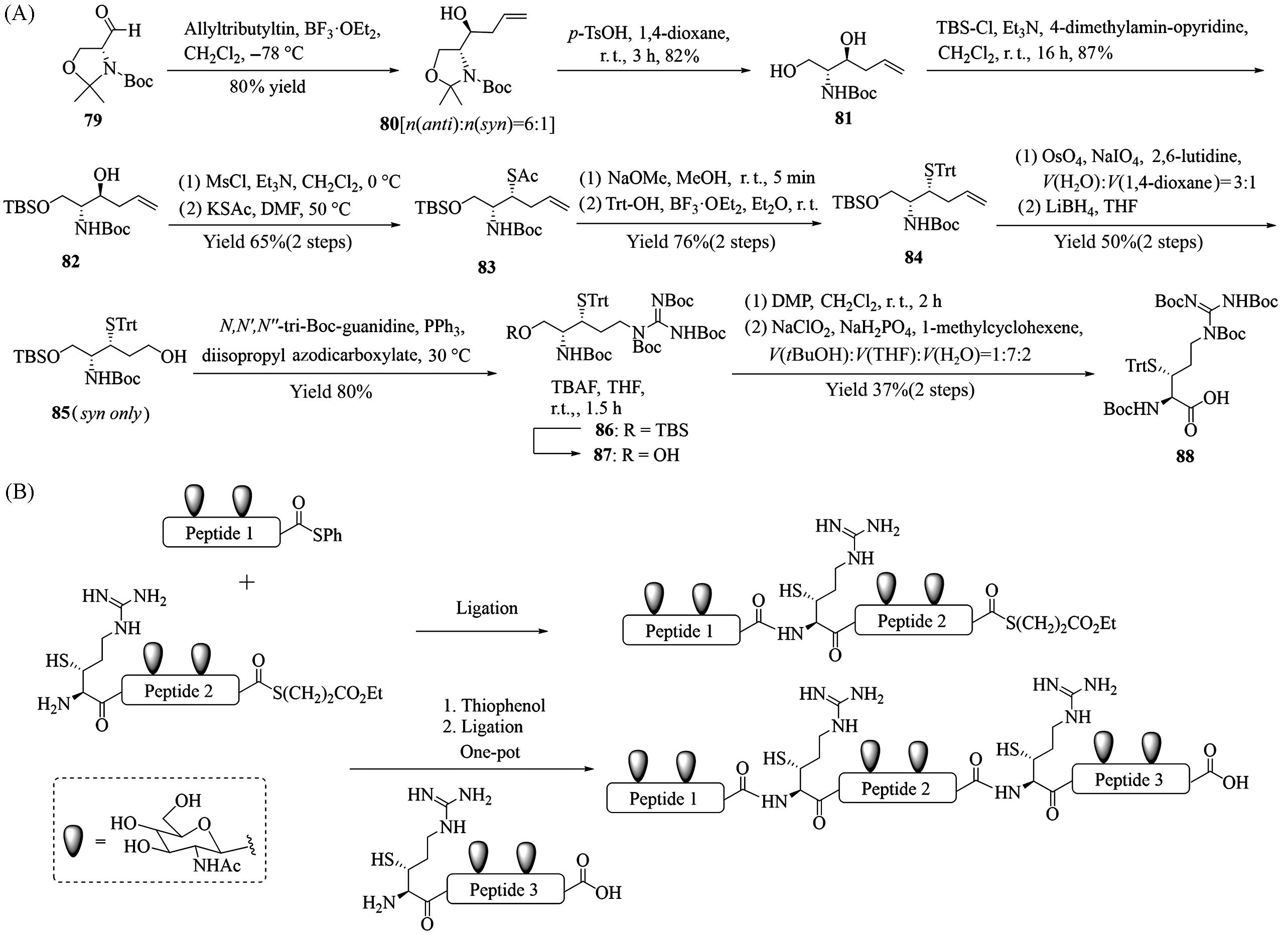
Fig.13 Synthesis of β⁃thioarginine(88, A), synthesis of MUC1 VNTR glycopeptides through a kinetically controlled ligation⁃desulfurization sequence using β⁃thioarginine(B)
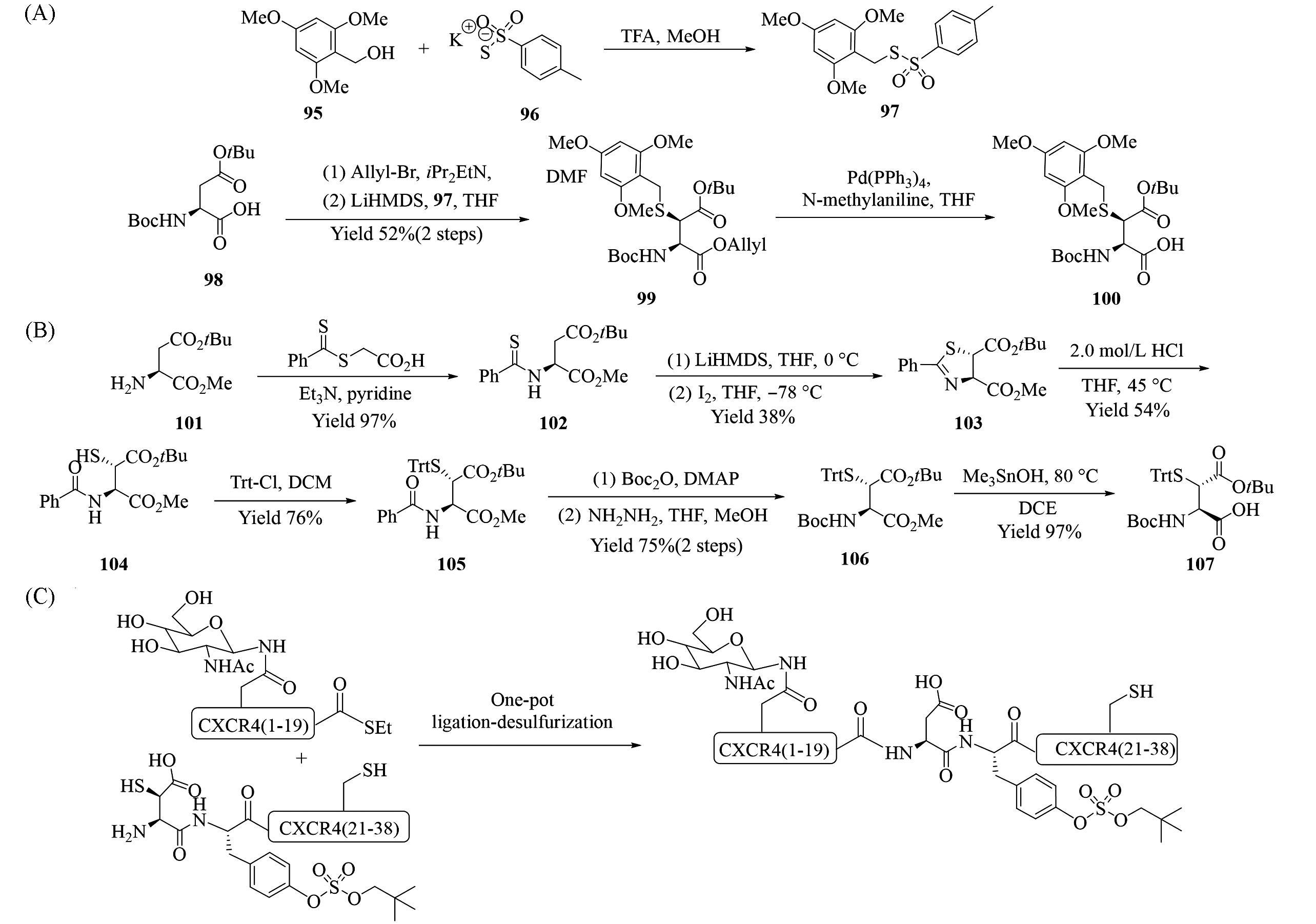
Fig.15 Synthesis of β⁃thioaspartic acid 100(A) and 107(B), β⁃thioaspartic acid used for one⁃pot protein ligation⁃ desulfurization to synthesize CXCR4 13(1-38)(C)
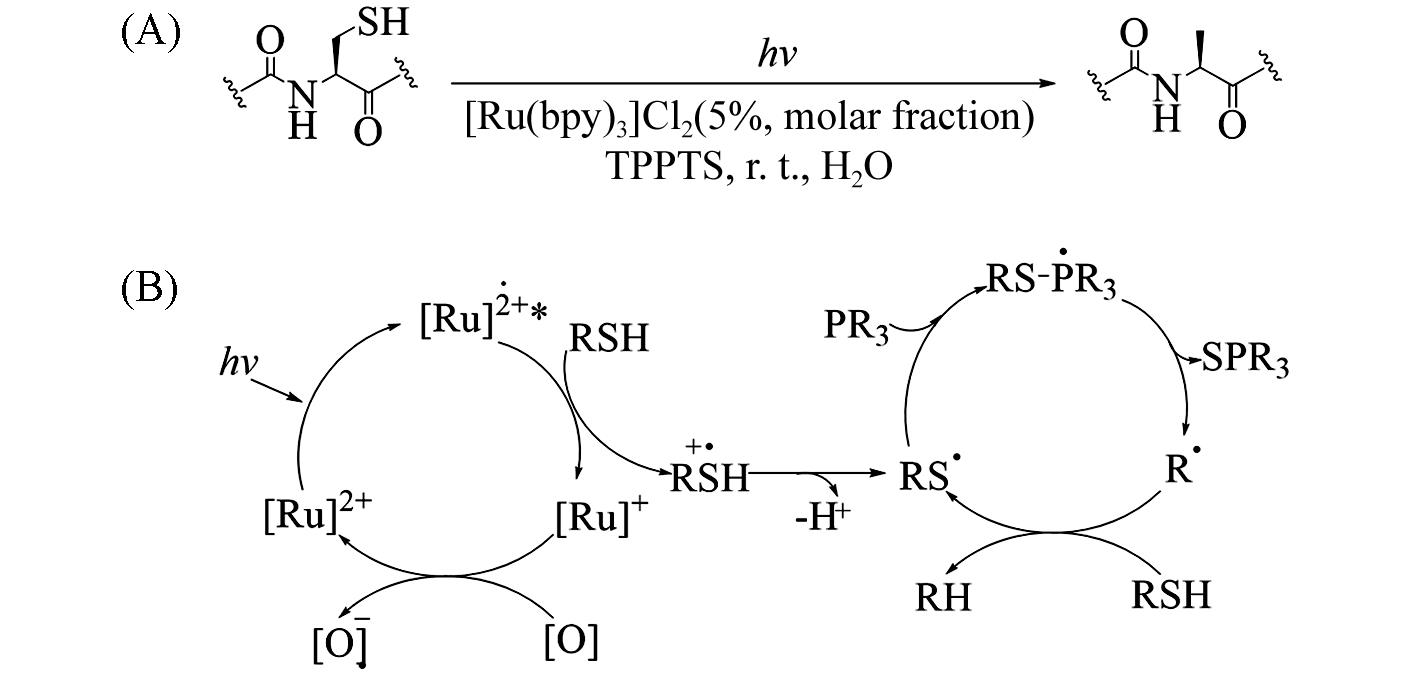
Fig.19 Visible⁃light⁃induced transformation of cysteinyl peptide to alanyl peptide(A) and proposed mechanism of [Ru(bpy)3]Cl2 catalyzed desulfurization(B)
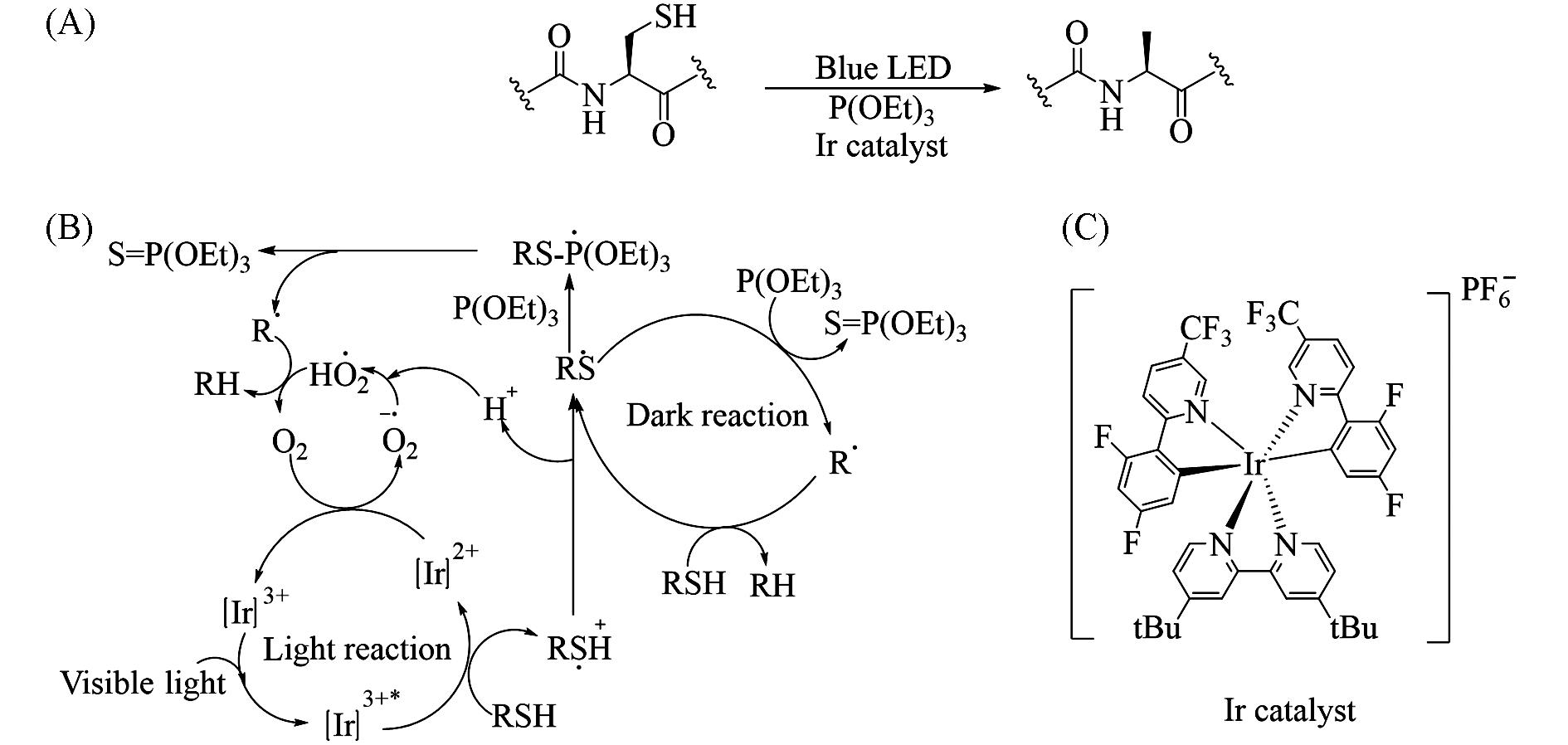
Fig.20 Visible⁃light⁃induced transformation of cysteinyl peptide to alanyl peptide(A) and proposed mechanism of [Ir(dF(CF3)ppy)2(dtbbpy)]PF6 catalyzed desulfurization(B) and structure of [Ir(dF(CF3)ppy)2(dtbbpy)]PF6(C)
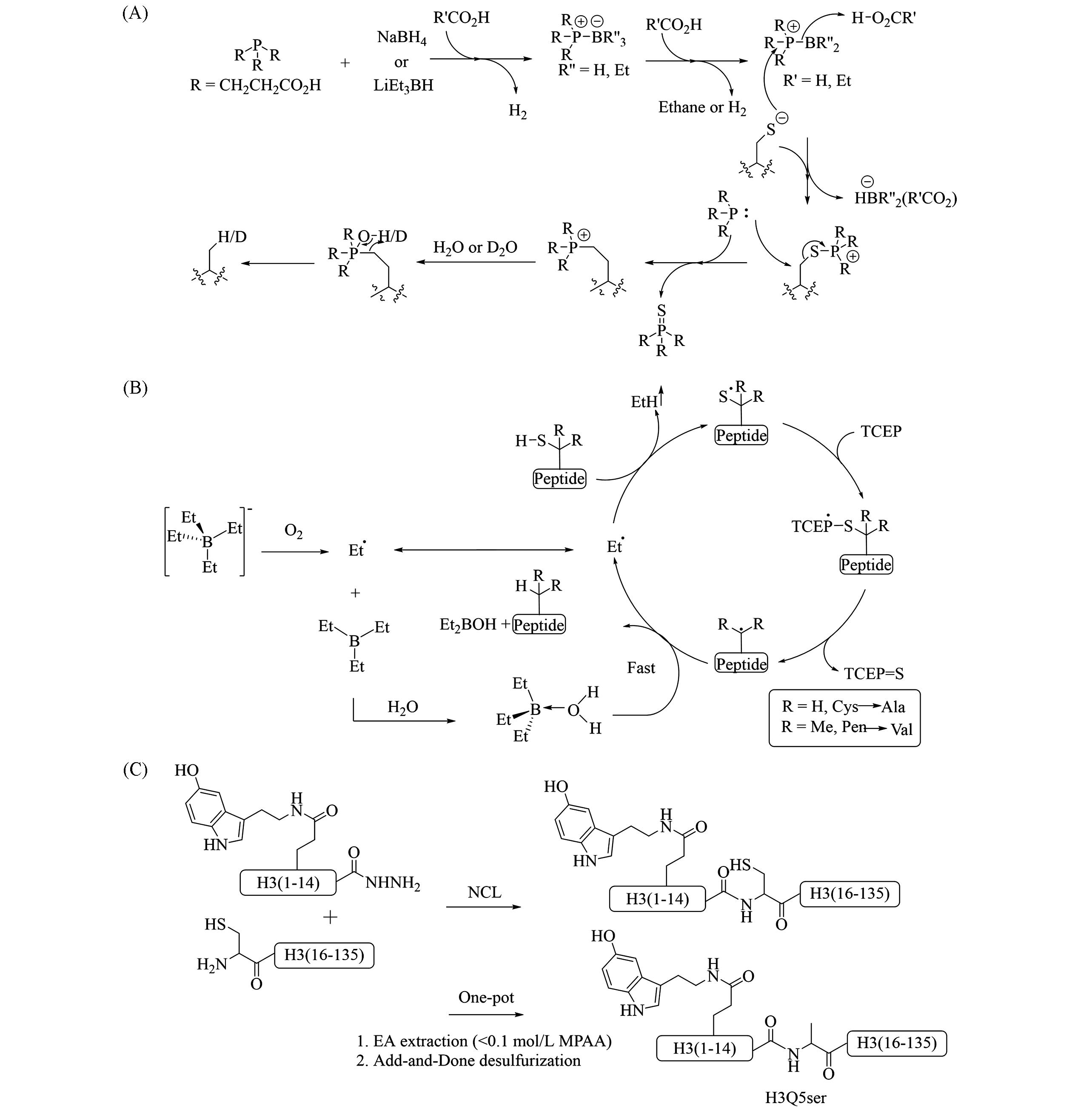
Fig.23 Proposed mechanism of NaBH4/LiBEt3H⁃mediated desulfurization(A) and NaBEt4⁃mediated desulfurization(B) and semisynthesis of H3Q5ser through one⁃pot EPL⁃ADD(C)
| 1 | Kent S. B. H., Chem. Soc. Rev., 2009, 38(2),338—351 |
| 2 | Dong S. W., Zheng J. S., Li Y. M., Wang H., Chen G., Chen Y. X., Fang G. M., Guo J., He C. M., Hu H. G., Li X. C., Li Y. M., Li Z. G., Pan M., Tang S., Tian C. L., Wang P., Wu B., Wu C. L., Zhao J. F., Liu L., Sci. China Chem., 2024, 67, 1060—1096 |
| 3 | Fischer E., Fourneau E., Ber. Dtsch. Chem. Ges., 1901, 34, 2868—2877 |
| 4 | Curtius T., Ber. Dtsch. Chem. Ges., 1904, 70(1),57—72 |
| 5 | Fischer E., Ber. Dtsch. Chem. Ges., 1905, 38(1),605 |
| 6 | Kent S., Sohma Y., Liu S. H., Bang D., Pentelute B., Mandal K., J. Pept. Sci., 2012, 18(7),428—436 |
| 7 | Merrifield R. B., J. Am. Chem. Soc., 1963, 85(14),2149—2154 |
| 8 | Merrifield R. B., Stewart J. M., Jernberg N., Anal. Chem., 1966, 38(13),1905—1914 |
| 9 | Merrifield R. B., Science, 1986, 232(4748),341—347 |
| 10 | Hartrampf N., Saebi A., Poskus M., Gates Z. P., Callahan A. J., Cowfer A. E., Hanna S., Antilla S., Schissel C. K., Quartararo A. J., Ye X., Mijalis A. J., Simon M. D., Loas A., Liu S., Jessen C., Nielsen T. E., Pentelute B. L., Science, 2020, 368(6494), 980—987 |
| 11 | Dawson P. E., Muir T. W., Clark⁃Lewis I., Kent S. B. H., Science, 1994, 266(5186),776—779 |
| 12 | Fang G. M., Li Y. M., Shen F., Huang Y. C., Li J. B., Lin Y., Cui H. K., Liu L., Angew. Chem. Int. Ed., 2011, 50(33), 7645—7649 |
| 13 | Fang G M., Wang J. X., Liu L., Angew. Chem. Int. Ed., 2012, 51(41),10347—10350 |
