

高等学校化学学报 ›› 2015, Vol. 36 ›› Issue (3): 505.doi: 10.7503/cjcu20140588
王娇1, 田克情2, 薛子桥1, 武云云1, 杨田1, 赵惠敏1, 张萍1( )
)
收稿日期:2014-06-27
出版日期:2015-03-10
发布日期:2015-01-23
作者简介:联系人简介: 张 萍, 女, 副教授, 主要从事有机化合物合成及抑菌活性研究. E-mail:
WANG Jiao1, TIAN Keqing2, XUE Ziqiao1, WU Yunyun1, YANG Tian1, ZHAO Huimin1, ZHANG Ping1,*( )
)
Received:2014-06-27
Online:2015-03-10
Published:2015-01-23
Contact:
ZHANG Ping
E-mail:zhangpingp@sina.com
摘要:
合成了七元环的杂?化合物2a~2k, 并发现化合物2a~2k会向其同分异构体六元环内酰胺3a~3k转化. 化合物2a~2k的结构通过红外光谱(IR)、 核磁共振波谱(NMR)、 质谱(MS)和元素分析确证. 初步的抑菌活性测试结果表明, 化合物2a~2k对白色念珠菌、 标准新生隐球菌、 临床新生隐球菌、 大肠杆菌和枯草牙孢杆菌均有较好的抑菌活性, 而六元环内酰胺3a~3k对所测菌种没有活性. 实验还测定了部分混合物(2a~2e和3a~3e)的抑菌活性, 结果表明化合物2a~2e和3a~3e混合物的抑真菌效果比单一的化合物2a~2e要好, 化合物3a~3e的存在增强了化合物2a~2e的抑菌活性.
中图分类号:
TrendMD:
王娇, 田克情, 薛子桥, 武云云, 杨田, 赵惠敏, 张萍. 4-取代苯基-1,5-苯并硫氮杂䓬-2-甲酸的合成及抑菌活性. 高等学校化学学报, 2015, 36(3): 505.
WANG Jiao, TIAN Keqing, XUE Ziqiao, WU Yunyun, YANG Tian, ZHAO Huimin, ZHANG Ping. Syntheses and Antimicrobial Activities of 4-Substituted Phenyl 1,5-Benzothiazepines-2-carboxylic Acids. Chem. J. Chinese Universities, 2015, 36(3): 505.
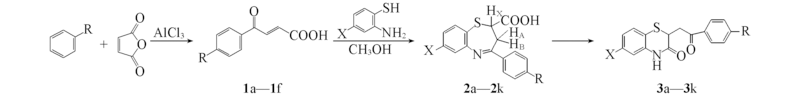
Scheme 1 Synthetic routes of compounds 2a—2k a: X=H, R=H; b:X=H, R=F; c: X=H, R=Cl; d: X=H, R=Br; e: X=H, R=CH3; f: X=H, R=OCH3;g: X=Cl, R=H; h: X=Cl, R=F; i: X=Cl, R=Cl; j: X=Cl, R=Br; k: X=Cl, R=CH3.
| Compd. | Appearance | Yield(%) | m. p./℃ | Elemental analysis(%, calcd. ) | MS([M+H]+), m/z | IR(KBr), | ||
|---|---|---|---|---|---|---|---|---|
| C | H | N | ||||||
| 2a | White solid | 53 | 186—189 | 67.70(67.84) | 4.68(4.59) | 4.84(4.95) | 284.2 | 2502,1705,1601 |
| 2b | White solid | 54 | 171—173 | 63.59(63.79) | 4.42(4.31) | 4.55(4.68) | 302.0 | 2509,1701,1601 |
| 2c | White solid | 55 | 167—169 | 60.35(60.47) | 3.61(3.78) | 4.31(4.41) | 318.7 | 2507,1713,1607 |
| 2d | White solid | 52 | 167—168 | 53.21(53.04) | 3.70(3.87) | 3.40(3.32) | 363.8 | 2504,1713,1705 |
| 2e | White solid | 49 | 153—156 | 68.48(68.67) | 5.16(5.05) | 4.63(4.71) | 298.9 | 2978,1693,1609 |
| 2f | White solid | 48 | 198—200 | 58.56(58.71) | 4.07(4.19) | 4.03(4.09) | 348.8 | 3503,1701,1601 |
| 2g | White solid | 53 | 183—186 | 60.32(60.47) | 3.61(3.78) | 4.39(4.41) | 318.8 | 3063,1705,1599 |
| 2h | White solid | 54 | 196—199 | 57.12(57.23) | 3.19(3.38) | 4.12(4.17) | 336.8 | 3074,1705,1601 |
| 2i | White solid | 51 | 170—172 | 54.40(54.55) | 3.02(3.13) | 3.85(3.98) | 352.7 | 3049,1709,1574 |
