

高等学校化学学报 ›› 2018, Vol. 39 ›› Issue (8): 1683.doi: 10.7503/cjcu20180081
万金林1, 巫受群1, 甘宜远2, 孟娇2, 王贞超2( ), 欧阳贵平2(
), 欧阳贵平2( )
)
收稿日期:2018-01-25
出版日期:2018-08-10
发布日期:2018-05-27
作者简介:联系人简介: 欧阳贵平, 男, 博士, 教授, 博士生导师, 主要从事新农药和新医药创制方面的研究. E-mail: 基金资助:
WAN Jinlin1, WU Shouqun1, GAN Yiyuan2, MENG Jiao2, WANG Zhenchao2*( ), OUYANG Guiping2*(
), OUYANG Guiping2*( )
)
Received:2018-01-25
Online:2018-08-10
Published:2018-05-27
Supported by:摘要:
以苯甲醛和1,3,4-噻二唑为起始原料, 设计合成了一系列新颖的含1,3,4-噻二唑结构的查尔酮缩氨基脲类化合物. 利用核磁共振波谱(NMR)和高分辨质谱(HRMS)对其结构进行了确证. 初步抑菌活性测试结果表明, 该类化合物对水稻白叶枯病菌(X. oryzae)、 烟草青枯病菌(R. solanacearum)和柑橘溃疡病菌(X. citri)均表现出一定的抑制活性, 其中化合物7h和7i在浓度为100 μg/mL时, 对以上3种植物病菌的抑制率均达到100%, EC50值分别为15.13, 30.90, 24.49和21.33, 24.54, 14.79 μg/mL, 均超过对照药叶枯唑(EC50值分别为92.23, 58.88和123.02 μg/mL).
TrendMD:
万金林, 巫受群, 甘宜远, 孟娇, 王贞超, 欧阳贵平. 含1,3,4-噻二唑结构的查尔酮缩氨基脲类化合物的合成及抗细菌活性. 高等学校化学学报, 2018, 39(8): 1683.
WAN Jinlin, WU Shouqun, GAN Yiyuan, MENG Jiao, WANG Zhenchao*, OUYANG Guiping*. Synthesis and Antibacterial Activities Evaluation of Chalconesemicarbazone Derivatives Bearing 1,3,4-Thiadiazole Moiety†. Chem. J. Chinese Universities, 2018, 39(8): 1683.
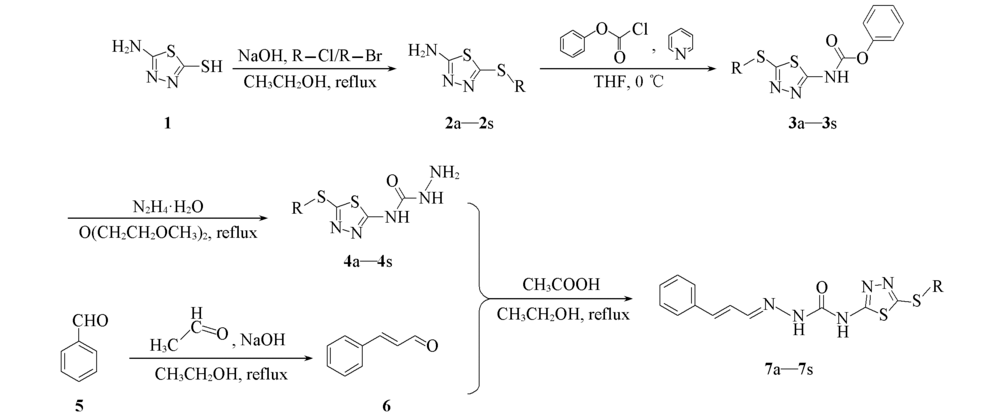
Scheme 1 Scheme 1 Synthetic routes of compounds 7a—7s7a: R=PhCH2; 7b: R=2-ClPhCH2; 7c: R=3-ClPhCH2; 7d: R=4-ClPhCH2; 7e: R=2-OCH3PhCH2; 7f: R=3-OCH3PhCH2; 7g: R=4-OCH3PhCH2; 7h: R=2-CH3PhCH2; 7i: R=3-CH3PhCH2; 7j: R=4-CH3PhCH2; 7k: R=2-FPhCH2; 7l: R=4-FPhCH2; 7m: R=2-CNPhCH2; 7n: R=2,4-diClPhCH2; 7o: R=CH3; 7p: R=C2H5; 7q: R=n-C3H7; 7r: R=n-C4H9; 7s: R=n-C5H11
| Compd. | m. p./℃ | Compd. | m. p./℃ | Compd. | m. p./℃ |
|---|---|---|---|---|---|
| 2a | 157—159(158[ | 2h | 119—121 | 2o | 177—179 |
| 2b | 151—153(151—152[ | 2i | 128—130(128—129[ | 2p | 136—137(136—137[ |
| 2c | 138—140(138—139[ | 2j | 181—183(181—182[ | 2q | 116—118(117—119[ |
| 2d | 167—169(168—169[ | 2k | 143—145(145—146[ | 2r | 103—105 |
| 2e | 100—102 | 2l | 148—150(149—150[ | 2s | 112—113(112—115[ |
| 2f | 148—150 | 2m | 104—106 | ||
| 2g | 151—153 | 2n | 133—135(133—134[ |
Table 1 Melting points of compounds 2a—2s
| Compd. | m. p./℃ | Compd. | m. p./℃ | Compd. | m. p./℃ |
|---|---|---|---|---|---|
| 2a | 157—159(158[ | 2h | 119—121 | 2o | 177—179 |
| 2b | 151—153(151—152[ | 2i | 128—130(128—129[ | 2p | 136—137(136—137[ |
| 2c | 138—140(138—139[ | 2j | 181—183(181—182[ | 2q | 116—118(117—119[ |
| 2d | 167—169(168—169[ | 2k | 143—145(145—146[ | 2r | 103—105 |
| 2e | 100—102 | 2l | 148—150(149—150[ | 2s | 112—113(112—115[ |
| 2f | 148—150 | 2m | 104—106 | ||
| 2g | 151—153 | 2n | 133—135(133—134[ |
| Compd. | m. p./℃ | HRMS(calcd.)*, m/z | 1H NMR(DMSO, 400 MHz), δ |
|---|---|---|---|
| 3a | 170—172 | 344.0517(344.0522) | 12.86(s, 1H), 7.48—7.38(m, 4H), 7.36—7.30(m, 3H), 7.29—7.25(m, 3H), 4.48(s, 2H) |
| 3b | 141—143 | 378.0130(378.0132) | 12.88(s, 1H), 7.52—7.47(m, 2H), 7.44(d, J=8.1 Hz, 2H), 7.36—7.25(m, 5H), 4.55(s, 2H) |
| 3c | 185—187 | 378.0122(378.0132) | 12.89(s, 1H), 7.50(d, J=1.4 Hz, 1H), 7.48—7.43(m, 2H), 7.40—7.30(m, 4H), 7.30—7.26(m, 2H), 4.49(s, 2H) |
| 3d | 172—174 | 378.0129(378.0132) | 12.88(s, 1H), 7.48—7.41(m, 4H), 7.41—7.36(m, 2H), 7.34—7.25(m, 3H), 4.48(s, 2H) |
| Compd. | m. p./℃ | HRMS(calcd.)*, m/z | 1H NMR(DMSO, 400 MHz), δ |
| 3e | 137—139 | 374.0620(374.0628) | 12.88(s, 1H), 7.45(t, J=7.7 Hz, 2H), 7.35—7.24(m, 5H), 7.01(d, J=8.1 Hz, 1H), 6.89(t, J=7.4 Hz, 1H), 4.41(s, 2H), 3.80(s, 3H) |
| 3f | 139—141 | 374.0620(374.0628) | 12.86(s, 1H), 7.45(t, J=7.9 Hz, 2H), 7.34—7.21(m, 4H), 6.98(t, J=5.6 Hz, 2H), 6.85(dd, J=8.2, 2.1 Hz, 1H), 4.45(s, 2H), 3.73(s, 3H) |
| 3g | 199—201 | 374.0619(374.0628) | 12.83(s, 1H), 7.48—7.42(m, 2H), 7.35—7.28(m, 5H), 7.28—7.24(m, 2H), 4.42(s, 2H), 3.73(s, 3H) |
| 3h | 137—139 | 358.0668(358.0678) | 12.84(s, 1H), 7.51—7.40(m, 2H), 7.33—7.25(m, 4H), 7.23—7.17(m, 2H), 7.16—7.10(m, 1H), 4.49(s, 2H), 2.37(s, 3H) |
| 3i | 152—154 | 358.0671(358.0678) | 12.88(s, 1H), 7.50—7.41(m, 2H), 7.33—7.25(m, 3H), 7.21(t, J=9.0, 2.7 Hz, 3H), 7.08(d, J=6.4 Hz, 1H), 4.44(s, 2H, CH2), 2.27(s, 3H, CH3) |
| 3j | 187—189 | 358.0669(358.0678) | 12.85(s, 1H), 7.45(t, J=7.8 Hz, 2H), 7.34—7.24(m, 5H), 7.13(d, J=7.6 Hz, 2H), 4.43(s, 2H), 2.27(s, 3H) |
| 3k | 144—146 | 362.0421(362.0428) | 12.90(s, 1H), 7.49—7.42(m, 3H), 7.38—7.25(m, 4H), 7.19—7.12(m, 2H), 4.48(s, 2H) |
| 3l | 161—163 | 362.0423(362.0428) | 12.86(s, 1H), 7.48—7.42(m, 4H), 7.34—7.25(m, 3H), 7.23—7.12(m, 2H), 4.48(s, 2H) |
| 3m | 120—122 | 369.0467(369.0474) | 12.90(s, 1H), 7.85(dd, J=7.7, 0.9 Hz, 1H), 7.70—7.61(m, 2H), 7.52—7.42(m, 3H), 7.34—7.25(m, 3H), 4.62(s, 2H) |
| 3n | 162—164 | 411.9735(411.9743) | 12.92(s, 1H), 7.63(d, J=12.1 Hz, 1H), 7.53(d, J=8.3 Hz, 1H), 7.46(t, J=7.0 Hz, 2H), 7.39(t, J=7.8 Hz, 1H), 7.30(dd, J=16.2, 8.3 Hz, 3H), 4.53(s, 2H) |
| 3o | 159—161 | 268.0204(268.0209) | 12.85(s, 1H), 7.45(t, J=7.6 Hz, 2H), 7.30(dd, J=16.4, 7.1 Hz, 3H), 2.71(s, 3H) |
| 3p | 206—209 | 282.0361(282.0365) | 8.46(s, 1H), 3.18(q, J=7.3 Hz, 2H), 1.33(t, J=7.3 Hz, 3H) |
