

高等学校化学学报 ›› 2014, Vol. 35 ›› Issue (12): 2574.doi: 10.7503/cjcu20140353
范世丽1, 张博1, 高丽叶1, 王兰芝1, 边艳青2( ), 李媛1(
), 李媛1( )
)
收稿日期:2014-04-14
出版日期:2014-12-10
发布日期:2014-11-29
作者简介:联系人简介: 李 媛, 女, 教授, 主要从事有机合成研究. E-mail:基金资助:
FAN Shili1, ZHANG Bo1, GAO Liye1, WANG Lanzhi1, BIAN Yanqing2,*( ), LI Yuan1,*(
), LI Yuan1,*( )
)
Received:2014-04-14
Online:2014-12-10
Published:2014-11-29
Contact:
BIAN Yanqing,LI Yuan
E-mail:353534578@qq.com;liyuanhbsd@163.com
Supported by:摘要:
以高活性的2-甲氧/乙氧羰基-4-(4-氟苯基)-1,5-苯并硫氮杂?A和B为模型化合物, 设计合成了11个含氟杂?衍生物3a~3k, 考察了它们对白色念珠菌和新生隐球菌的抑菌活性. 研究结果表明, 2-甲氧/乙氧羰基-4-(2-氟苯基)/(3-氟苯基)/(2,4-二氟苯基)-1,5-苯并硫氮杂?3a, 3b, 3d~3f对新生隐球菌有很强的抑菌活性, 3c的活性中等, 而7位氯代杂?3g~3k基本无活性; 上述杂?对白色念珠菌均无活性. 在此基础上, 进一步测试了高活性杂?3a, 3b, 3d~3f对新生隐球菌的抑菌浓度梯度、 最小抑菌浓度(MIC)和最小杀菌浓度(MFC), 发现其MIC和MFC均远低于对照药氟康唑. 为了考察杂?3a~3f的药效基团, 又设计合成了4类杂?衍生物4a~4f, 5a~5f, 6a~6f和7a~7c, 通过对其抑菌活性的评价, 发现分子中2-甲氧/乙氧羰基和亚胺官能团对杂?3a~3f的抑真菌(新生隐球菌)活性起关键作用, 硫原子被氧原子或氮原子代替后原杂?的活性降低.
中图分类号:
TrendMD:
范世丽, 张博, 高丽叶, 王兰芝, 边艳青, 李媛. 2-甲氧/乙氧羰基-4-氟苯基-1,5-苯并硫氮杂䓬的合成、抑真菌活性及构效关系. 高等学校化学学报, 2014, 35(12): 2574.
FAN Shili, ZHANG Bo, GAO Liye, WANG Lanzhi, BIAN Yanqing, LI Yuan. Synthesis, Antifungal Activity and Structure-activity Relationship of 2-Methoxycarbonyl/ethoxycarbonyl-4-fluorophenyl-1,5-benzothiazepines†. Chem. J. Chinese Universities, 2014, 35(12): 2574.
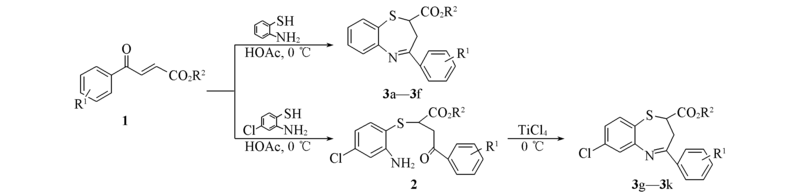
Scheme 1 Synthetic routes of 1,5-benzothiazepines 3a—3k 3a—3c: R2=CH3, R1=o-F(3a), m-F(3b), 2,4-F2(3c); 3d—3f: R2=CH2CH3, R1=o-F(3d), m-F(3e), 2,4-F2(3f); 3g: R2=CH3, R1=p-F; 3h—3k: R2=CH2CH3, R1=p-F(3h), o-F(3i), m-F(3j), 2,4-F2(3k).