| 14 | Zheng J. S., Tang S., Qi Y. K., Wang Z. P., Liu L., Nat. Protoc., 2013, 8(12),2483—2495 |
| 15 | Moura A., Savageau M. A., Alves R., PloS One, 2013, 8(10),e77319 |
| 16 | Loibl S. F., Harpaz Z., Seitz O., Angew. Chem. Int. Ed., 2015, 54(50), 15055—15059 |
| 17 | Offer J., Biopolymers, 2010, 94(4), 530—541 |
| 18 | Lutsky M. Y., Nepomniaschiy N., Brik A., Chem. Commun., 2008, 10, 1229—1231 |
| 19 | Li X. C., Lam H. Y., Zhang Y. F., Chan C. K., Org. Lett., 2010, 12(8), 1724—1727 |
| 20 | Li T. L., Liu H., Li X. C., Org. Lett., 2016, 18(22), 5944—5947 |
| 21 | Lee C. L., Liu H., Wong C. T., Chow H. Y., Li X. C., J. Am. Chem. Soc., 2016, 138(33), 10477—10484 |
| 22 | Bode J. W., Fox R. M., Baucom K. D., Angew. Chem. Int. Ed., 2006, 45(8), 1248—1252 |
| 23 | Pusterla I., Bode J. W., Angew. Chem. Int. Ed., 2011, 51(2), 513—516 |
| 24 | Harmand T. J., Murar C. E., Bode J. W., Nat. Protoc., 2016, 11(6), 1130—1147 |
| 25 | Schmidt M., Toplak A., Quaedflieg P. J., Nuijens T., Curr. Opin. Chem. Biol., 2017, 38, 1—7 |
| 26 | Han D. Y., Ren Y. X., Yang Z. Y, Huang H., Zheng J. S., Chem. J. Chinese Universities, 2023, 44(10), 20230188 |
| 韩东阳, 任宇祥, 杨子毅, 黄赫, 郑基深. 高等学校化学学报, 2023, 44(10), 20230188 | |
| 27 | Yan L. Z., Dawson P. E., J. Am. Chem. Soc., 2001, 123(4), 526—533 |
| 28 | Rohde H., Seitz O., Biopolymers, 2010, 94(4),551—559 |
| 29 | Deng Z. H., Ai H. S., Sun M. S., Tong Z. B., Du Y. X., Qu Q., Zhang L. Y., Xu Z. Y., Tao S. X., Shi Q., Li J. B., Pan M., Liu L., Mol. Cell., 2023, 83(17),3080—3094 |
| 30 | Shin Y., Winans K. A., Backes B. J., Kent S. B. H., Ellman J. A., Bertozzi C. R., J. Am. Chem. Soc., 1999, 121(50),11684—11689 |
| 31 | Marcaurelle L. A., Mizoue L. S., Wilken J., Oldham L., Kent S. B. H., Handel T. M., Bertozzi C. R., Chem. Eur. J., 2001, 7(5),1129—1132 |
| 32 | Mezzato S., Schaffrath M., Unverzagt C., Angew. Chem. Int. Ed., 2005, 44(11),1650—1654 |
| 33 | Yamamoto N., Tanabe Y., Okamoto R., Dawson P. E., Kajihara Y., J. Am. Chem. Soc., 2008, 130(2),501—510 |
| 34 | Aussedat B., Fasching B., Johnston E., Sane N., Nagorny P., Danishefsky S. J., J. Am. Chem. Soc., 2012, 134(7),3532—3541 |
| 35 | Fernández⁃Tejada A., Vadola P. A., Danishefsky S. J., J. Am. Chem. Soc., 2014, 136(23),8450—8458 |
| 36 | Wilkinson B. L., Stone R. S., Capicciotti C. J., Thaysen⁃Andersen M., Matthews J. M., Packer N. H., Ben R. N., Payne R. J., Angew. Chem., 2012, 124(15),3666—3670 |