| 2j | White solid | 52 | 178—180 | 48.57(48.42) | 2.83(2.77) | 3.40(3.53) | 397.7 | 2880,1709,1574 |
| 2k | White solid | 49 | 236—238 | 61.41(61.54) | 4.12(4.22) | 4.34(4.22) | 332.5 | 3049,1701,1607 |
Table 1 Appearance, yields, melting points, elemental analysis, MS and IR data for compounds 2a—2k
| Compd. | Appearance | Yield(%) | m. p./℃ | Elemental analysis(%, calcd. ) | MS([M+H]+), m/z | IR(KBr), | ||
|---|---|---|---|---|---|---|---|---|
| C | H | N | ||||||
| 2a | White solid | 53 | 186—189 | 67.70(67.84) | 4.68(4.59) | 4.84(4.95) | 284.2 | 2502,1705,1601 |
| 2b | White solid | 54 | 171—173 | 63.59(63.79) | 4.42(4.31) | 4.55(4.68) | 302.0 | 2509,1701,1601 |
| 2c | White solid | 55 | 167—169 | 60.35(60.47) | 3.61(3.78) | 4.31(4.41) | 318.7 | 2507,1713,1607 |
| 2d | White solid | 52 | 167—168 | 53.21(53.04) | 3.70(3.87) | 3.40(3.32) | 363.8 | 2504,1713,1705 |
| 2e | White solid | 49 | 153—156 | 68.48(68.67) | 5.16(5.05) | 4.63(4.71) | 298.9 | 2978,1693,1609 |
| 2f | White solid | 48 | 198—200 | 58.56(58.71) | 4.07(4.19) | 4.03(4.09) | 348.8 | 3503,1701,1601 |
| 2g | White solid | 53 | 183—186 | 60.32(60.47) | 3.61(3.78) | 4.39(4.41) | 318.8 | 3063,1705,1599 |
| 2h | White solid | 54 | 196—199 | 57.12(57.23) | 3.19(3.38) | 4.12(4.17) | 336.8 | 3074,1705,1601 |
| 2i | White solid | 51 | 170—172 | 54.40(54.55) | 3.02(3.13) | 3.85(3.98) | 352.7 | 3049,1709,1574 |
| 2j | White solid | 52 | 178—180 | 48.57(48.42) | 2.83(2.77) | 3.40(3.53) | 397.7 | 2880,1709,1574 |
| 2k | White solid | 49 | 236—238 | 61.41(61.54) | 4.12(4.22) | 4.34(4.22) | 332.5 | 3049,1701,1607 |
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ | |
|---|---|---|---|
| 2a | 4.52(dd, 1H, JXA=12.5 Hz, JXB=5.5 Hz, HX), 3.42(dd, 1H, JAX=12.5 Hz, JAB=13.5 Hz, HA), 3.04(dd, 1H, JBX=5.5 Hz, JBA=13.5 Hz, HB), 11.15(s, 1H, —COOH), 7.12—8.22(m, 9H, ArH) | ||
| 2b | 4.52(dd, 1H, JXA=12.5 Hz, JXB=5.5 Hz, HX), 3.42(dd, 1H, JAX=12.5 Hz, JAB=13.5 Hz, HA), 3.04(dd, 1H, JBX=5.5 Hz, JBA=13.5 Hz, HB), 11.20(s, 1H, —COOH), 7.12—8.31(m, 8H, ArH) | ||
| 2c | 4.53(dd, 1H, JXA=12.8 Hz, JXB=5.4 Hz, HX), 3.40(dd, 1H, JAX=12.8 Hz, JAB=13.5 Hz, HA), 3.04(dd, 1H, JBX=5.4 Hz, JBA=13.5 Hz, HB), 11.17(s, 1H, —COOH), 7.12—8.17(m, 8H, ArH) | 171.6, 169.9, 138.2, 136.6, 133.1, 131.8, 130.7, 126.6, 126.0, 122.7, 57.1, 32.4 | |