| 3q | 190—192 | 296.0518(296.0522) | 8.46(s, 1H), 3.15(t, J=7.1 Hz, 2H), 1.76—1.63(m, 2H), 0.98(t, J=7.3 Hz, 3H) |
| 3r | 181—183 | 310.0669(310.0678) | 8.46(s, 1H), 3.17(t, J=7.3 Hz, 2H), 1.71—1.60(m, 2H), 1.46—1.35(m, 2H), 0.89(t, J=7.4 Hz, 3H) |
| 3s | 108—110 | 324.0826(324.0835) | 12.86(s, 1H), 7.49—7.42(m, 2H), 7.34—7.25(m, 3H), 3.20(t, J=14.6 Hz, 2H), 1.77—1.61(m, 2H), 1.42—1.24(m, 4H), 0.86(t, J=7.2 Hz, 3H) |
| 4a | 196—198 | 282.0469(282.0478) | 8.45(s, 1H), 7.41—7.37(m, 2H), 7.35—7.30(m, 2H), 7.29—7.23(m, 1H), 4.43(s, 2H) |
| 4b | 195—197 | 337.9907(337.9908) | 8.51(s, 1H), 7.51—7.46(m, 2H), 7.37—7.27(m, 2H), 4.51(s, 2H) |
| 4c | 185—187 | 316.0082(316.0088) | 8.45(s, 1H), 7.47(s, 1H), 7.38—7.30(m, 3H), 4.44(s, 2H) |
| 4d | 212—213 | 337.9905(337.9908) | 8.46(s, 1H), 7.43—7.36(m, 4H), 4.43(s, 2H) |
| 4e | 201—203 | 312.0577(312.0583) | 8.49(s, 1H), 7.29(t, J=7.9 Hz, 2H), 7.01(d, J=8.1 Hz, 1H), 6.89(t, J=7.4 Hz, 1H), 4.37(s, 2H), 3.81(s, 3H) |
| 4f | 191—194 | 312.0579(312.0583) | 8.45(s, 1H), 7.24(t, J=8.0 Hz, 1H), 6.95(d, J=7.4 Hz, 2H), 6.84(ddd, J=8.2, 2.5, 1.0 Hz, 1H), 4.40(s, 2H), 3.73(s, 3H) |
| 4g | 211—213 | 312.0577(312.0583) | 8.45(s, 1H), 7.31(d, J=8.7 Hz, 2H), 6.88(d, J=8.7 Hz, 2H), 4.38(s, 2H), 3.73(s, 3H) |
| 4h | 200—202 | 296.0631(296.0634) | 8.48(s, 1H), 7.29(d, J=7.3 Hz, 1H), 7.20(d, J=3.7 Hz, 2H), 7.15(dd, J=11.0, 5.7 Hz, 1H), 4.44(s, 2H), 2.37(s, 3H) |
| 4i | 207—209 | 296.0632(296.0634) | 8.44(s, 1H), 7.23—7.15(m, 3H), 7.08(d, J=7.3 Hz, 1H), 4.39(s, 2H), 2.28(s, 3H) |
| 4j | 189—192 | 318.0450(318.0454) | 8.46(s, 1H), 7.27(d, J=8.0 Hz, 2H), 7.13(d, J=7.8 Hz, 2H), 4.39(s, 2H), 2.27(s, 3H) |
| 4k | 171—173 | 300.0377(300.0384) | 8.48(s, 1H), 7.43(t, J=7.7 Hz, 1H), 7.35(dt, J=13.6, 6.6 Hz, 2H), 7.24—7.13(m, 3H), 4.43(s, 2H) |
| 4l | 197—199 | 300.0377(300.0384) | 8.48(s, 1H), 7.46—7.41(m, 2H), 7.19—7.12(m, 2H), 4.44(s, 2H) |
| 4m | 188—190 | 307.0425(307.0430) | 8.51(s, 1H), 7.85(dd, J=7.7, 1.0 Hz, 1H), 7.69—7.65(m, 1H), 7.64—7.59(m, 1H), 7.49(m, 1H), 4.58(s, 2H) |
| 4n | 183—185 | 371.9517(371.9518) | 7.65(d, J=2.2 Hz, 1H), 7.48(d, J=8.3 Hz, 1H), 7.40(dd, J=8.3, 2.1 Hz, 1H), 4.47(s, 2H) |
| 4o | 209—211 | 228.9980(228.9984) | 8.46(s, 1H), 2.68(s, 3H) |
| Compd. | m. p./℃ | HRMS(calcd.)*, m/z | 1H NMR(DMSO, 400 MHz), δ |
| 4p | 206—209 | 220.0320(220.0321) | 8.46(s, 1H), 3.18(q, J=7.3 Hz, 2H), 1.33(t, J=7.3 Hz, 3H) |
| 4q | 190—192 | 234.0475(234.0478) | 8.46(s, 1H), 3.15(t, J=7.1 Hz, 2H), 1.76—1.63(m, 2H), 0.98(t, J=7.3 Hz, 3H) |
| 4r | 181—183 | 248.0631(248.0634) | 8.46(s, 1H), 3.17(t, J=7.3 Hz, 2H), 1.71—1.60(m, 2H), 1.46—1.40(m, 2H), 0.89(t, J=7.4 Hz, 3H) |
| 4s | 194—196 | 262.0784(262.0791) | 8.45(s, 1H), 3.16(t, J=7.3 Hz, 2H), 1.69—1.64(m, 2H), 1.43—1.24(m, 4H, CH2), 0.86(t, J=7.2 Hz, 3H) |
| 6 | 223—225 | 180.0210(180.0211) | 13.05(s, 1H), 9.99(s, 1H), 8.11—8.01(m, 1H), 7.45—7.38(m, 1H), 7.28—7.20(m, 2H) |
Table 2 Melting points, HRMS and 1H NMR data of compounds 3a—3s, 4a—4s and 6
| Compd. | m. p./℃ | HRMS(calcd.)*, m/z | 1H NMR(DMSO, 400 MHz), δ |
|---|---|---|---|
| 3a | 170—172 | 344.0517(344.0522) | 12.86(s, 1H), 7.48—7.38(m, 4H), 7.36—7.30(m, 3H), 7.29—7.25(m, 3H), 4.48(s, 2H) |
| 3b | 141—143 | 378.0130(378.0132) | 12.88(s, 1H), 7.52—7.47(m, 2H), 7.44(d, J=8.1 Hz, 2H), 7.36—7.25(m, 5H), 4.55(s, 2H) |
| 3c | 185—187 | 378.0122(378.0132) | 12.89(s, 1H), 7.50(d, J=1.4 Hz, 1H), 7.48—7.43(m, 2H), 7.40—7.30(m, 4H), 7.30—7.26(m, 2H), 4.49(s, 2H) |
| 3d | 172—174 | 378.0129(378.0132) | 12.88(s, 1H), 7.48—7.41(m, 4H), 7.41—7.36(m, 2H), 7.34—7.25(m, 3H), 4.48(s, 2H) |
| Compd. | m. p./℃ | HRMS(calcd.)*, m/z | 1H NMR(DMSO, 400 MHz), δ |
| 3e | 137—139 | 374.0620(374.0628) | 12.88(s, 1H), 7.45(t, J=7.7 Hz, 2H), 7.35—7.24(m, 5H), 7.01(d, J=8.1 Hz, 1H), 6.89(t, J=7.4 Hz, 1H), 4.41(s, 2H), 3.80(s, 3H) |
| 3f | 139—141 | 374.0620(374.0628) | 12.86(s, 1H), 7.45(t, J=7.9 Hz, 2H), 7.34—7.21(m, 4H), 6.98(t, J=5.6 Hz, 2H), 6.85(dd, J=8.2, 2.1 Hz, 1H), 4.45(s, 2H), 3.73(s, 3H) |
| 3g | 199—201 | 374.0619(374.0628) | 12.83(s, 1H), 7.48—7.42(m, 2H), 7.35—7.28(m, 5H), 7.28—7.24(m, 2H), 4.42(s, 2H), 3.73(s, 3H) |
| 3h | 137—139 | 358.0668(358.0678) | 12.84(s, 1H), 7.51—7.40(m, 2H), 7.33—7.25(m, 4H), 7.23—7.17(m, 2H), 7.16—7.10(m, 1H), 4.49(s, 2H), 2.37(s, 3H) |
| 3i | 152—154 | 358.0671(358.0678) | 12.88(s, 1H), 7.50—7.41(m, 2H), 7.33—7.25(m, 3H), 7.21(t, J=9.0, 2.7 Hz, 3H), 7.08(d, J=6.4 Hz, 1H), 4.44(s, 2H, CH2), 2.27(s, 3H, CH3) |
| 3j | 187—189 | 358.0669(358.0678) | 12.85(s, 1H), 7.45(t, J=7.8 Hz, 2H), 7.34—7.24(m, 5H), 7.13(d, J=7.6 Hz, 2H), 4.43(s, 2H), 2.27(s, 3H) |
| 3k | 144—146 | 362.0421(362.0428) | 12.90(s, 1H), 7.49—7.42(m, 3H), 7.38—7.25(m, 4H), 7.19—7.12(m, 2H), 4.48(s, 2H) |
| 3l | 161—163 | 362.0423(362.0428) | 12.86(s, 1H), 7.48—7.42(m, 4H), 7.34—7.25(m, 3H), 7.23—7.12(m, 2H), 4.48(s, 2H) |
| 3m | 120—122 | 369.0467(369.0474) | 12.90(s, 1H), 7.85(dd, J=7.7, 0.9 Hz, 1H), 7.70—7.61(m, 2H), 7.52—7.42(m, 3H), 7.34—7.25(m, 3H), 4.62(s, 2H) |