| 37 | Wang P., Dong S. W., Shieh J. H., Peguero E., Hendrickson R., Moore M. A. S., Danishefsky S. J., Science, 2013, 342(6164), 1357—1360 |
| 38 | Zhao J., Liu X. L., Liu J. L., Ye F. R., Wei B. C., Deng M. G., Li T. H., Huang P., Wang P., J. Am. Chem. Soc., 2024, 146(4),2615—2623 |
| 39 | Sakamoto I., Tezuka K., Fukae K., Ishii K., Taduru K., Maeda M., Ouchi M., Yoshida K., Nambu Y., Igarashi J., Hayashi N., Tsuji T., Kajihara Y., J. Am. Chem. Soc., 2012, 134(12), 5428—5431 |
| 40 | van de Langemheen H., van Hoeke M., van Ufford H. C. Q., Kruijtzer J. A. W., Liskamp R. M. J., Org. Biomol. Chem., 2014, 12(25), 4471—4478 |
| 41 | Lee J., Oh E. T., Lee E., Park H. J., Kim C., New J. Chem., 2022, 46(32),15551—15556 |
| 42 | Yim V. V., Kavianinia I., Cameron A. J., Harris P. W., Brimble M. A., Org. Biomol. Chem., 2020, 18(15),2838—2844 |
| 43 | Chaudhuri D., Ganesan R., Vogelaar A., Dughbaj M. A., Beringer P. M., Camarero J. A., J. Org. Chem., 2021, 86(21),15242—15246 |
| 44 | Wierzbicka M., Waliczek M., Dziadecka A., Stefanowicz P., J. Org. Chem., 2021, 86(17),12292—12299 |
| 45 | Levinson A. M., McGee J. H., Roberts A. G., Creech G. S., Wang T., Peterson M. T., Hendrickson R. C., Verdine G. L., Danishefsky S. J., J. Am. Chem. Soc., 2017, 139(22), 7632—7639 |
| 46 | Bunker R. D., Mandal K., Bashiri G., Chaston J. J., Pentelute B. L., Lott J. S., Kent S. B. H., Baker E. N., Proc. Natl. Acad. Sci. USA, 2015, 112(14), 4310—4315 |
| 47 | Mandal K., Uppalapati M., Ault⁃Riché D., Kenney J., Lowitz J., Sidhu S. S., Kent S. B. H., Proc. Natl. Acad. Sci. USA, 2012, 109(37),14779—14784 |
| 48 | Weidmann J., Schnölzer M., Dawson P. E., Hoheisel J. D., Cell Chem. Biol., 2019, 26(5),645—651 |
| 49 | Muir T. W., Sondhi D., Cole P. A., Proc. Natl. Acad. Sci. USA, 1998, 95(12),6705—6710 |
| 50 | Muir T. W., Annu. Rev. Biochem., 2003, 72(1),249—289 |
| 51 | Bilbrough T., Piemontese E., Seitz O., Chem. Soc. Rev., 2022, 51(13),5691—5730 |
| 52 | Shivatare S. S., Shivatare V. S., Wong C. H., Chem. Rev., 2022, 122(20), 15603—15671 |
| 53 | Tashiro K., Mohapatra J., Brautigam C. A., Liszczak G., ACS Chem. Biol., 2022, 17(4),810—815 |
| 54 | Hanna C. C., Kriegesmann J., Dowman L. J., Becker C. F. W., Payne R. J., Angew. Chem. Int. Ed., 2022, 61(15), e202111266 |
| 55 | Tolbert T. J., Wong C. H., J. Am. Chem. Soc., 2000, 122(23),5421—5428 |
| 56 | Macmillan D., Bertozzi C. R., Angew. Chem. Int. Ed., 2004, 43(11),1355—1359 |