| Compd. | Isolated yield(%) | m. p./℃ | HRMS([M+H]+), m/z | IR(KBr), |
|---|---|---|---|---|
| 3a | 48.0 | 85—88 | 316.0806(316.0808) | 1736.0, 1610.6 |
| 3b | 72.5 | 102—104 | 316.0804(316.0808) | 1728.3, 1614.5 |
| 3c | 73.2 | 79—81 | 334.0712(334.0713) | 1702.6, 1602.9 |
| 3d | 70.1 | 58—60 | 330.0962(330.0964) | 1716.7, 1601.0 |
| 3e | 50.2 | 73—75 | 330.0958(330.0964) | 1724.4, 1614.5 |
| 3f | 53.2 | 71—73 | 348.0864(348.0870) | 1716.7, 1604.8 |
| 3g | 72.4 | 95—97 | 350.0412(350.0418) | 1735.8, 1600.8 |
| 3h | 75.8 | 104—106 | 364.0569(364.0574) | 1718.6, 1599.0 |
| 3i | 75.4 | 87—89 | 364.0569(364.0574) | 1720.4, 1600.8 |
| 3j | 77.1 | 107—108 | 364.0569(364.0574) | 1720.6, 1618.3 |
| 3k | 81.2 | 111—113 | 382.0745(382.0486) | 1716.5, 1602.7 |
Table 1 Yields, melting points, HRMS and IR data for compounds 3a—3k
| Compd. | Isolated yield(%) | m. p./℃ | HRMS([M+H]+), m/z | IR(KBr), |
|---|---|---|---|---|
| 3a | 48.0 | 85—88 | 316.0806(316.0808) | 1736.0, 1610.6 |
| 3b | 72.5 | 102—104 | 316.0804(316.0808) | 1728.3, 1614.5 |
| 3c | 73.2 | 79—81 | 334.0712(334.0713) | 1702.6, 1602.9 |
| 3d | 70.1 | 58—60 | 330.0962(330.0964) | 1716.7, 1601.0 |
| 3e | 50.2 | 73—75 | 330.0958(330.0964) | 1724.4, 1614.5 |
| 3f | 53.2 | 71—73 | 348.0864(348.0870) | 1716.7, 1604.8 |
| 3g | 72.4 | 95—97 | 350.0412(350.0418) | 1735.8, 1600.8 |
| 3h | 75.8 | 104—106 | 364.0569(364.0574) | 1718.6, 1599.0 |
| 3i | 75.4 | 87—89 | 364.0569(364.0574) | 1720.4, 1600.8 |
| 3j | 77.1 | 107—108 | 364.0569(364.0574) | 1720.6, 1618.3 |
| 3k | 81.2 | 111—113 | 382.0745(382.0486) | 1716.5, 1602.7 |
| Compd. | 1H NMR(500 MHz), δ | 13C NMR(125 MHz), δ |
|---|---|---|
| 3aa | 8.07—7.11(m, 8H, PhH), 4.52(dd, J=6.0, 11.5 Hz, 1H, SCH), 3.74(s, 3H, CH3), 3.15(dd, J=11.5, 13.0 Hz, 1H, SCCH), 3.10(dd, J=6.0, 13.0 Hz, 1H, SCCH) | 170.95,167.92(d, J=1.62 Hz), 162.38, 160.39, 151.62, 137.97, 135.49, 132.59(d, J=8.88 Hz), 131.43, 130.76(d, J=3.00 Hz), 130.66, 130.51, 126.90(d, J=11.50 Hz), 125.72, 124.95, 124.66(d, J=3.25 Hz), 124.53, 121.50, 118.29, 116.68, 116.40(d, J=10.38 Hz), 115.05, 55.45, 55.41, 52.62, 34.94, 34.90, 29.72 |
| 3ba | 7.80—7.08(m, 8H, PhH), 4.36(dd, J=6.0, 12.0 Hz, 1H, SCH), 3.77(s, 3H, CH3), 3.17(dd, J=6.0, 13.5 Hz, 1H, SCCH), 3.13(dd, J=12.0, 13.5 Hz, 1H, SCCH) | 170.73, 167.90(d, J=2.50 Hz), 164.13, 162.17, 152.30, 139.80(d, J=7.12 Hz), 135.50, 130.61, 130.34(d, J=7.88 Hz), 125.65, 125.01, 123.00(d, J=2.62 Hz), 120.93, 118.25(d, J=21.38 Hz), 114.21(d, J=22.88 Hz), 55.78, 52.75, 31.56 |
| 3ca | 8.13—6.88(m, 7H, PhH), 4.48(dd, J=6.0, 12.5 Hz, 1H, SCH), 3.75(s, 3H, CH3), 3.13(dd, J=12.5, 13.0 Hz, 1H, SCCH), 3.07(dd, J=6.0, 13.0 Hz, 1H, SCCH) | 170.88, 166.66, 164.65(dd, J=12.75, 252.88 Hz), 161.72(dd, J=12.00, 251.50 Hz), 151.51, 135.52, 132.24(dd, J=4.50, 9.75 Hz), 130.57, 125.79, 124.91, 123.24(dd, J=3.62, 11.12 Hz), 121.44, 112.28(dd, J=3.00, 20.88 Hz), 104.57(dd, J=25.62, 26.75 Hz), 55.41, 55.37, 52.67, 34.81, 34.76 |