| 2d | 4.54(dd, 1H, JXA=12.5 Hz, JXB=5.5 Hz, HX), 3.40(dd, 1H, JAX=12.5 Hz, JAB=13.3 Hz, HA), 3.04(dd, 1H, JBX=5.5 Hz, JBA=13.3 Hz, HB), 11.18(s, 1H, —COOH), 7.13—8.17(m, 8H, ArH), | 171.8, 169.5, 153.7, 137.7, 136.2, 132.6, 131.3, 130.3, 126.1, 125.5, 122.3, 56.8, 32.0 | |
| 2e | 4.48(dd, 1H, JXA=12.8 Hz, JXB=5.8 Hz, HX), 3.40(dd, 1H, JAX=12.5 Hz, JAB=13.3 Hz, HA), 3.06(dd, 1H, JBX=5.8 Hz, JBA=13.3 Hz, HB), 11.16(s, 1H, —COOH), 7.10—8.12(m, 8H, ArH), 2.42(s, 3H, —CH3) | ||
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ | |
| 2f | 4.47(dd, 1H, JXA=12.8 Hz, JXB=5.3 Hz, HX), 3.43(dd,1H, JAX=12.8 Hz, JAB=13.5 Hz, HA), 2.99(dd, 1H, JBX=5.3 Hz, JBA=13.5 Hz, HB), 11.20(s, 1H, —COOH), 7.05—8.18(m,7H, ArH), 3.89(s, 3H, —OCH3) | ||
| 2g | 4.55(dd, 1H, JXA=12.5 Hz, JXB=5.5 Hz, HX), 3.48(dd, 1H, JAX=12.5 Hz, JAB=13.5 Hz, HA), 3.04(dd, 1H, JBX=5.5 Hz, JBA=13.5 Hz, HB), 11.27(s, 1H, —COOH), 7.16—8.23(m, 8H, ArH) | ||
| 2h | 4.56(dd, 1H, JXA=12.5 Hz, JXB=5.5 Hz , HX), 3.41(dd, 1H, JAX=12.5 Hz, JAB=13.8 Hz, HA), 3.05(dd, 1H, JBX=5.5 Hz, JBA=13.8 Hz, HB), 11.27(s, 1H, —COOH), 7.17—8.23(m, 7H, ArH) | ||
| 2i | 4.56(dd, 1H, JXA=12.5 Hz, JXB=5.5 Hz, HX), 3.45(dd, 1H, JAX=12.5 Hz, JAB=13.5 Hz, HA), 3.05(dd, 1H, JBX=5.5 Hz, JBA=13.5 Hz , HB), 11.25(s, 1H, —COOH), 7.17—8.24(m, 7H, ArH) | 171.7, 169.5, 138.9, 136.1, 133.6, 131.2, 130.7, 125.9, 125.5, 122.2, 58.1, 31.0 | |
| 2j | 4.57(dd, 1H, JXA=12.8 Hz, JXB=5.3 Hz, HX), 3.46(dd, 1H, JAX=12.8 Hz, JAB=13.8 Hz, HA), 3.05(dd, 1H, JBX=5.3 Hz, JBA=13.8 Hz, HB), 11.28(s, 1H, —COOH), 7.18—8.18(m, 7H, ArH) | 171.8, 169.6, 152.6, 137.8, 136.4, 131.4, 131.3, 130.2, 126.8, 125.6, 122.6, 55.4, 32.5 | |
| 2k | 4.49(dd, 1H, JXA=12.8 Hz, JXB=5.8 Hz, HX), 3.43(dd, 1H, JAX=12.8 Hz, JAB=13.5 Hz, HA), 3.00(dd, 1H, JBX=5.8 Hz, JBA=13.5 Hz, HB), 11.2(s, 1H, —COOH), 7.13—8.11(m, 7H, ArH), 2.41(s, 3H, CH3) | ||
Table 2 1H NMR and 13C NMR data for compounds 2a—2k
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ | |
|---|---|---|---|
| 2a | 4.52(dd, 1H, JXA=12.5 Hz, JXB=5.5 Hz, HX), 3.42(dd, 1H, JAX=12.5 Hz, JAB=13.5 Hz, HA), 3.04(dd, 1H, JBX=5.5 Hz, JBA=13.5 Hz, HB), 11.15(s, 1H, —COOH), 7.12—8.22(m, 9H, ArH) | ||
| 2b | 4.52(dd, 1H, JXA=12.5 Hz, JXB=5.5 Hz, HX), 3.42(dd, 1H, JAX=12.5 Hz, JAB=13.5 Hz, HA), 3.04(dd, 1H, JBX=5.5 Hz, JBA=13.5 Hz, HB), 11.20(s, 1H, —COOH), 7.12—8.31(m, 8H, ArH) | ||