| 57 | Reif A., Siebenhaar S., Tröster A., Schmälzlein M., Lechner C., Velisetty P., Gottwald K., Pöhner C., Boos I., Schubert V., Rose‐John S., Unverzagt C., Angew. Chem. Int. Ed., 2014, 53(45), 12125—12131 |
| 58 | Ye F. R., Zhao J., Xu P., Liu X. L., Yu J., Shangguan W., Liu J. Z., Luo X. S., Li C., Ying T. L., Wang J., Yu B., Wang P., Angew. Chem. Int. Ed., 2021, 60(23),12904—12910 |
| 59 | Murakami M., Kiuchi T., Nishihara M., Tezuka K., Okamoto R., Izumi M., Kajihara Y., Sci. Adv., 2016, 2(1), e1500678 |
| 60 | Tan X. L., Pan M., Zheng Y., Gao S., Liang L. J., Li Y. M., Chem. Sci., 2017, 8(10),6881—6887 |
| 61 | Li Y. T., Yi C., Chen C. C., Lan H., Pan M., Zhang S. J., Huang Y. C., Guan C. J., Li Y. M., Yu L., Liu L., Nat. Commun., 2017, 8(1),14846 |
| 62 | Pan M., Gao S., Zheng Y., Tan X. D., Lan H., Tan X. L., Sun D. M., Lu L. N., Wang T., Zheng Q. Y., Huang Y. C., Wang J. W., Liu L., J. Am. Chem. Soc., 2016, 138(23), 7429—7435 |
| 63 | Gao S., Pan M., Zheng Y., Huang Y. C., Zheng Q. Y., Sun D. M., Lu L. N., Tan X. D., Tan X. L., Lan H., Wang J. X., Wang T., Wang J. W., Liu L., J. Am. Chem. Soc., 2016, 138(43),14497—14502 |
| 64 | Tang S., Liang L. J., Si Y. Y., Gao S., Wang J. X., Liang J., Mei Z. Q., Zheng J. S., Liu L., Angew. Chem. Int. Ed., 2017, 56(43),13333—13337 |
| 65 | Pan M., Zheng Q. Y., Ding S., Zhang L. J., Qu Q., Wang T., Hong D. N., Ren Y. J., Liang L. J., Chen C. L., Mei Z. Q., Liu L., Angew. Chem. Int. Ed., 2019, 58(9),2627—2631 |
| 66 | Chu G. C., Pan M., Li J. B., Liu S. L., Zuo C., Tong Z. B., Bai J. S., Gong Q. Y., Ai H. S., Fan J., Meng X. B., Huang Y. C., Shi J., Deng H. T., Tian C. L., Li Y. M., Liu L., J. Am. Chem. Soc., 2019, 141(8),3654—3663 |
| 67 | Ai H. S., Guo Y., Sun D. M., Liu S. L., Qi Y. K., Guo J., Qu Q., Gong Q. Y., Zhao S. W., Li J. B., Liu L., ChemBioChem, 2019, 20(2),221—229 |
| 68 | Ai H. S., Peng S., Li J. B., J. Pept. Sci., 2022, 28(5), e3381 |
| 69 | Ai H. S., Chu G. C., Gong Q. Y., Tong Z. B., Deng Z. H., Liu X., Yang F., Xu Z. Y., Li J. B., Tian C. L., Liu L., J. Am. Chem. Soc., 2022, 144(40), 18329—18337 |
| 70 | Ai H. S., Sun M. S., Liu A. J., Sun Z. X., Liu T. T., Cao L., Liang L. J., Qu Q., Li Z. C., Deng Z. H., Tong Z. B., Chu G. C., Tian X. L., Deng H. T., Zhao S. W., Li J. B., Lou Z. Y., Liu L., Nat. Chem. Biol., 2022, 18(9),972—980 |
| 71 | Pan M., Zheng Q. Y., Wang T., Liang L. J., Mao J. X., Zuo C., Ding R. C., Ai H. S., Xie Y., Si D., Yu Y. Y., Liu L., Zhao M. L., Nature, 2021, 600(7888),334—338 |