| 3n | 162—164 | 411.9735(411.9743) | 12.92(s, 1H), 7.63(d, J=12.1 Hz, 1H), 7.53(d, J=8.3 Hz, 1H), 7.46(t, J=7.0 Hz, 2H), 7.39(t, J=7.8 Hz, 1H), 7.30(dd, J=16.2, 8.3 Hz, 3H), 4.53(s, 2H) |
| 3o | 159—161 | 268.0204(268.0209) | 12.85(s, 1H), 7.45(t, J=7.6 Hz, 2H), 7.30(dd, J=16.4, 7.1 Hz, 3H), 2.71(s, 3H) |
| 3p | 206—209 | 282.0361(282.0365) | 8.46(s, 1H), 3.18(q, J=7.3 Hz, 2H), 1.33(t, J=7.3 Hz, 3H) |
| 3q | 190—192 | 296.0518(296.0522) | 8.46(s, 1H), 3.15(t, J=7.1 Hz, 2H), 1.76—1.63(m, 2H), 0.98(t, J=7.3 Hz, 3H) |
| 3r | 181—183 | 310.0669(310.0678) | 8.46(s, 1H), 3.17(t, J=7.3 Hz, 2H), 1.71—1.60(m, 2H), 1.46—1.35(m, 2H), 0.89(t, J=7.4 Hz, 3H) |
| 3s | 108—110 | 324.0826(324.0835) | 12.86(s, 1H), 7.49—7.42(m, 2H), 7.34—7.25(m, 3H), 3.20(t, J=14.6 Hz, 2H), 1.77—1.61(m, 2H), 1.42—1.24(m, 4H), 0.86(t, J=7.2 Hz, 3H) |
| 4a | 196—198 | 282.0469(282.0478) | 8.45(s, 1H), 7.41—7.37(m, 2H), 7.35—7.30(m, 2H), 7.29—7.23(m, 1H), 4.43(s, 2H) |
| 4b | 195—197 | 337.9907(337.9908) | 8.51(s, 1H), 7.51—7.46(m, 2H), 7.37—7.27(m, 2H), 4.51(s, 2H) |
| 4c | 185—187 | 316.0082(316.0088) | 8.45(s, 1H), 7.47(s, 1H), 7.38—7.30(m, 3H), 4.44(s, 2H) |
| 4d | 212—213 | 337.9905(337.9908) | 8.46(s, 1H), 7.43—7.36(m, 4H), 4.43(s, 2H) |
| 4e | 201—203 | 312.0577(312.0583) | 8.49(s, 1H), 7.29(t, J=7.9 Hz, 2H), 7.01(d, J=8.1 Hz, 1H), 6.89(t, J=7.4 Hz, 1H), 4.37(s, 2H), 3.81(s, 3H) |
| 4f | 191—194 | 312.0579(312.0583) | 8.45(s, 1H), 7.24(t, J=8.0 Hz, 1H), 6.95(d, J=7.4 Hz, 2H), 6.84(ddd, J=8.2, 2.5, 1.0 Hz, 1H), 4.40(s, 2H), 3.73(s, 3H) |
| 4g | 211—213 | 312.0577(312.0583) | 8.45(s, 1H), 7.31(d, J=8.7 Hz, 2H), 6.88(d, J=8.7 Hz, 2H), 4.38(s, 2H), 3.73(s, 3H) |
| 4h | 200—202 | 296.0631(296.0634) | 8.48(s, 1H), 7.29(d, J=7.3 Hz, 1H), 7.20(d, J=3.7 Hz, 2H), 7.15(dd, J=11.0, 5.7 Hz, 1H), 4.44(s, 2H), 2.37(s, 3H) |
| 4i | 207—209 | 296.0632(296.0634) | 8.44(s, 1H), 7.23—7.15(m, 3H), 7.08(d, J=7.3 Hz, 1H), 4.39(s, 2H), 2.28(s, 3H) |
| 4j | 189—192 | 318.0450(318.0454) | 8.46(s, 1H), 7.27(d, J=8.0 Hz, 2H), 7.13(d, J=7.8 Hz, 2H), 4.39(s, 2H), 2.27(s, 3H) |
| 4k | 171—173 | 300.0377(300.0384) | 8.48(s, 1H), 7.43(t, J=7.7 Hz, 1H), 7.35(dt, J=13.6, 6.6 Hz, 2H), 7.24—7.13(m, 3H), 4.43(s, 2H) |
| 4l | 197—199 | 300.0377(300.0384) | 8.48(s, 1H), 7.46—7.41(m, 2H), 7.19—7.12(m, 2H), 4.44(s, 2H) |
| 4m | 188—190 | 307.0425(307.0430) | 8.51(s, 1H), 7.85(dd, J=7.7, 1.0 Hz, 1H), 7.69—7.65(m, 1H), 7.64—7.59(m, 1H), 7.49(m, 1H), 4.58(s, 2H) |
| 4n | 183—185 | 371.9517(371.9518) | 7.65(d, J=2.2 Hz, 1H), 7.48(d, J=8.3 Hz, 1H), 7.40(dd, J=8.3, 2.1 Hz, 1H), 4.47(s, 2H) |
| 4o | 209—211 | 228.9980(228.9984) | 8.46(s, 1H), 2.68(s, 3H) |
| Compd. | m. p./℃ | HRMS(calcd.)*, m/z | 1H NMR(DMSO, 400 MHz), δ |
| 4p | 206—209 | 220.0320(220.0321) | 8.46(s, 1H), 3.18(q, J=7.3 Hz, 2H), 1.33(t, J=7.3 Hz, 3H) |
| 4q | 190—192 | 234.0475(234.0478) | 8.46(s, 1H), 3.15(t, J=7.1 Hz, 2H), 1.76—1.63(m, 2H), 0.98(t, J=7.3 Hz, 3H) |
| 4r | 181—183 | 248.0631(248.0634) | 8.46(s, 1H), 3.17(t, J=7.3 Hz, 2H), 1.71—1.60(m, 2H), 1.46—1.40(m, 2H), 0.89(t, J=7.4 Hz, 3H) |
| 4s | 194—196 | 262.0784(262.0791) | 8.45(s, 1H), 3.16(t, J=7.3 Hz, 2H), 1.69—1.64(m, 2H), 1.43—1.24(m, 4H, CH2), 0.86(t, J=7.2 Hz, 3H) |
| 6 | 223—225 | 180.0210(180.0211) | 13.05(s, 1H), 9.99(s, 1H), 8.11—8.01(m, 1H), 7.45—7.38(m, 1H), 7.28—7.20(m, 2H) |
| Compd. | Appearance | Yield(%) | m. p. /℃ | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 7a | White solid | 85 | 189—191 | 396.0940(396.0947) |
| 7b | White solid | 83 | 180—181 | 430.0548(430.0558) |
| 7c | White solid | 78 | 172—173 | 430.0547(430.0558) |
| 7d | White solid | 74 | 200—201 | 430.0549(430.0558) |
| 7e | White solid | 83 | 173—175 | 426.1042(426.1053) |
| 7f | White solid | 81 | 159—161 | 426.1042(426.1053) |
| 7g | White solid | 76 | 177—178 | 426.1043(426.1053) |
| 7h | White solid | 90 | 172—174 | 410.1097(410.1104) |
| 7i | White solid | 89 | 171—173 | 410.1099(410.1104) |
| 7j | White solid | 70 | 173—175 | 410.1101(410.1104) |
| 7k | White solid | 84 | 167—168 | 414.0841(414.0853) |
| 7l | White solid | 79 | 210—211 | 414.0843(414.0853) |
| 7m | Yellow solid | 86 | 165—167 | 421.0889(421.0899) |
| 7n | Yellow solid | 79 | 185—187 | 464.0161(464.0168) |
| 7o | Yellow solid | 77 | 189—191 | 320.0641(320.0634) |
| 7p | Yellow solid | 83 | 197—199 | 334.0784(334.0791) |
| 7q | Yellow solid | 82 | 184—186 | 348.0938(348.0947) |
| 7r | Yellow solid | 81 | 138—140 | 362.1095(362.1104) |
| 7s | Yellow solid | 48 | 139—141 | 376.1250(376.1260) |
Table 3 Appearance, yields, melting points and HRMS data for compounds 7a—7s
| Compd. | Appearance | Yield(%) | m. p. /℃ | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 7a | White solid | 85 | 189—191 | 396.0940(396.0947) |
| 7b | White solid | 83 | 180—181 | 430.0548(430.0558) |
| 7c | White solid | 78 | 172—173 | 430.0547(430.0558) |
| 7d | White solid | 74 | 200—201 | 430.0549(430.0558) |