| 2c | 4.53(dd, 1H, JXA=12.8 Hz, JXB=5.4 Hz, HX), 3.40(dd, 1H, JAX=12.8 Hz, JAB=13.5 Hz, HA), 3.04(dd, 1H, JBX=5.4 Hz, JBA=13.5 Hz, HB), 11.17(s, 1H, —COOH), 7.12—8.17(m, 8H, ArH) | 171.6, 169.9, 138.2, 136.6, 133.1, 131.8, 130.7, 126.6, 126.0, 122.7, 57.1, 32.4 | |
| 2d | 4.54(dd, 1H, JXA=12.5 Hz, JXB=5.5 Hz, HX), 3.40(dd, 1H, JAX=12.5 Hz, JAB=13.3 Hz, HA), 3.04(dd, 1H, JBX=5.5 Hz, JBA=13.3 Hz, HB), 11.18(s, 1H, —COOH), 7.13—8.17(m, 8H, ArH), | 171.8, 169.5, 153.7, 137.7, 136.2, 132.6, 131.3, 130.3, 126.1, 125.5, 122.3, 56.8, 32.0 | |
| 2e | 4.48(dd, 1H, JXA=12.8 Hz, JXB=5.8 Hz, HX), 3.40(dd, 1H, JAX=12.5 Hz, JAB=13.3 Hz, HA), 3.06(dd, 1H, JBX=5.8 Hz, JBA=13.3 Hz, HB), 11.16(s, 1H, —COOH), 7.10—8.12(m, 8H, ArH), 2.42(s, 3H, —CH3) | ||
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ | |
| 2f | 4.47(dd, 1H, JXA=12.8 Hz, JXB=5.3 Hz, HX), 3.43(dd,1H, JAX=12.8 Hz, JAB=13.5 Hz, HA), 2.99(dd, 1H, JBX=5.3 Hz, JBA=13.5 Hz, HB), 11.20(s, 1H, —COOH), 7.05—8.18(m,7H, ArH), 3.89(s, 3H, —OCH3) | ||
| 2g | 4.55(dd, 1H, JXA=12.5 Hz, JXB=5.5 Hz, HX), 3.48(dd, 1H, JAX=12.5 Hz, JAB=13.5 Hz, HA), 3.04(dd, 1H, JBX=5.5 Hz, JBA=13.5 Hz, HB), 11.27(s, 1H, —COOH), 7.16—8.23(m, 8H, ArH) | ||
| 2h | 4.56(dd, 1H, JXA=12.5 Hz, JXB=5.5 Hz , HX), 3.41(dd, 1H, JAX=12.5 Hz, JAB=13.8 Hz, HA), 3.05(dd, 1H, JBX=5.5 Hz, JBA=13.8 Hz, HB), 11.27(s, 1H, —COOH), 7.17—8.23(m, 7H, ArH) | ||
| 2i | 4.56(dd, 1H, JXA=12.5 Hz, JXB=5.5 Hz, HX), 3.45(dd, 1H, JAX=12.5 Hz, JAB=13.5 Hz, HA), 3.05(dd, 1H, JBX=5.5 Hz, JBA=13.5 Hz , HB), 11.25(s, 1H, —COOH), 7.17—8.24(m, 7H, ArH) | 171.7, 169.5, 138.9, 136.1, 133.6, 131.2, 130.7, 125.9, 125.5, 122.2, 58.1, 31.0 | |
| 2j | 4.57(dd, 1H, JXA=12.8 Hz, JXB=5.3 Hz, HX), 3.46(dd, 1H, JAX=12.8 Hz, JAB=13.8 Hz, HA), 3.05(dd, 1H, JBX=5.3 Hz, JBA=13.8 Hz, HB), 11.28(s, 1H, —COOH), 7.18—8.18(m, 7H, ArH) | 171.8, 169.6, 152.6, 137.8, 136.4, 131.4, 131.3, 130.2, 126.8, 125.6, 122.6, 55.4, 32.5 | |
| 2k | 4.49(dd, 1H, JXA=12.8 Hz, JXB=5.8 Hz, HX), 3.43(dd, 1H, JAX=12.8 Hz, JAB=13.5 Hz, HA), 3.00(dd, 1H, JBX=5.8 Hz, JBA=13.5 Hz, HB), 11.2(s, 1H, —COOH), 7.13—8.11(m, 7H, ArH), 2.41(s, 3H, CH3) | ||
| Entry | T/℃ | Catalyst | t/min | Product | Yield(%) |
|---|---|---|---|---|---|
| 1 | Reflux | 30 | 3a | 47 | |
| 2 | Reflux | HAc | 120 | 3a | 56 |
| 3 | Reflux | HOTs | 60 | 3a | 72 |
| 4 | 0 | 300 | 2a | 45 | |