| 72 | Liang L. J., Chu G. C., Qu Q., Zuo C., Mao J. X., Zheng Q. Y., Chen J. N., Meng X. B., Jing Y., Deng H. T., Li Y. M., Liu L., Angew. Chem. Int. Ed., 2021, 60(31),17171—17177 |
| 73 | Xu L., Fan J., Wang Y., Zhang Z. P., Fu Y., Li Y. M., Shi J., Chem. Commun., 2019, 55(49),7109—7112 |
| 74 | Zuo C., Shi W. W., Chen X. X., Glatz M., Riedl B., Flamme I., Pook E., Wang J. W., Fang G. M., Bierer D., Liu L., Sci. China Chem., 2019, 62(10),1371—1378 |
| 75 | Chang H. N., Liu B. Y., Qi Y. K., Zhou Y., Chen Y. P., Pan K. M., Li W. W., Zhou X. M., Ma W. W., Fu C. Y., Qi Y. M., Liu L., Gao Y. F., Angew. Chem. Int. Ed., 2015, 54(40), 11760—11764 |
| 76 | Zhang L., Shen H., Gong Y. Y., Pang X. J., Yi M. Q., Guo L., Li J., Arroyo S., Lu X., Ovchinnikov S., Cheng G., Liu X. D., Jiang X., Feng S., Deng H. T., Chem. Sci., 2019, 10(11),3271—3280 |
| 77 | Zhao R., Shi P., Wei X. X., Xia Z. M., Shi C. W., Shi J., Org. Lett., 2023, 25(35),6544—6548 |
| 78 | Premdjee B., Andersen A. S., Larance M., Conde⁃Frieboes K. W., Payne R. J., J. Am. Chem. Soc., 2021, 143(14), 5336—5342 |
| 79 | Wang J. X., Fang G. M., He Y., Qu D. L., Yu M., Hong Z. Y., Liu L., Angew. Chem. Int. Ed., 2015, 54(7), 2194—2198 |
| 80 | Wang Z. M., Xu W. L., Liu L., Zhu T. F., Nat. Chem., 2016, 8(7),698—704 |
| 81 | Pech A., Achenbach J., Jahnz M., Schülzchen S., Jarosch F., Bordusa F., Klussmann S., Nucleic Acids Res., 2017, 45(7),3997—4005 |
| 82 | Jiang W. J., Zhang B. C., Fan C. Y., Wang M., Wang J. X., Deng Q., Liu X. Y., Chen J., Zheng J. S., Liu L., Zhu T. F., Cell Discov., 2017, 3(1),1—7 |
| 83 | Zhou X. N., Zuo C., Li W. Q., Shi W. W., Zhou X. W., Wang H. F., Chen S. M., Du J. F., Chen G.Y., Zhai W. J., Zhao W. S., Wu Y. H., Qi Y. M., Liu L., Gao Y. F., Angew. Chem. Int. Ed., 2020, 59(35),15114—15118 |
| 84 | Zheng J. S., Yu M., Qi Y. K., Tang S., Shen F., Wang Z. P., Xiao L., Zhang L. H., Tian C. L., Liu L., J. Am. Chem. Soc., 2014, 136(9),3695—3704 |
| 85 | Li Y. M., Li Y. T., Pan M., Kong X. Q., Huang Y. C., Hong Z. Y., Liu L., Angew. Chem. Int. Ed., 2014, 53(8), 2198—2202 |
| 86 | Wan Q., Danishefsky S. J., Angew. Chem. Int. Ed., 2007, 46(48), 9248—9252 |
| 87 | Yang R. L., Pasunooti K. K., Li F. P., Liu X. W., Liu C. F., Chem. Commun., 2010, 46(38), 7199—7201 |
| 88 | Yu Y. Y., Zheng Q. Y., Erramilli S. K., Pan M., Park S., Xie Y., Li J. X., Fei J. Y., Kossiakoff A. A., Liu L., Zhao M. L., Nat. Chem. Biol., 2021, 17(8), 896—905 |