| 7e | White solid | 83 | 173—175 | 426.1042(426.1053) |
| 7f | White solid | 81 | 159—161 | 426.1042(426.1053) |
| 7g | White solid | 76 | 177—178 | 426.1043(426.1053) |
| 7h | White solid | 90 | 172—174 | 410.1097(410.1104) |
| 7i | White solid | 89 | 171—173 | 410.1099(410.1104) |
| 7j | White solid | 70 | 173—175 | 410.1101(410.1104) |
| 7k | White solid | 84 | 167—168 | 414.0841(414.0853) |
| 7l | White solid | 79 | 210—211 | 414.0843(414.0853) |
| 7m | Yellow solid | 86 | 165—167 | 421.0889(421.0899) |
| 7n | Yellow solid | 79 | 185—187 | 464.0161(464.0168) |
| 7o | Yellow solid | 77 | 189—191 | 320.0641(320.0634) |
| 7p | Yellow solid | 83 | 197—199 | 334.0784(334.0791) |
| 7q | Yellow solid | 82 | 184—186 | 348.0938(348.0947) |
| 7r | Yellow solid | 81 | 138—140 | 362.1095(362.1104) |
| 7s | Yellow solid | 48 | 139—141 | 376.1250(376.1260) |
| Compd. | 1H NMR(DMSO, 400 MHz), δ | 13C NMR(DMSO, 100 MHz), δ |
|---|---|---|
| 7a | 11.17(d, J=127.5 Hz, 2H), 7.85(d, J=8.0 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.41(dt, J=7.5, 3.9 Hz, 4H), 7.38—7.31(m, 3H), 7.30—7.24(m, 1H), 7.08—6.95(m, 2H), 4.48(s, 2H) | 160.8,158.0, 152.6, 146.2, 138.9,137.2, 136.4, 129.4, 129.0, 128.0, 127.4, 125.6, 38.1 |
| 7b | 11.23(d, J=129.6 Hz, 2H), 7.87(d, J=7.4 Hz, 1H), 7.58(d, J=7.4 Hz, 2H), 7.53—7.48(m, 2H), 7.41(t, J=7.4 Hz, 2H), 7.33(ddd, J=12.3, 6.0, 2.3 Hz, 3H), 7.08—6.97(m, 2H), 4.55(s, 2H) | 161.2, 157.1, 152.5, 146.2, 138.9, 136.3, 134.7, 133.8, 131.9, 130.1, 129.3, 127.8, 127.4, 125.6, 36.3 |
| 7c | 11.22(d, J=118.1 Hz, 2H), 7.87(d, J=7.3 Hz, 1H), 7.58(d, J=7.4 Hz, 2H), 7.50(s, 1H), 7.43—7.33(m, 6H), 7.08—6.95(m, 2H), 4.49(s, 2H) | 161.0, 157.4, 152.5, 146.1, 140.1, 138.9, 136.3, 133.5, 130.8, 129.3, 128.2, 127.9, 127.4, 125.6, 37.2 |
| Compd. | 1H NMR(DMSO, 400 MHz), δ | 13C NMR(DMSO, 100 MHz), δ |
| 7d | 11.20(d, J=130.9 Hz, 2H), 7.86(d, J=7.9 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.42(td, J=8.4, 1.6 Hz, 6H), 7.34(d, J=7.2 Hz, 1H), 7.08—6.95(m, 2H), 4.48(s, 2H) | 160.9, 157.5, 152.5, 146.1, 138.9, 136.6, 136.3, 132.6, 131.3, 129.3, 129.0, 127.4, 125.6, 37.2 |
| 7e | 11.16(d, J=129.2 Hz, 2H), 7.85(d, J=7.5 Hz, 1H), 7.58(d, J=6.9 Hz, 2H), 7.41(t, J=7.4 Hz, 2H), 7.37—7.24(m, 3H), 7.09—6.95(m, 3H), 6.93—6.87(m, 1H), 4.40(s, 2H), 3.81(s, 3H) | 160.9, 158.3, 157.6, 152.6, 146.1, 138.8, 136.4, 130.8, 129.8, 129.3, 127.4, 125.6, 124.8, 120.7, 111.6, 56.0, 33.6 |
| 7f | 11.23(d, J=139.4 Hz, 2H), 7.87(d, J=6.5 Hz, 1H), 7.58(d, J=6.7 Hz, 2H), 7.37(dd, J=21.8, 6.6 Hz, 3H), 7.25(t, J=7.4 Hz, 1H), 7.00(t, J=10.5 Hz, 4H), 6.86(d, J=7.3 Hz, 1H), 4.45(s, 2H), 3.74(s, 3H) | 160.9, 159.7, 157.9, 152.5, 146.2, 138.8, 138.7, 136.4, 130.1, 129.3, 129.3, 127.4, 125.6, 121.6, 115.1, 113.5, 55.5, 38.1 |
| 7g | 11.22(d, J=128.7 Hz, 2H), 7.86(d, J=7.7 Hz, 1H), 7.57(d, J=7.4 Hz, 1H), 7.47—7.37(m, 2H), 7.32(t, J=8.4 Hz, 3H), 7.08—6.96(m, 2H), 6.89(dd, J=7.9, 4.2 Hz, 3H), 4.38(s, 2H), 3.73(s, 3H). | 160.8, 159.1, 156.1, 152.5, 146.2, 138.7, 136.4, 130.8, 129.4, 127.4, 125.6, 114.4, 55.5, 37.8 |
| 7h | 11.21(d, J=136.7 Hz, 2H), 7.86(d, J=6.9 Hz, 1H), 7.56(d, J=7.4 Hz, 2H), 7.39(t, J=7.4 Hz, 2H), 7.31(dd, J=9.8, 7.4 Hz, 2H), 7.22—7.10(m, 3H), 7.06—6.94(m, 2H), 4.47(s, 2H), 2.37(s, 3H) | 160.9, 157.7, 152.6, 146.1, 138.8, 137.2, 136.4, 134.6, 129.4, 129.3, 128.4, 127.4, 126.5, 125.6, 36.7, 19.2 |
| 7i | 11.20(d, J=128.1 Hz, 2H), 7.86(d, J=7.4 Hz, 1H), 7.57(d, J=7.2 Hz, 2H), 7.41(t, J=7.4 Hz, 2H), 7.33(t, J=7.3 Hz, 1H), 7.25—7.18(m, 3H), 7.12—7.05(m, 1H), 7.04—6.95(m, 2H), 4.44(s, 2H), 2.28(s, 3H) | 160.8, 158.0, 152.6, 146.1, 138.8, 138.2, 137.0, 136.3, 130.1, 129.4, 129.3, 128.9, 128.7, 127.4, 126.6, 125.6, 38.1, 21.4 |
| 7j | 11.17(d, J=134.6 Hz, 2H), 7.85(d, J=7.4 Hz, 1H), 7.56(d, J=7.1 Hz, 2H), 7.40(t, J=7.2 Hz, 2H), 7.31(dd, J=20.4, 7.4 Hz, 3H), 7.13(d, J=7.5 Hz, 2H), 7.09—6.93(m, 2H), 4.42(s, 2H), 2.27(s, 3H) | 160.9, 157.7, 152.6, 146.1, 130.9, 130.4, 129.3, 128.4, 127.4, 126.5, 125.6, 36.7, 19.2 |
| 7k | 11.22(d, J=107.8 Hz, 2H), 7.85(d, J=7.3 Hz, 1H), 7.56(d, J=7.3 Hz, 2H), 7.45(td, J=7.7, 1.6 Hz, 1H), 7.39(t, J=7.5 Hz, 2H), 7.34(dt, J=5.2, 2.0 Hz, 2H), 7.24—7.12(m, 2H), 7.06—6.95(m, 2H), 4.47(s, 2H) | 160.9, 161.2, 157.0, 152.6, 146.2, 138.9, 136.3, 131.8, 130.4, 129.3, 127.4, 125.6, 125.0, 124.3, 116.1, 115.9, 32.0 |
| 7l | 11.20(d, J=138.6 Hz, 2H), 7.87(d, J=7.5 Hz, 1H), 7.58(d, J=7.5 Hz, 2H), 7.50—7.44(m, 2H), 7.41(t, J=7.5 Hz, 2H), 7.35(d, J=7.2 Hz, 1H), 7.17(t, J=8.8 Hz, 2H), 7.08—6.95(m, 2H), 4.48(s, 2H) | 163.2, 160.8, 157.6, 152.6, 146.1, 138.8, 136.3, 133.6, 131.5, 129.3, 127.4, 125.6, 115.9, 115.7, 37.3 |
| 7m | 11.22(d, J=108.4 Hz, 2H), 7.86(dd, J=4.5, 3.6 Hz, 2H), 7.70—7.61(m, 2H), 7.57(d, J=7.3 Hz, 2H), 7.50(td, J=7.5, 1.4 Hz, 1H), 7.41(t, J=7.4 Hz, 2H), 7.34(d, J=7.2 Hz, 1H), 7.10—6.95(m, 2H), 4.61(s, 2H) | 161.5, 156.2, 152.5, 146.2, 140.9, 138.9, 136.3, 133.8, 131.0, 129.3, 129.0, 127.4, 125.6, 117.7, 112.3, 36.7 |