| 5 | 0 | HAc | 300 | 2a | 56 |
| 6 | 0 | HOTs | 120 | 3a | 64 |
| 7 | Room temperature | 2 | 2a | 53 | |
| 8 | Room temperature | HAc | 2 | 2a | 64 |
| 9 | Room temperature | HOTs | 2 | 3a | 76 |
Table 3 Experimental conditions of compound 1a with o-aminothiophenol
| Entry | T/℃ | Catalyst | t/min | Product | Yield(%) |
|---|---|---|---|---|---|
| 1 | Reflux | 30 | 3a | 47 | |
| 2 | Reflux | HAc | 120 | 3a | 56 |
| 3 | Reflux | HOTs | 60 | 3a | 72 |
| 4 | 0 | 300 | 2a | 45 | |
| 5 | 0 | HAc | 300 | 2a | 56 |
| 6 | 0 | HOTs | 120 | 3a | 64 |
| 7 | Room temperature | 2 | 2a | 53 | |
| 8 | Room temperature | HAc | 2 | 2a | 64 |
| 9 | Room temperature | HOTs | 2 | 3a | 76 |
| Temperature/℃ | 20 | 30 | 40 | 50 |
|---|---|---|---|---|
| Integral area ratio of 2c/3c | 1.03:1 | 0.78:1 | 0.43:1 | 0.30:1 |
Table 4 Integral area ratio of 2c/3c on VT 1H NMR
| Temperature/℃ | 20 | 30 | 40 | 50 |
|---|---|---|---|---|
| Integral area ratio of 2c/3c | 1.03:1 | 0.78:1 | 0.43:1 | 0.30:1 |
| Dose/(μg·disc-1) | Zone of inhibition*/mm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C.albicans | C.neoformans(stand) | B.subtilis | ||||||||||
| 200 | 100 | 50 | 25 | 200 | 100 | 50 | 25 | 200 | 100 | 50 | 25 | |
| 2a | 17.5 | 16.6 | 8.9 | 7.0 | 18.4 | 11.5 | 7.0 | 6.0 | 15.8 | 8.7 | 7.0 | 6.0 |
| 2b | 11.1 | 10.2 | 6.9 | 6.9 | 12.5 | 6.0 | 6.0 | 6.0 | 7.8 | 6.0 | 6.0 | 6.0 |
| 2c | 16.9 | 14.8 | 9.9 | 7.0 | 12.2 | 9.6 | 6.0 | 6.0 | 15.1 | 9.0 | 6.0 | 6.0 |
| 2d | 12.8 | 10.9 | 7.0 | 6.0 | 16.4 | 9.3 | 7.0 | 7.0 | 14.4 | 9.9 | 7.0 | 6.0 |
| 2e | 14.1 | 7.8 | 7.0 | 6.0 | 21.2 | 6.0 | 6.0 | 6.0 | 12.4 | 7.5 | 6.0 | 6.0 |
| 2f | 25.9 | 22.7 | 17.9 | 10.2 | 27.1 | 23.5 | 12.5 | 8.0 | 14.7 | 7.7 | 6.0 | 6.0 |
| 2g | 23.9 | 21.4 | 17.4 | 9.6 | 25.7 | 16.7 | 7.0 | 6.0 | 11.9 | 7.0 | 6.0 | 6.0 |
| 2h | 19.0 | 15.6 | 12.8 | 9.4 | 26.9 | 16.8 | 12.9 | 6.0 | 13.2 | 7.5 | 6.0 | 6.0 |
| 2i | 16.5 | 13.3 | 7.0 | 7.0 | 21.2 | 17.0 | 6.0 | 6.0 | 9.30 | 6.0 | 6.0 | 6.0 |
| 2j | 29.5 | 17.0 | 18.5 | 9.8 | 28.4 | 24.7 | 15.3 | 6.0 | 11.1 | 6.0 | 6.0 | 6.0 |
| 2k | 17.8 | 11.5 | 9.5 | 7.0 | 25.9 | 20.9 | 14.4 | 6.0 | 14.0 | 7.6 | 6.0 | 6.0 |
| 2a+3a | 20.9 | 42.5 | ||||||||||
| 2b+3b | 18.5 | 21.3 | ||||||||||
| 2c+3c | 22.7 | 32.9 | ||||||||||
| 2d+3d | 19.8 | 32.8 | ||||||||||
| 2e+3e | 21.1 | 34.8 | ||||||||||
Table 5 Antimicrobial activities of compounds 2a—2k