| 89 | Bondalapati S., Mansour W., Nakasone M. A., Maity S. K., Glickman M. H., Brik A., Chem. Eur. J., 2015, 21(20), 7360—7364 |
| 90 | Qu Q., Pan M., Gao S., Zheng Q. Y., Yu Y. Y., Su J. C., Li X., Hu H. G., Adv. Sci., 2018, 5(7), 1800234 |
| 91 | Lewis Y. E., Galesic A., Levine P. M., de Leon C. A., Lamiri N., Brennan C. K., Pratt M. R., ACS Chem. Biol., 2017, 12(4), 1020—1027 |
| 92 | Levine P. M., de Leon C. A., Galesic A., Balana A., Marotta N. P., Lewis Y. E., Pratt M. R., Bioorg. Med. Chem., 2017, 25(18),4977—4982 |
| 93 | Dikiy I., Fauvet B., Jovičić A., Mahul⁃Mellier A. L., Desobry C., El⁃Turk F., Gitler A. D., Lashuel H. A., Eliezer D., ACS Chem. Biol., 2016, 11(9),2428—2437 |
| 94 | Hejjaoui M., Butterfield S., Fauvet B., Vercruysse F., Cui J., Dikiy I., Prudent M., Olschewski D., Zhang Y., Eliezer D., Lashuel H. A., J. Am. Chem. Soc., 2012, 134(11),5196—5210 |
| 95 | Gatzemeier L. M., Meyer F., Diederichsen U., Outeiro T. F., Chem. Eur. J., 2023, 29(33), e202300649 |
| 96 | Jing Y. H., Ding D. B., Tian G. F., Kwan K. C. J., Liu Z., Ishibashi T., Li X. D., Nucleic Acids Res., 2020, 48(17),9538—9549 |
| 97 | Qi Y. K., He Q. Q., Ai H. S., Guo J., Li J. B., Chem. Commun., 2017, 53(29),4148—4151 |
| 98 | Li J. B., He Q., Liu Y. T., Liu S. L., Tang S., Li C. M., Sun D. M., Li X. R., Zhou M., Zhu P., Bi G. Q., Zhou Z. H., Zheng J. S., Tian C. L., ChemBioChem, 2017, 18(2),176—180 |
| 99 | Li J. B., Qi Y. K., He Q. Q., Ai H. S., Liu S. L., Wang J. X., Zheng J. S., Liu L., Tian C. L., Cell Res., 2018, 28(2), 257—260 |
| 100 | Li J. B., Liang J., Tian C. L., Meth. Enzymol., 2020, 639, 263—287 |
| 101 | He Q. Q., Li J. B., Qi Y. K., Wang Z. P., Huang Y., Liu L., Sci. China Chem., 2017, 60(5), 621—627 |
| 102 | Crich D., Banerjee A., J. Am. Chem. Soc., 2007, 129(33),10064—10065 |
| 103 | Easton C. J., Hutton C. A., Roselt P. D., Tiekink E. R., Tetrahedron, 1994, 50(24),7327—7340 |
| 104 | Malins L. R., Giltrap A. M., Dowman L. J., Payne R. J., Org. Lett., 2015, 17(9),2070—2073 |
| 105 | Chen J., Wan Q., Yuan Y., Zhu J. L., Danishefsky S. J., Angew. Chem. Int. Ed., 2008, 47(44),8521—8524 |
| 106 | Yang R. L., Pasunooti K. K., Li F. P., Liu X. W., Liu C. F., J. Am. Chem. Soc., 2009, 131(38),13592—13593 |
| 107 | Marin J., Didierjean C., Aubry A., Casimir J. R., Briand J. P., Guichard G., J. Org. Chem., 2004, 69(1),130—141 |
| 108 | Merkx R., de Bruin G., Kruithof A., van den Bergh T., Snip E., Lutz M., Oualid F. E., Ovaa H., Chem. Sci., 2013, 4(12),4494—4498 |