| 7n | 11.23(d, J=113.3 Hz, 2H), 7.86(d, J=8.0 Hz, 1H), 7.66(d, J=2.1 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.52(d, J=8.3 Hz, 1H), 7.44—7.37(m, 3H), 7.35(d, J=7.2 Hz, 1H), 7.08—6.95(m, 2H), 4.52(s, 2H) | 161.3, 156.7, 152.6, 146.1, 138.9, 136.3, 134.8, 134.1, 133.7, 133.1, 129.5, 129.3, 128.0, 127.4, 125.6, 35.7 |
| 7o | 11.21(d, J=147.4 Hz, 2H), 7.87(d, J=7.3 Hz, 1H), 7.58(d, J=7.4 Hz, 2H), 7.42(dt, J=14.7, 7.3 Hz, 2H), 7.38—7.23(m, 1H), 7.18—6.93(m, 2H), 2.71(s, 3H) | 160.1, 158.6, 152.6, 146.0, 138.8, 136.4, 129.4, 129.3 127.4, 125.6, 16.4 |
| 7p | 11.18(d, J=144.3 Hz, 2H), 7.86(d, J=7.6 Hz, 1H), 7.58(d, J=7.3 Hz, 2H), 7.41(t, J=7.4 Hz, 2H), 7.34(d, J=7.2 Hz, 1H), 7.09—6.95(m, 2H), 3.21(q, J=7.3 Hz, 2H), 1.35(t, J=7.3 Hz, 3H) | 160.4, 158.4, 152.6, 146.0, 138.8, 136.4, 129.3, 127.4, 125.6, 28.5, 15.3 |
| 7q | 11.20(d, J=148.3 Hz, 2H), 7.87(d, J=7.4 Hz, 1H), 7.58(d, J=7.7 Hz, 2H), 7.41(t, J=7.5 Hz, 2H), 7.34(d, J=7.3 Hz, 1H), 7.08—6.95(m, 2H), 3.18(t, J=7.1 Hz, 2H), 1.78—1.65(m, 2H), 0.99(t, J=7.3 Hz, 3H) | 160.5, 158.6, 152.6, 146.1, 138.8, 136.5, 129.3, 127.4, 125.6, 36.0, 22.9, 13.4 |
| 7r | 11.16(d, J=141.1 Hz, 2H), 7.85(d, J=7.7 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.42—7.39(m, 2H), 7.34(d, J=7.3 Hz, 1H), 7.08—6.96(m, 2H), 3.20(t, J=7.3 Hz, 2H), 1.67(dt, J=14.8, 7.4 Hz, 2H), 1.41(dq, J=14.6, 7.3 Hz, 2H), 0.90(t, J=7.4 Hz, 3H) | 160.4, 158.7, 152.5, 146.1, 138.8, 136.4, 129.4, 127.4, 15.8, 33.8, 31.5, 21.6, 13.9 |
| 7s | 11.20(d, J=145.6 Hz, 2H), 7.86(d, J=7.5 Hz, 1H), 7.58(d, J=7.2 Hz, 2H), 7.44—7.38(m, 3H), 7.08—6.96(m, 2H), 3.19(t, J=7.3 Hz, 2H), 1.74—1.63(m, 2H), 1.41—1.25(m, 4H), 0.87(t, J=7.2 Hz, 3H) | 160.4, 158.7, 152.6, 146.1, 138.8, 129.3, 128.0, 127.4, 125.7, 125.6, 34.0, 30.6, 29.1, 22.1, 14.3 |
Table 4 1H NMR and 13C NMR data of compounds 7a—7s
| Compd. | 1H NMR(DMSO, 400 MHz), δ | 13C NMR(DMSO, 100 MHz), δ |
|---|---|---|
| 7a | 11.17(d, J=127.5 Hz, 2H), 7.85(d, J=8.0 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.41(dt, J=7.5, 3.9 Hz, 4H), 7.38—7.31(m, 3H), 7.30—7.24(m, 1H), 7.08—6.95(m, 2H), 4.48(s, 2H) | 160.8,158.0, 152.6, 146.2, 138.9,137.2, 136.4, 129.4, 129.0, 128.0, 127.4, 125.6, 38.1 |
| 7b | 11.23(d, J=129.6 Hz, 2H), 7.87(d, J=7.4 Hz, 1H), 7.58(d, J=7.4 Hz, 2H), 7.53—7.48(m, 2H), 7.41(t, J=7.4 Hz, 2H), 7.33(ddd, J=12.3, 6.0, 2.3 Hz, 3H), 7.08—6.97(m, 2H), 4.55(s, 2H) | 161.2, 157.1, 152.5, 146.2, 138.9, 136.3, 134.7, 133.8, 131.9, 130.1, 129.3, 127.8, 127.4, 125.6, 36.3 |
| 7c | 11.22(d, J=118.1 Hz, 2H), 7.87(d, J=7.3 Hz, 1H), 7.58(d, J=7.4 Hz, 2H), 7.50(s, 1H), 7.43—7.33(m, 6H), 7.08—6.95(m, 2H), 4.49(s, 2H) | 161.0, 157.4, 152.5, 146.1, 140.1, 138.9, 136.3, 133.5, 130.8, 129.3, 128.2, 127.9, 127.4, 125.6, 37.2 |
| Compd. | 1H NMR(DMSO, 400 MHz), δ | 13C NMR(DMSO, 100 MHz), δ |
| 7d | 11.20(d, J=130.9 Hz, 2H), 7.86(d, J=7.9 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.42(td, J=8.4, 1.6 Hz, 6H), 7.34(d, J=7.2 Hz, 1H), 7.08—6.95(m, 2H), 4.48(s, 2H) | 160.9, 157.5, 152.5, 146.1, 138.9, 136.6, 136.3, 132.6, 131.3, 129.3, 129.0, 127.4, 125.6, 37.2 |
| 7e | 11.16(d, J=129.2 Hz, 2H), 7.85(d, J=7.5 Hz, 1H), 7.58(d, J=6.9 Hz, 2H), 7.41(t, J=7.4 Hz, 2H), 7.37—7.24(m, 3H), 7.09—6.95(m, 3H), 6.93—6.87(m, 1H), 4.40(s, 2H), 3.81(s, 3H) | 160.9, 158.3, 157.6, 152.6, 146.1, 138.8, 136.4, 130.8, 129.8, 129.3, 127.4, 125.6, 124.8, 120.7, 111.6, 56.0, 33.6 |
| 7f | 11.23(d, J=139.4 Hz, 2H), 7.87(d, J=6.5 Hz, 1H), 7.58(d, J=6.7 Hz, 2H), 7.37(dd, J=21.8, 6.6 Hz, 3H), 7.25(t, J=7.4 Hz, 1H), 7.00(t, J=10.5 Hz, 4H), 6.86(d, J=7.3 Hz, 1H), 4.45(s, 2H), 3.74(s, 3H) | 160.9, 159.7, 157.9, 152.5, 146.2, 138.8, 138.7, 136.4, 130.1, 129.3, 129.3, 127.4, 125.6, 121.6, 115.1, 113.5, 55.5, 38.1 |
| 7g | 11.22(d, J=128.7 Hz, 2H), 7.86(d, J=7.7 Hz, 1H), 7.57(d, J=7.4 Hz, 1H), 7.47—7.37(m, 2H), 7.32(t, J=8.4 Hz, 3H), 7.08—6.96(m, 2H), 6.89(dd, J=7.9, 4.2 Hz, 3H), 4.38(s, 2H), 3.73(s, 3H). | 160.8, 159.1, 156.1, 152.5, 146.2, 138.7, 136.4, 130.8, 129.4, 127.4, 125.6, 114.4, 55.5, 37.8 |
| 7h | 11.21(d, J=136.7 Hz, 2H), 7.86(d, J=6.9 Hz, 1H), 7.56(d, J=7.4 Hz, 2H), 7.39(t, J=7.4 Hz, 2H), 7.31(dd, J=9.8, 7.4 Hz, 2H), 7.22—7.10(m, 3H), 7.06—6.94(m, 2H), 4.47(s, 2H), 2.37(s, 3H) | 160.9, 157.7, 152.6, 146.1, 138.8, 137.2, 136.4, 134.6, 129.4, 129.3, 128.4, 127.4, 126.5, 125.6, 36.7, 19.2 |
| 7i | 11.20(d, J=128.1 Hz, 2H), 7.86(d, J=7.4 Hz, 1H), 7.57(d, J=7.2 Hz, 2H), 7.41(t, J=7.4 Hz, 2H), 7.33(t, J=7.3 Hz, 1H), 7.25—7.18(m, 3H), 7.12—7.05(m, 1H), 7.04—6.95(m, 2H), 4.44(s, 2H), 2.28(s, 3H) | 160.8, 158.0, 152.6, 146.1, 138.8, 138.2, 137.0, 136.3, 130.1, 129.4, 129.3, 128.9, 128.7, 127.4, 126.6, 125.6, 38.1, 21.4 |
| 7j | 11.17(d, J=134.6 Hz, 2H), 7.85(d, J=7.4 Hz, 1H), 7.56(d, J=7.1 Hz, 2H), 7.40(t, J=7.2 Hz, 2H), 7.31(dd, J=20.4, 7.4 Hz, 3H), 7.13(d, J=7.5 Hz, 2H), 7.09—6.93(m, 2H), 4.42(s, 2H), 2.27(s, 3H) | 160.9, 157.7, 152.6, 146.1, 130.9, 130.4, 129.3, 128.4, 127.4, 126.5, 125.6, 36.7, 19.2 |