| Dose/(μg·disc-1) | Zone of inhibition*/mm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C.albicans | C.neoformans(stand) | B.subtilis | ||||||||||
| 200 | 100 | 50 | 25 | 200 | 100 | 50 | 25 | 200 | 100 | 50 | 25 | |
| 2a | 17.5 | 16.6 | 8.9 | 7.0 | 18.4 | 11.5 | 7.0 | 6.0 | 15.8 | 8.7 | 7.0 | 6.0 |
| 2b | 11.1 | 10.2 | 6.9 | 6.9 | 12.5 | 6.0 | 6.0 | 6.0 | 7.8 | 6.0 | 6.0 | 6.0 |
| 2c | 16.9 | 14.8 | 9.9 | 7.0 | 12.2 | 9.6 | 6.0 | 6.0 | 15.1 | 9.0 | 6.0 | 6.0 |
| 2d | 12.8 | 10.9 | 7.0 | 6.0 | 16.4 | 9.3 | 7.0 | 7.0 | 14.4 | 9.9 | 7.0 | 6.0 |
| 2e | 14.1 | 7.8 | 7.0 | 6.0 | 21.2 | 6.0 | 6.0 | 6.0 | 12.4 | 7.5 | 6.0 | 6.0 |
| 2f | 25.9 | 22.7 | 17.9 | 10.2 | 27.1 | 23.5 | 12.5 | 8.0 | 14.7 | 7.7 | 6.0 | 6.0 |
| 2g | 23.9 | 21.4 | 17.4 | 9.6 | 25.7 | 16.7 | 7.0 | 6.0 | 11.9 | 7.0 | 6.0 | 6.0 |
| 2h | 19.0 | 15.6 | 12.8 | 9.4 | 26.9 | 16.8 | 12.9 | 6.0 | 13.2 | 7.5 | 6.0 | 6.0 |
| 2i | 16.5 | 13.3 | 7.0 | 7.0 | 21.2 | 17.0 | 6.0 | 6.0 | 9.30 | 6.0 | 6.0 | 6.0 |
| 2j | 29.5 | 17.0 | 18.5 | 9.8 | 28.4 | 24.7 | 15.3 | 6.0 | 11.1 | 6.0 | 6.0 | 6.0 |
| 2k | 17.8 | 11.5 | 9.5 | 7.0 | 25.9 | 20.9 | 14.4 | 6.0 | 14.0 | 7.6 | 6.0 | 6.0 |
| 2a+3a | 20.9 | 42.5 | ||||||||||
| 2b+3b | 18.5 | 21.3 | ||||||||||
| 2c+3c | 22.7 | 32.9 | ||||||||||
| 2d+3d | 19.8 | 32.8 | ||||||||||
| 2e+3e | 21.1 | 34.8 | ||||||||||
| Dose/(μg·disc-1) | Zone of inhibition*/mm | |||||||
|---|---|---|---|---|---|---|---|---|
| C.neoformans(clinical) | E. coli | |||||||
| 200 | 100 | 50 | 25 | 200 | 100 | 50 | 25 | |
| 2a | 15.4 | 14.4 | 7.0 | 6.0 | 16.2 | 8.9 | 7.0 | 6.0 |
| 2b | 10.9 | 6.0 | 6.0 | 6.0 | 8.2 | 6.0 | 6.0 | 6.0 |
| 2c | 8.1 | 6.0 | 6.0 | 6.0 | 15.9 | 7.0 | 6.0 | 6.0 |
| 2d | 11.1 | 7.0 | 6.0 | 6.0 | 14.9 | 7.8 | 6.0 | 6.0 |
| 2e | 23.6 | 6.0 | 6.0 | 6.0 | 12.2 | 7.8 | 6.0 | 6.0 |
| 2f | 24.7 | 22.1 | 17.1 | 12.5 | 11.4 | 7.0 | 7.0 | 6.0 |
| 2g | 25.0 | 21.0 | 17.5 | 10.1 | 14.1 | 9.2 | 7.0 | 6.0 |
| 2h | 19.2 | 16.8 | 14.4 | 9.8 | 11.2 | 7.0 | 6.0 | 6.0 |
| 2i | 16.8 | 13.4 | 6.0 | 6.0 | 7.0 | 6.0 | 6.0 | 6.0 |
| 2j | 23.4 | 16.2 | 18.9 | 6.0 | 16.8 | 10.2 | 7.0 | 6.0 |
| 2k | 20.1 | 14.1 | 9.0 | 6.0 | 8.9 | 7.0 | 6.0 | 6.0 |
| 2a+3a | 30.4 | |||||||
| 2b+3b | 16.2 | |||||||
| 2c+3c | 25.1 | |||||||
| 2d+3d | 30.4 | |||||||
| 2e+3e | 22.2 | |||||||
Table 6 Antimicrobial activities of the mixtures of 2a—2e and 3a—3e