| 109 | van der Heden van Noort G. J., Kooij R., Elliott P. R., Komander D., Ovaa H., Org. Lett., 2017, 19(24),6490—6493 |
| 110 | Ajish Kumar KS., Haj⁃Yahya M., Olschewski D., Lashuel H. A., Brik A., Angew. Chem. Int. Ed., 2009, 48(43),8090—8094 |
| 111 | Padrón J. M., Kokotos G., Martı́n T., Markidis T., Gibbons W. A., Martı́n V. S., Tetrahedron: Asymmetr., 1998, 9(19),3381—3394 |
| 112 | El Oualid F., Merkx R., Ekkebus R., Hameed D. S., Smit J. J., de Jong A., Hilkmann H., Sixma T. K., Ovaa H., Angew. Chem., 2010, 122(52),10347—10351 |
| 113 | Dao Y. K., Han L., Wang H. X., Dong S. W., Org. Lett., 2019, 21(9),3265—3270 |
| 114 | Zhang X., King⁃Smith E., Renata H., Angew. Chem. Int. Ed., 2018, 57(18),5037—5041 |
| 115 | Chen J., Wang P., Zhu J. L., Wan Q., Danishefsky S. J., Tetrahedron, 2010, 66(13),2277—2283 |
| 116 | Harpaz Z., Siman P., Kumar K. A., Brik A., ChemBioChem, 2010, 11(9), 1232—1235 |
| 117 | Tan Z. P., Shang S. Y., Danishefsky S. J., Angew. Chem. Int. Ed., 2010, 49(49),9500—9503 |
| 118 | Ding H., Shigenaga A., Sato K., Morishita K., Otaka A., Org. Lett., 2011, 13(20),5588—5591 |
| 119 | Siman P., Karthikeyan S. V., Brik A., Org. Lett., 2012, 14(6), 1520—1523 |
| 120 | Hernández J. N., Ramírez M. A., Martín V. S., J. Org. Chem., 2003, 68(3),743—746 |
| 121 | Malins L. R., Cergol K. M., Payne R. J., ChemBioChem, 2013, 14(5),559—563 |
| 122 | Pasunooti K. K., Banerjee B., Yap T., Jiang Y. J., Liu C. F., Org. Lett., 2015, 17(24),6094—6097 |
| 123 | Pasunooti K. K., Yang R. L., Banerjee B., Yap T., Liu C. F., Org. Lett., 2016, 18(11),2696—2699 |
| 124 | Thompson R. E., Chan B., Radom L., Jolliffe K. A., Payne R. J., Angew. Chem., 2013, 37(125),9905—9909 |
| 125 | Guan X. Y., Drake M. R., Tan Z. P., Org. Lett., 2013, 15(24),6128—6131 |
| 126 | Cergol K. M., Thompson R. E., Malins L. R., Turner P., Payne R. J., Org. Lett., 2014, 16(1),290—293 |
| 127 | Malins L. R., Cergol K. M., Payne R. J., Chem. Sci., 2014, 5(1),260—266 |
| 128 | Sayers J., Thompson R. E., Perry K. J., Malins L. R., Payne R. J., Org. Lett., 2015, 17(19),4902—4905 |
| 129 | Gao X. F., Du J. J., Liu Z., Guo J., Org. Lett., 2016, 18(5),1166—1169 |
| 130 | Lee M., Neukirchen S., Cabrele C., Reiser O., J. Pept. Sci., 2017, 23(7/8),556—562 |
| 131 | Qiu W. T., Shi S., Li R. N., Lin X. F., Rao L. M., Sun Z. K., Chin. J. Chem., 2021, 39(5), 1255—1258 |
| 132 | Venneti N. M., Samala G., Morsy R. M. I., Mendoza L. G., Isidro⁃Llobet A., Tom J. K., Mukherjee S., Kopach M. E., Stockdill J. L., J. Am. Chem. Soc., 2023, 145(2),1053—1061 |