| 7k | 11.22(d, J=107.8 Hz, 2H), 7.85(d, J=7.3 Hz, 1H), 7.56(d, J=7.3 Hz, 2H), 7.45(td, J=7.7, 1.6 Hz, 1H), 7.39(t, J=7.5 Hz, 2H), 7.34(dt, J=5.2, 2.0 Hz, 2H), 7.24—7.12(m, 2H), 7.06—6.95(m, 2H), 4.47(s, 2H) | 160.9, 161.2, 157.0, 152.6, 146.2, 138.9, 136.3, 131.8, 130.4, 129.3, 127.4, 125.6, 125.0, 124.3, 116.1, 115.9, 32.0 |
| 7l | 11.20(d, J=138.6 Hz, 2H), 7.87(d, J=7.5 Hz, 1H), 7.58(d, J=7.5 Hz, 2H), 7.50—7.44(m, 2H), 7.41(t, J=7.5 Hz, 2H), 7.35(d, J=7.2 Hz, 1H), 7.17(t, J=8.8 Hz, 2H), 7.08—6.95(m, 2H), 4.48(s, 2H) | 163.2, 160.8, 157.6, 152.6, 146.1, 138.8, 136.3, 133.6, 131.5, 129.3, 127.4, 125.6, 115.9, 115.7, 37.3 |
| 7m | 11.22(d, J=108.4 Hz, 2H), 7.86(dd, J=4.5, 3.6 Hz, 2H), 7.70—7.61(m, 2H), 7.57(d, J=7.3 Hz, 2H), 7.50(td, J=7.5, 1.4 Hz, 1H), 7.41(t, J=7.4 Hz, 2H), 7.34(d, J=7.2 Hz, 1H), 7.10—6.95(m, 2H), 4.61(s, 2H) | 161.5, 156.2, 152.5, 146.2, 140.9, 138.9, 136.3, 133.8, 131.0, 129.3, 129.0, 127.4, 125.6, 117.7, 112.3, 36.7 |
| 7n | 11.23(d, J=113.3 Hz, 2H), 7.86(d, J=8.0 Hz, 1H), 7.66(d, J=2.1 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.52(d, J=8.3 Hz, 1H), 7.44—7.37(m, 3H), 7.35(d, J=7.2 Hz, 1H), 7.08—6.95(m, 2H), 4.52(s, 2H) | 161.3, 156.7, 152.6, 146.1, 138.9, 136.3, 134.8, 134.1, 133.7, 133.1, 129.5, 129.3, 128.0, 127.4, 125.6, 35.7 |
| 7o | 11.21(d, J=147.4 Hz, 2H), 7.87(d, J=7.3 Hz, 1H), 7.58(d, J=7.4 Hz, 2H), 7.42(dt, J=14.7, 7.3 Hz, 2H), 7.38—7.23(m, 1H), 7.18—6.93(m, 2H), 2.71(s, 3H) | 160.1, 158.6, 152.6, 146.0, 138.8, 136.4, 129.4, 129.3 127.4, 125.6, 16.4 |
| 7p | 11.18(d, J=144.3 Hz, 2H), 7.86(d, J=7.6 Hz, 1H), 7.58(d, J=7.3 Hz, 2H), 7.41(t, J=7.4 Hz, 2H), 7.34(d, J=7.2 Hz, 1H), 7.09—6.95(m, 2H), 3.21(q, J=7.3 Hz, 2H), 1.35(t, J=7.3 Hz, 3H) | 160.4, 158.4, 152.6, 146.0, 138.8, 136.4, 129.3, 127.4, 125.6, 28.5, 15.3 |
| 7q | 11.20(d, J=148.3 Hz, 2H), 7.87(d, J=7.4 Hz, 1H), 7.58(d, J=7.7 Hz, 2H), 7.41(t, J=7.5 Hz, 2H), 7.34(d, J=7.3 Hz, 1H), 7.08—6.95(m, 2H), 3.18(t, J=7.1 Hz, 2H), 1.78—1.65(m, 2H), 0.99(t, J=7.3 Hz, 3H) | 160.5, 158.6, 152.6, 146.1, 138.8, 136.5, 129.3, 127.4, 125.6, 36.0, 22.9, 13.4 |
| 7r | 11.16(d, J=141.1 Hz, 2H), 7.85(d, J=7.7 Hz, 1H), 7.57(d, J=7.3 Hz, 2H), 7.42—7.39(m, 2H), 7.34(d, J=7.3 Hz, 1H), 7.08—6.96(m, 2H), 3.20(t, J=7.3 Hz, 2H), 1.67(dt, J=14.8, 7.4 Hz, 2H), 1.41(dq, J=14.6, 7.3 Hz, 2H), 0.90(t, J=7.4 Hz, 3H) | 160.4, 158.7, 152.5, 146.1, 138.8, 136.4, 129.4, 127.4, 15.8, 33.8, 31.5, 21.6, 13.9 |
| 7s | 11.20(d, J=145.6 Hz, 2H), 7.86(d, J=7.5 Hz, 1H), 7.58(d, J=7.2 Hz, 2H), 7.44—7.38(m, 3H), 7.08—6.96(m, 2H), 3.19(t, J=7.3 Hz, 2H), 1.74—1.63(m, 2H), 1.41—1.25(m, 4H), 0.87(t, J=7.2 Hz, 3H) | 160.4, 158.7, 152.6, 146.1, 138.8, 129.3, 128.0, 127.4, 125.7, 125.6, 34.0, 30.6, 29.1, 22.1, 14.3 |
| Compd. | Inhibition rate(%) | |||||
|---|---|---|---|---|---|---|
| X. oryzae | R. solanacearum | X. citri | ||||
| 200 μg/mL | 100 μg/mL | 200 μg/mL | 100 μg/mL | 200 μg/mL | 100 μg/mL | |
| 7a | 81.02±1.15 | 72.38±0.35 | 90.78±0.47 | 61.56±0.38 | 61.72±2.45 | 36.35±0.32 |
| 7b | 88.34±0.65 | 67.62±1.36 | 71.35±4.43 | 57.32±0.58 | 46.26±1.25 | 28.11±2.23 |
| 7c | 72.13±1.25 | 47.04±1.21 | 58.63±1.19 | 31.25±1.28 | 58.47±0.57 | 39.04±0.92 |
| 7d | 81.56±0.11 | 59.92±0.45 | 66.27±0.66 | 51.33±0.58 | 41.35±6.34 | 19.06±0.35 |
| 7e | 90.00±0.23 | 82.11±1.32 | 91.92±1.03 | 78.83±1.24 | 89.62±2.15 | 72.37±7.14 |
| 7f | 74.56±0.12 | 43.22±0.72 | 69.33±1.11 | 56.46±0.26 | 66.25±1.02 | 34.32±0.15 |
| 7g | 96.00±0.33 | 73.11±0.26 | 93.22±0.66 | 88.17±0.15 | 75.11±0.24 | 41.31±0.44 |
| 7h | 92.12±0.15 | 80.05±0.65 | 88.13±1.15 | 62.05±0.45 | 79.63±0.65 | 58.59±6.42 |
| 7i | 100.03±0.24 | 100.17±1.44 | 91.03±8.24 | 79.82±4.06 | 87.25±6.44 | 66.93±1.43 |
| 7j | 100.82±0.75 | 100.08±1.02 | 100.18±5.45 | 100.25±8.15 | 100.06±8.25 | 100.32±4.36 |
| 7k | 100.02±1.16 | 100.15±1.21 | 100.34±2.83 | 100.01±6.54 | 100.25±1.93 | 100.17±8.04 |
| 7l | 54.13±0.61 | 39.57±2.35 | 81.03±8.20 | 57.24±0.26 | 100.02±2.92 | 100.28±3.15 |
| 7m | 62.78±0.72 | 39.52±1.22 | 82.93±4.21 | 64.82±5.42 | 66.46±9.01 | 47.82±4.33 |
| 7n | 100.33±0.43 | 100.01±1.15 | 100.32±0.14 | 100.35±5.00 | 62.39±6.34 | 35.52±0.25 |
| 7o | 71.22±0.15 | 42.38±0.55 | 60.88±0.37 | 31.66±1.38 | 62.52±1.45 | 31.35±0.22 |
| 7p | 56.04±1.25 | 39.88±0.75 | 100.09±4.34 | 100.62±4.13 | 59.77±2.36 | 38.36±9.23 |
| 7q | 67.26±0.55 | 59.65±2.42 | 100.26±5.82 | 100.35±6.74 | 100.05±3.11 | 100.14±2.17 |
| 7r | 100.06±1.66 | 100.27±1.03 | 100.02±0.32 | 100.52±2.11 | 63.22±0.23 | 39.37±0.11 |
| 7s | 61.11±0.34 | 42.66±2.16 | 100.61±1.23 | 100.86±8.07 | 67.42±0.14 | 48.73±8.14 |
| Bismerthiazolb | 74.25±0.45 | 53.11±0.65 | 100.26±1.17 | 100.02±4.53 | 70.22±6.23 | 48.85±7.42 |
| Thiodiazole-copperb | 70.17±1.35 | 41.56 ±1.01 | 55.63±1.20 | 41.32±5.39 | 63.51±8.15 | 44.27±3.46 |
Table 5 Inhibition rates of the title compounds 7a—7pa
| Compd. | Inhibition rate(%) | |||||
|---|---|---|---|---|---|---|
| X. oryzae | R. solanacearum | X. citri | ||||
| 200 μg/mL | 100 μg/mL | 200 μg/mL | 100 μg/mL | 200 μg/mL | 100 μg/mL | |