| Dose/(μg·disc-1) | Zone of inhibition*/mm | |||||||
|---|---|---|---|---|---|---|---|---|
| C.neoformans(clinical) | E. coli | |||||||
| 200 | 100 | 50 | 25 | 200 | 100 | 50 | 25 | |
| 2a | 15.4 | 14.4 | 7.0 | 6.0 | 16.2 | 8.9 | 7.0 | 6.0 |
| 2b | 10.9 | 6.0 | 6.0 | 6.0 | 8.2 | 6.0 | 6.0 | 6.0 |
| 2c | 8.1 | 6.0 | 6.0 | 6.0 | 15.9 | 7.0 | 6.0 | 6.0 |
| 2d | 11.1 | 7.0 | 6.0 | 6.0 | 14.9 | 7.8 | 6.0 | 6.0 |
| 2e | 23.6 | 6.0 | 6.0 | 6.0 | 12.2 | 7.8 | 6.0 | 6.0 |
| 2f | 24.7 | 22.1 | 17.1 | 12.5 | 11.4 | 7.0 | 7.0 | 6.0 |
| 2g | 25.0 | 21.0 | 17.5 | 10.1 | 14.1 | 9.2 | 7.0 | 6.0 |
| 2h | 19.2 | 16.8 | 14.4 | 9.8 | 11.2 | 7.0 | 6.0 | 6.0 |
| 2i | 16.8 | 13.4 | 6.0 | 6.0 | 7.0 | 6.0 | 6.0 | 6.0 |
| 2j | 23.4 | 16.2 | 18.9 | 6.0 | 16.8 | 10.2 | 7.0 | 6.0 |
| 2k | 20.1 | 14.1 | 9.0 | 6.0 | 8.9 | 7.0 | 6.0 | 6.0 |
| 2a+3a | 30.4 | |||||||
| 2b+3b | 16.2 | |||||||
| 2c+3c | 25.1 | |||||||
| 2d+3d | 30.4 | |||||||
| 2e+3e | 22.2 | |||||||
| [1] | Attia A., Abdel-Salam O. I., Abo-Ghalia M. H., Amr A. E., Egypt J. Chem., 1995, 38(5), 543—553 |
| [2] | De Sarro G., Chimirri A., De Sarro A., Gitto R., Grasso S., Zappala M., Eur. J. Med. Chem., 1995, 30(12), 925—929 |
| [3] | Floyd D. M., Kimball D. S., Krapcho J., J. Med. Chem., 1992, 35(4), 756—772 |
| [4] | Ltao Y., Venhuis B. J., Rodenhuis N., J. Med. Chem., 1999, 42(12), 2235—2244 |
| [5] | Hester R. K., Shibata S., Cardiovasc. Drugs. Ther., 1990, 4(5), 1345—1354 |
| [6] | Qiu Z. L., Li W. H., Zhu H. F., Liu Q., Li Y., Chem. J. Chinese Universities,2013, 34(3), 579—589 |
| (邱召来, 李文红, 朱海菲, 刘倩, 李媛. 高等学校化学学报, 2013, 34(3), 579—589) | |
| [7] | Bariwal J. B., Upadhyay K. D., Manvar A. T., Trivedi J. C., Singh J. S., Jain K. S., Shah A. K., Eur. J. Med. Chem., 2008, 43(11), 2279—2290 |
| [8] | Xu J. X., Zuo G., Liang B., Chem. Res. Chinese Universities,2005, 21(3), 274—279 |
| [9] | Yadav A., Awasthi A., Rao N. K., Eur. J. Med. Chem., 2009, 44(1), 1—6 |
| [10] | Schwarz M., Tumelty D., J. Org. Chem., 1999, 64(7), 2219—2231 |
| [11] | Liu D., Fan Z. C., Jiang J. M., Wei J., Xin J. C., Chem. Res. Chinese Universities,2013, 29(4), 706—709 |
| [12] | Xu H., Hu X. H., Zou X. M., Zhu Y. Q., Liu B., Hu F. Z., Yang H. Z., Chem. Res. Chinese Universities,2012, 28(5), 824—827 |
| [13] | Fred K. K., John A., J. Am. Chem. Soc., 1959, 81(7), 1721—1726 |