| 133 | Jing R., Walczak M. A., Org. Lett., 2024, 26(13), 2590—2595 |
| 134 | Jin K., Li T. L., Chow H. Y., Liu H., Li X. C., Angew. Chem. Int. Ed., 2017, 56(46),14607—14611 |
| 135 | Sun Z. Q., Ma W. J., Cao Y. H., Wei T. Y., Mo X. Y., Chow H. Y., Tan Y., Cheung C. H. P., Liu J. M., Lee H. K., Tse E. C. M., Liu H., Li X. C., Chem., 2022, 8(9), 2542—2557 |
| 136 | Desmet R., Boidin⁃Wichlacz C., Mhidia R., Tasiemski A., Agouridas V., Melnyk O., Angew. Chem. Int. Ed., 2023, 62(18),e202302648 |
| 137 | Mitchell N. J., Malins L. R., Liu X., Thompson R. E., Chan B., Radom L., Payne R. J., J. Am. Chem. Soc., 2015, 137(44), 14011—14014 |
| 138 | Mueller L. K., Baumruck A. C., Zhdanova H., Tietze A. A., Front. Bioeng. Biotechnol., 2020, 8, 162 |
| 139 | Shi W. W., Shi C. W., Wang T. Y., Li Y. L., Zhou Y. K., Zhang X. H., Bierer D., Zheng J. S., Liu L., J. Am. Chem. Soc., 2022, 144(1), 349—357 |
| [1] | 韩旭, 白雪, 张仲, 杨琰莉, 崔红, 刘术侠. PW11M@Cu3(BTC)2杂化物的合成及燃料油深度脱硫活性[J]. 高等学校化学学报, 2023, 44(4): 20220702. |
| [2] | 韩东阳, 任宇祥, 杨子毅, 黄赫, 郑基深. 分裂内含肽: 一种高效的无痕多肽片段连接工具[J]. 高等学校化学学报, 2023, 44(10): 20230188. |
| [3] | 孟祥龙, 杨歌, 郭海玲, 刘晨光, 柴永明, 王纯正, 郭永梅. 纳米分子筛的合成及硫化氢吸附性能[J]. 高等学校化学学报, 2022, 43(3): 20210687. |
| [4] | 张仁丽, 王瑶, 遇治权, 孙志超, 王安杰, 刘颖雅. 氟改性UiO-66固载钼基过氧化物催化氧化含硫化合物[J]. 高等学校化学学报, 2021, 42(6): 1914. |
| [5] | 汪潇,金彪,王宇斌,徐卓越,张鲁,张小婷,杨留栓. 阴离子在脱硫石膏晶须水热结晶中的作用机理[J]. 高等学校化学学报, 2020, 41(3): 473. |
| [6] | 张丽,钱明超,刘雪珂,高帅涛,余江,谢海深,王宏斌,孙风江,苏向红. 铁基离子液体/NHD吸收氧化H2S的反应动力学[J]. 高等学校化学学报, 2020, 41(2): 317. |
| [7] | 王影, 孙传胤, 王润伟, 张震东, 张大明, 张宗弢, 裘式纶. 双亲型Ti/ZSM-5分子筛催化剂的制备及氧化脱硫性能[J]. 高等学校化学学报, 2019, 40(6): 1265. |
| [8] | 高晓明, 代源, 费娇, 张裕, 付峰. n-p异质结型CdS/BiOBr复合光催化剂的制备及性能[J]. 高等学校化学学报, 2017, 38(7): 1249. |
| [9] | 于凤丽, 王庆玉, 李桂晓, 袁冰, 解从霞, 于世涛. Brönsted-Lewis有机-无机杂多酸催化模拟催化裂化汽油的烷基化脱硫反应[J]. 高等学校化学学报, 2017, 38(1): 72. |
| [10] | 宋华, 于祺, 徐晓伟, 宋华林, 姜楠, 李锋, 陈彦广. 钇和柠檬酸对非负载磷化镍催化剂加氢脱硫性能的影响[J]. 高等学校化学学报, 2016, 37(8): 1528. |
| [11] | 刘治庆, 薛飞, 雷振凯, 刘晨江. 离子液体1-烷基-3-羧甲基苯并咪唑双三氟甲磺酰亚胺盐的合成及在油品中的脱硫应用[J]. 高等学校化学学报, 2016, 37(5): 886. |
| [12] | 于凤丽, 谢盼辉, 朱国强, 袁冰, 解从霞, 于世涛. 有机-无机杂多酸类离子液体催化汽油超声氧化脱硫[J]. 高等学校化学学报, 2016, 37(12): 2184. |
| [13] | 王业海, 孔一夫, 陈晨晨, 李宜明. K33位乙酰化修饰SUMO蛋白的化学合成[J]. 高等学校化学学报, 2016, 37(11): 1987. |
| [14] | 薛飞, 麻荣, 孙亚栋, 阿布力米提·阿布都卡德, 张永红, 刘晨江. 羧基功能化苯并三氮唑类离子液体的合成及在萃取-氧化脱硫中的应用[J]. 高等学校化学学报, 2015, 36(7): 1298. |
| [15] | 程尚增, 郭倩倩, 黄张根, 申文忠, 韩小金. 纤维素制备渗氮炭材料用于脱除烟气中的SO2[J]. 高等学校化学学报, 2015, 36(6): 1126. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||