| 7a | 81.02±1.15 | 72.38±0.35 | 90.78±0.47 | 61.56±0.38 | 61.72±2.45 | 36.35±0.32 |
| 7b | 88.34±0.65 | 67.62±1.36 | 71.35±4.43 | 57.32±0.58 | 46.26±1.25 | 28.11±2.23 |
| 7c | 72.13±1.25 | 47.04±1.21 | 58.63±1.19 | 31.25±1.28 | 58.47±0.57 | 39.04±0.92 |
| 7d | 81.56±0.11 | 59.92±0.45 | 66.27±0.66 | 51.33±0.58 | 41.35±6.34 | 19.06±0.35 |
| 7e | 90.00±0.23 | 82.11±1.32 | 91.92±1.03 | 78.83±1.24 | 89.62±2.15 | 72.37±7.14 |
| 7f | 74.56±0.12 | 43.22±0.72 | 69.33±1.11 | 56.46±0.26 | 66.25±1.02 | 34.32±0.15 |
| 7g | 96.00±0.33 | 73.11±0.26 | 93.22±0.66 | 88.17±0.15 | 75.11±0.24 | 41.31±0.44 |
| 7h | 92.12±0.15 | 80.05±0.65 | 88.13±1.15 | 62.05±0.45 | 79.63±0.65 | 58.59±6.42 |
| 7i | 100.03±0.24 | 100.17±1.44 | 91.03±8.24 | 79.82±4.06 | 87.25±6.44 | 66.93±1.43 |
| 7j | 100.82±0.75 | 100.08±1.02 | 100.18±5.45 | 100.25±8.15 | 100.06±8.25 | 100.32±4.36 |
| 7k | 100.02±1.16 | 100.15±1.21 | 100.34±2.83 | 100.01±6.54 | 100.25±1.93 | 100.17±8.04 |
| 7l | 54.13±0.61 | 39.57±2.35 | 81.03±8.20 | 57.24±0.26 | 100.02±2.92 | 100.28±3.15 |
| 7m | 62.78±0.72 | 39.52±1.22 | 82.93±4.21 | 64.82±5.42 | 66.46±9.01 | 47.82±4.33 |
| 7n | 100.33±0.43 | 100.01±1.15 | 100.32±0.14 | 100.35±5.00 | 62.39±6.34 | 35.52±0.25 |
| 7o | 71.22±0.15 | 42.38±0.55 | 60.88±0.37 | 31.66±1.38 | 62.52±1.45 | 31.35±0.22 |
| 7p | 56.04±1.25 | 39.88±0.75 | 100.09±4.34 | 100.62±4.13 | 59.77±2.36 | 38.36±9.23 |
| 7q | 67.26±0.55 | 59.65±2.42 | 100.26±5.82 | 100.35±6.74 | 100.05±3.11 | 100.14±2.17 |
| 7r | 100.06±1.66 | 100.27±1.03 | 100.02±0.32 | 100.52±2.11 | 63.22±0.23 | 39.37±0.11 |
| 7s | 61.11±0.34 | 42.66±2.16 | 100.61±1.23 | 100.86±8.07 | 67.42±0.14 | 48.73±8.14 |
| Bismerthiazolb | 74.25±0.45 | 53.11±0.65 | 100.26±1.17 | 100.02±4.53 | 70.22±6.23 | 48.85±7.42 |
| Thiodiazole-copperb | 70.17±1.35 | 41.56 ±1.01 | 55.63±1.20 | 41.32±5.39 | 63.51±8.15 | 44.27±3.46 |
| Compd. | X. oryzae | R. solanacearu | X. citri | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EC50/ (μg·mL-1) | Regression equation | r | EC50/ (μg·mL-1) | Regression equation | r | EC50/ (μg·mL-1) | Regression equation | r | |
| 7e | 38.02±2.15 | y=1.7584x+2.2116 | 0.97 | 47.86±0.64 | y=2.1183x+1.4312 | 0.97 | 67.20±2.24 | y=1.7527x+1.8076 | 0.98 |
| 7g | 36.88±0.29 | y=2.0126x+1.8664 | 0.95 | 63.09±0.45 | y=1.7527x+1.8076 | 0.96 | — | — | — |
| 7h | 41.30±2.51 | y=1.8947x+1.9382 | 0.98 | — | — | — | — | — | — |
| 7i | 63.09±1.71 | y=2.3573x+0.7284 | 0.96 | — | — | — | — | — | — |
| 7j | 15.13±0.64 | y=2.9303x+1.5332 | 0.96 | 30.90±1.03 | y=1.9523x+2.0932 | 0.96 | 24.49±0.61 | y=1.5289x+2.8762 | 0.99 |
| 7k | 21.33±1.44 | y=1.1424x+3.4811 | 0.97 | 24.54±0.53 | y=1.5243x+2.8742 | 0.97 | 14.79±1.82 | y=3.4379x+0.9771 | 0.98 |
| 7l | — | — | — | — | — | — | 51.28±3.21 | y=1.4098x+2.5822 | 0.99 |
| 7n | 75.36±1.73 | y=2.1535x+0.9522 | 0.96 | 86.09±2.13 | y=1.4033x+2.2843 | 0.98 | — | — | — |
| 7p | — | — | — | 37.15±2.35 | y=1.2443x+3.0454 | 0.97 | — | — | — |
| 7q | — | — | — | 23.98±1.41 | y=2.8346x+1.0746 | 0.98 | 48.86±2.21 | y=1.9589x+1.6904 | 0.97 |
| 7r | 36.89±2.12 | y=2.5243x+1.0433 | 0.97 | 39.81±2.82 | y=1.3243x+2.8753 | 0.96 | — | — | — |
| 7s | — | — | — | 56.75±0.61 | y=1.8564x+1.7434 | 0.98 | — | — | — |
| Bismert- | 92.23±0.7 | y=1.5038x+2.0433 | 0.98 | 58.88±1.01 | y=1.2144x+2.8443 | 0.99 | 123.02±1.11 | y=1.5143x+1.8278 | 0.99 |
| hiazol | |||||||||
Table 6 Antibacterial activities of some title compounds
| Compd. | X. oryzae | R. solanacearu | X. citri | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EC50/ (μg·mL-1) | Regression equation | r | EC50/ (μg·mL-1) | Regression equation | r | EC50/ (μg·mL-1) | Regression equation | r | |
| 7e | 38.02±2.15 | y=1.7584x+2.2116 | 0.97 | 47.86±0.64 | y=2.1183x+1.4312 | 0.97 | 67.20±2.24 | y=1.7527x+1.8076 | 0.98 |
| 7g | 36.88±0.29 | y=2.0126x+1.8664 | 0.95 | 63.09±0.45 | y=1.7527x+1.8076 | 0.96 | — | — | — |
| 7h | 41.30±2.51 | y=1.8947x+1.9382 | 0.98 | — | — | — | — | — | — |
| 7i | 63.09±1.71 | y=2.3573x+0.7284 | 0.96 | — | — | — | — | — | — |
| 7j | 15.13±0.64 | y=2.9303x+1.5332 | 0.96 | 30.90±1.03 | y=1.9523x+2.0932 | 0.96 | 24.49±0.61 | y=1.5289x+2.8762 | 0.99 |
| 7k | 21.33±1.44 | y=1.1424x+3.4811 | 0.97 | 24.54±0.53 | y=1.5243x+2.8742 | 0.97 | 14.79±1.82 | y=3.4379x+0.9771 | 0.98 |
| 7l | — | — | — | — | — | — | 51.28±3.21 | y=1.4098x+2.5822 | 0.99 |
| 7n | 75.36±1.73 | y=2.1535x+0.9522 | 0.96 | 86.09±2.13 | y=1.4033x+2.2843 | 0.98 | — | — | — |
| 7p | — | — | — | 37.15±2.35 | y=1.2443x+3.0454 | 0.97 | — | — | — |
| 7q | — | — | — | 23.98±1.41 | y=2.8346x+1.0746 | 0.98 | 48.86±2.21 | y=1.9589x+1.6904 | 0.97 |
| 7r | 36.89±2.12 | y=2.5243x+1.0433 | 0.97 | 39.81±2.82 | y=1.3243x+2.8753 | 0.96 | — | — | — |
| 7s | — | — | — | 56.75±0.61 | y=1.8564x+1.7434 | 0.98 | — | — | — |
| Bismert- | 92.23±0.7 | y=1.5038x+2.0433 | 0.98 | 58.88±1.01 | y=1.2144x+2.8443 | 0.99 | 123.02±1.11 | y=1.5143x+1.8278 | 0.99 |
| hiazol | |||||||||