| [14] | Beryozkina T. V., Kolos N. N., Orlov V. D., Zubatyuk R. I., Shishkin O. V., Phosphorus Sulfur and Silicon,2004, 179(10), 2153—2162 |
| [15] | Mani U., Seema P., Anshu D., Umeshc P., Phosphorus Sulfur and Silicon,1996, 113(1), 165—171 |
| [16] | Umeshc P., Mani U., Seema P., Anshu D., Phosphorus Sulfur and Silicon,1997, 126(8), 193—199 |
| [17] | Anshu D., Ruby S., Sarita K., J. Fluorine Chem., 2007, 128(9), 524—529 |
| [18] | Kapil A., Anshu D., Bioorg. Med. Chem. Lett., 2008, 18(5), 114—119 |
| [19] | Zhang P., Du N., Wang L.Z., Li Y.,Acta Crystallographica Section E, 2008, E64, o746 |
| [20] | Drakulic' B. J., Juranic' Z. D., Stanojkovic' T. P., Juranic' I. O., J. Med. Chem., 2005, 48, 5600—5603 |
| [21] | Bianchit M., Butti A., Christidis Y., Perronnet J., Barzaghi F., Cesana R., Nencioni A., Eur. J. Med. Chem., 1988, 23, 45—52 |
| [1] | 张晓斐, 刘佳鑫. 可见光诱导邻烯基甲酰苯胺环化合成2-喹啉酮[J]. 高等学校化学学报, 2022, 43(10): 20220274. |
| [2] | 黄秋红, 李文军, 李鑫. 有机催化靛红衍生酮亚胺与噁唑酮的不对称Mannich型加成反应[J]. 高等学校化学学报, 2022, 43(8): 20220131. |
| [3] | 葛怡聪, 聂万丽, 孙国峰, 陈稼轩, 田冲. 银催化2-烯基苯胺与苯并异噁唑的[5+1]环化反应[J]. 高等学校化学学报, 2022, 43(8): 20220142. |
| [4] | 李晶, 苏伟, 王学元, 傅鹏, 孙艳. 降压药阿雷地平及其相关杂质的合成与表征[J]. 高等学校化学学报, 2022, 43(2): 20210663. |
| [5] | 赵莹, 乔玲, 赵国锋, 陈莉. 含苹果酸酯的石蒜碱衍生物的合成及生物活性[J]. 高等学校化学学报, 2021, 42(9): 2789. |
| [6] | 李鹏杰, 周春妮, 王泽田, 郑子昂, 张玉敏, 王亮, 肖标. 铑催化吲哚与乙烯基三乙氧基硅烷的C—H烯基化反应[J]. 高等学校化学学报, 2021, 42(8): 2450. |
| [7] | 董心睿, 夏喆, 王桢学, 边强, 李华斌. 含1,2,4,5-四取代苯基的吡唑-4-甲酰胺类化合物的设计、 合成及生物活性[J]. 高等学校化学学报, 2020, 41(12): 2759. |
| [8] | 南江, 陈璞, 马养民. 酸促进2-乙烯基苯胺与重氮的[5+1]环化合成2-芳基喹啉[J]. 高等学校化学学报, 2020, 41(11): 2457. |
| [9] | 潘一骁, 李艳稳, 韩佳宏, 赵浩强, 冯宇, 丁相元, 徐立进, 范青华, 时茜. 环化及亚胺/酰胺部分氢化一锅法串联反应合成1,2,3,4⁃四氢喹喔啉[J]. 高等学校化学学报, 2020, 41(10): 2239. |
| [10] | 马静雨, 刘双磊, 张振国, 金俊阳, 贾振华. B(C6F5)3催化合成二吲哚甲烷类化合物的研究[J]. 高等学校化学学报, 2020, 41(10): 2225. |
| [11] | 陈淡宜, 张福梅, 何丹, 张紫媚, 钟芬, 文思妙妙, 刘祈星, 周海峰. 钌催化的手性苯基/苯并噻唑甲醇的转移氢化合成[J]. 高等学校化学学报, 2020, 41(10): 2264. |
| [12] | 刘畅, 张鹏飞, 李鹏飞. 不对称有机催化MBH碳酸酯与噻唑基烯酮的[1+4]环化反应构建含有噻唑骨架的手性二氢呋喃衍生物[J]. 高等学校化学学报, 2020, 41(10): 2272. |
| [13] | 任玉双, 郭园园, 刘学怡, 宋杰, 张川. 顺铂前药接枝修饰硫代DNA及其自组装靶向纳米药物研究[J]. 高等学校化学学报, 2020, 41(8): 1721. |
| [14] | 张成路, 孙越冬, 王静, 何钰, 张彦鹏, 张璐, 宋府璐. 高选择性检测Hg2+的喹啉酮衍生物荧光探针的合成及应用[J]. 高等学校化学学报, 2020, 41(8): 1785. |
| [15] | 周春妮, 郑子昂, 彭望明, 王洪波, 张玉敏, 王亮, 肖标. 微波辅助下铑催化二芳基膦酰胺与炔烃的C—H活化/环化反应[J]. 高等学校化学学报, 2020, 41(4): 726. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||