| [1] | Niu C., Li G., Mai D.N., Chem. J. Chinese Universities, 2014, 35(6), 1204—1211 |
| (牛超, 李根, 买迪娜. 高等学校化学学报, 2014, 35(6), 1204—1211) | |
| [2] | Beaudoin D., Maris T., Wuest J.D., J. Chem. Pharm. Res., 2013, 5(11), 830—834 |
| [3] | Singh H.P., Pandeya S. N., Chandra C. S., Sharma C. S, Med. Chem. Res., 2011, 20(1), 74—80 |
| [4] | Singh H.P., Chanuhan C. S., Pandeya S. N., Sharma C. S., Srivastava B., Singhal M, Der. Pharm. Lett., 2010, 2(2), 460—462 |
| [5] | Singhal M., Paul A.,Singh P.H., Dubey S. K., Songara R. K., Int. J. Pharmaceut. Sci. Drug Res., 2011, 3(2), 150—154 |
| [6] | Jafri L., Ansari F.L., Jamil M., Kalsoom S., Qureishi S., Mirza B, Chem. Biol. Drug Des., 2012, 79(6), 950—959 |
| [7] | Singhal M., Paul A., Internal. J. Chem. Pharm. Res., 2011, 10(2), 2602—2604 |
| [8] | Demirbas N., Karaoglu S.A., Demirbas A., Sanca K., Eur. J. Med. Chem., 2004, 39(9), 793—804 |
| [9] | Wang M.J., Lu J. R., Xin C. W., Liu J. B., Mu J. B., Zhang H., Zhang R. B., Yang X. Y., Wang H. W., Chem. J. Chinese Universities, 2015, 36(3), 469—476 |
| (王美君, 卢俊瑞, 辛春伟, 刘金彪, 穆江蓓, 张贺, 张瑞波, 杨旭云, 王宏韫. 高等学校化学学报, 2015, 36(3), 469—476) | |
| [10] | Azam M.A., Kumar B. R. P., Shalinis S., Surech B., Reddy T. K., Reddy C. D., Indian. J. Pharm. Sci., 2008, 70(5), 672—677 |
| [11] | Foroumadi A., Emami S., Hassanzadeh A., Rajaee M., Sokhanvar K., Moshafi M.H., Bioorg. Med. Chem. Lett., 2005, 15(20), 4488—4492 |
| [12] | Chou J.Y., Lai S. Y., Pan S. L., Jow G. M., Chern J. W., Guh J. H, Biochem. Pharmacol., 2003, 66(1), 115—124 |
| [13] | Martinez A., Alonso D., Castro A., Aran V.J., Cardelus I., Banos E, Arch. Pharm., 2010, 332(6), 191—194 |
| [14] | Jin G.Y., Hou Z., Zhao G. F., Cao C. Y., Li Y. C., Chem. J. Chinese Universities, 1997, 18(4), 409—412 |
| (金桂玉, 侯震, 赵国峰, 曹春阳, 李煜昶. 高等学校化学学报, 1997, 18(4), 409—412) | |
| [15] | Raj M.M., Patel H. V., Raj L. M., Patel N. K., Int. J. Pharm, Chem. Biol. Sci., 2013, 3(3), 814—819 |
| [16] | Zhao J., Chen B.Q., Shi Y. P., Shi Y. P., Liu Y. M., Zhao H. C., Cheng J., Chinese Chem. Lett., 2012, 23(7), 817—819 |
| [17] | Sheng C.Q., Che X. Y., Wang W. Y., Wang S. Z., Cao Y. B., Miao Z. Y., Yao J. Z., Zhang W. N., Eur. J. Med. Chem., 2011, 46(11), 5276—5282 |
| [18] | Ma J.J., Bao G. L., Wang L. M., Li W. T., Xu B. X., Du B. Q., Lv J., Zhai X., Gong P., Eur. J. Med. Chem., 2015, 96, 173—186 |
| [19] | Mortimer D., Whiting M., Harrity J.P. A., Jones S., Coldham L, Tetrahedron Lett., 2014, 55(6), 1255—1257 |
| [20] | Cami G.E., González M. L., Ruiz F. S., Pedregosa J. C., J. Phys. Chem. Solids, 2005, 66(6), 936—945 |
| [21] | Liu H.L., Wu R. M., Sun Y. Y., Ye Y., Chen J., Luo X. M., Shen X., Liu H, Bioorg. Med. Chem., 2014, 22(22), 6344—6352 |
| [22] | Shen L.H., Li H. Y., Shang H. X., Tian S. T., Lai Y. S., Liu L. J., Chinese Chem. Lett., 2013, 24(4), 299—302 |
| [23] | Zhao J., Xuan L.N., Zhao H. C., Cheng J., Fu X. Y., Li S., Jing F., Liu Y. M., Chen B. Q., Chem. Res. Chinese Universities, 2014, 30(5), 764—769 |
| [24] | Foroumadi A., Firoozpour L., Emami S., Mansouri S., Ebrahimabadi A.H., Asadipour A., Amini M., Saeid-Adeli N., Shafiee A, Arch. Pharm. Res., 2007, 30(2), 138—145 |
| [25] | Zhao J., Chen B.Q., Shi Y. P., Liu Y. M., Zhao H. C., Cheng J., Chinese Chem. Lett., 2012, 23(7), 817—819 |
| [26] | Su S.H., Zhou X., Liao G. P., Qi P. Y., Jin L. H., Molecules, 2016, 22(1), 64—81 |
| [1] | 左怀龙, 雷思敏, 张锐, 李玉新, 陈伟. 新型异喹啉衍生物的设计合成及抑菌活性[J]. 高等学校化学学报, 2021, 42(9): 2766. |
| [2] | 胡皓程, 李文利, 张嘉宁, 刘宇博. 黑木耳寡糖的提取、 结构表征及生物活性[J]. 高等学校化学学报, 2021, 42(8): 2465. |
| [3] | 李普, 陈英, 夏榕娇, 郭涛, 张敏, 仕春, 汤旭, 贺鸣, 薛伟. 含喹喔啉杨梅素衍生物的合成及生物活性[J]. 高等学校化学学报, 2019, 40(5): 909. |
| [4] | 李冰, 王学敏, 白凤英, 刘淑清. 稀土氮杂环配合物的合成、 结构及抑菌活性[J]. 高等学校化学学报, 2019, 40(4): 632. |
| [5] | 常俊朋, 赵佳瑞, 陈思佳, 孟凯, 石微妮, 李瑞芳. 抗菌肽SAMP1及其类似肽的构效关系[J]. 高等学校化学学报, 2019, 40(4): 705. |
| [6] | 谭英, 肖梦武, 叶姣, 胡艾希, 曾子清, 欧晓明. (Z)-3,3-二甲基-1-(1H-1,2,4-三唑-1-基)-2-丁酮肟(5-芳基-1,3,4-噁二唑-2-基)甲基醚的合成和抑菌活性[J]. 高等学校化学学报, 2017, 38(8): 1375. |
| [7] | 肖维, 阮祥辉, 李琴, 张菊平, 钟新敏, 谢艳, 王晓斌, 黄民国, 薛伟. 酰胺类杨梅素衍生物的合成及抑菌活性[J]. 高等学校化学学报, 2017, 38(1): 35. |
| [8] | 贾长青, 杨冬燕, 车传亮, 马永强, 芮昌辉, 闫晓静, 覃兆海. 1H-1,2,4-三唑-5-氨基甲酸酯类化合物的合成、 结构表征及杀虫、 抑菌活性[J]. 高等学校化学学报, 2016, 37(5): 892. |
| [9] | 陈伟, 魏巍, 刘明, 华学文, 李玉新, 李永红, 张晓, 李正名. 新型含二甲氧基甲基嘧啶基磺酰脲衍生物的合成及生物活性[J]. 高等学校化学学报, 2015, 36(7): 1291. |
| [10] | 陈伟, 魏巍, 李玉新, 万莹莹, 刘巧霞, 李永红, 于淑晶, 李正名. 2-甲基-6-硝基苯磺酰脲衍生物的合成及生物活性[J]. 高等学校化学学报, 2015, 36(5): 907. |
| [11] | 陈伟, 魏巍, 周莎, 李永红, 张晓, 童军, 李玉新, 李正名. 新型含苯基取代嘧啶基磺酰脲衍生物的设计、 合成及生物活性[J]. 高等学校化学学报, 2015, 36(4): 672. |
| [12] | 王娇, 田克情, 薛子桥, 武云云, 杨田, 赵惠敏, 张萍. 4-取代苯基-1,5-苯并硫氮杂䓬-2-甲酸的合成及抑菌活性[J]. 高等学校化学学报, 2015, 36(3): 505. |
| [13] | 武瑶, 梁雯婧, 李成, 商旭, 丛丽娜. 热处理对海参溶菌酶C端多肽的抑菌活性和结构的影响[J]. 高等学校化学学报, 2014, 35(5): 1044. |
| [14] | 范世丽, 张博, 高丽叶, 王兰芝, 边艳青, 李媛. 2-甲氧/乙氧羰基-4-氟苯基-1,5-苯并硫氮杂䓬的合成、抑真菌活性及构效关系[J]. 高等学校化学学报, 2014, 35(12): 2574. |
| [15] | 刘卓, 潘里, 于淑晶, 李正名. N-(4'-芳环取代嘧啶基-2'-基)-2-乙氧羰基苯磺酰脲衍生物的合成及抑菌活性[J]. 高等学校化学学报, 2013, 34(8): 1868. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||