| 3da | 8.06—7.10(m, 8H, PhH), 4.48(dd, J=6.0, 12.0 Hz, 1H, SCH), 4.19(q, J=7.0 Hz, 2H, OCH2), 3.15(dd, J=12.5, 12.5 Hz, 1H, SCCH), 3.10(dd, J=6.0, 13.0 Hz, 1H, SCCH), 1.28(t, J=7.0 Hz, 1H, CH3) | 170.41, 168.02, 162.36, 160.37, 151.74, 135.51, 132.49(d, J=8.62 Hz), 130.75(d, J=2.88 Hz), 130.44, 127.05(d, J=11.5 Hz), 125.62, 124.89, 124.63(d, J=3.38 Hz), 121.59, 116.37(d, J=23.00 Hz), 61.51, 55.75, 55.71, 34.93, 34.89, 14.08 |
| 3ea | 7.80—7.10(m, 8H, PhH), 4.33(dd, J=6.5, 11.5 Hz, 1H, SCH), 4.21(q, J=7.0 Hz, 2H, OCH2), 3.16(dd, J=6.0, 13.0 Hz, 1H, SCCH), 3.12(dd, J=11.5, 13.0 Hz, 1H, SCCH), 1.30(t, J=7.0 Hz, 3H, CH3) | 170.15, 167.99(d, J=2.50 Hz), 164.13, 162.17, 152.37, 139.93(d, J=7.12 Hz), 135.51, 130.51, 130.28(d, J=7.75 Hz), 125.55, 124.94, 123.01, 121.11, 118.15(d, J=21.38 Hz), 114.21(d, J=22.75 Hz), 61.66, 56.11, 31.60, 14.08 |
| 3fa | 8.14—6.88(m, 7H, PhH), 4.45(dd, J=5.5, 12.0 Hz, 1H, SCH), 4.21—4.17(m, 2H, OCH2), 3.14(dd, J=12.0, 13.0 Hz, 1H, SCCH), 3.07(dd, J=5.5, 13.0 Hz, 1H, SCCH), 1.28(t, J=7.0 Hz, 3H, CH3) | 170.30, 166.82, 164.63(dd, J=12.25, 252.50 Hz), 161.70(dd, J=12.12, 251.75 Hz), 151.56, 135.54, 132.24(dd, J=4.62, 9.88 Hz), 130.50, 125.71, 124.85, 123.34(dd, J=3.75, 11.25 Hz), 121.57, 112.23(dd, J=3.00, 21.00 Hz), 104.74, 104.53(dd, J=25.50, 26.88 Hz), 61.56, 55.72, 55.69, 34.81, 34.76, 14.08 |
| 3ga | 8.06—7.10(m, 7H, PhH), 4.34(dd, J=5.5, 12.5 Hz, 1H, SCH), 3.77(s, 3H, CH3), 3.19(dd, J=5.5, 13.5 Hz, 1H, SCCH), 3.13(dd, J=12.5, 13.5 Hz, 1H, SCCH) | 170.76, 167.87, 165.83, 163.83, 152.45, 136.37(d, J=17.88 Hz), 133.26(d, J=2.63 Hz), 130.57, 129.65(d, J=8.75 Hz), 125.14(d, J=66.12 Hz), 120.92, 115.93(d, J=21.62 Hz), 55.70, 52.70, 31.47 |
| 3ha | 8.05—7.08(m, 7H, PhH), 4.30(dd, J=5.5, 12.5 Hz, 1H, SCH), 4.21(q, J=7.0 Hz, 2H, OCH2), 3.19(dd, J=5.5, 13.5 Hz, 1H, SCCH), 3.10(dd, J=12.5, 13.0 Hz, 1H, SCCH), 1.29(t, J=7.0 Hz, 3H, CH3) | 170.01, 168.96, 166.00, 163.99, 153.57, 136.39, 136.32, 133.73(d, J=8.75 Hz), 130.95(d, J=2.38 Hz), 129.63, 126.68(d, J=10.88 Hz), 125.24(d, J=3.00 Hz), 119.54, 117.06(d, J=22.50 Hz), 61.79, 55.85, 31.54, 14.09 |
| 3ib | 7.98—7.24(m, 7H, PhH), 4.62(dd, J=6.0, 12.0 Hz, 1H, SCH), 4.13—4.07(m, 2H, OCH2), 3.16(dd, J=6.0, 13.5 Hz, 1H, SCCH), 2.94(dd, J=12.0, 13.0 Hz, 1H, SCCH), 2.50(t, J=2.0 Hz, 3H, CH3) | 170.00, 169.50, 162.19, 160.20, 153.17, 136.39, 136.32, 135.62, 133.32(d, J=2.62 Hz), 129.66(d, J=8.62 Hz), 126.72, 126.63, 125.10(d, J=66.12 Hz), 120.45, 117.15, 115.92(d, J=21.62 Hz), 61.63, 55.90, 55.87, 34.98, 34.94, 14.34 |
| 3ja | 7.79—7.08(m, 7H, PhH), 4.32(dd, J=5.5, 12.5 Hz, 1H, SCH), 4.21(q, J=7.0 Hz, 2H, OCH2), 3.17(dd, J=6.0, 13.5 Hz, 1H, SCCH), 3.10(dd, J=12.5, 13.5 Hz, 1H, SCCH), 1.30(t, J=7.0 Hz, 3H, CH3) | 169.93, 169.09(d, J=2.62 Hz), 164.13, 162.17, 153.33, 139.43(d, J=7.12 Hz), 136.44, 136.35, 130.37(d, J=8.00 Hz), 125.59, 124.88, 123.09(d, J=2.75 Hz), 119.61, 118.56(d, J=21.12 Hz), 114.29(d, J=22.75 Hz), 61.81, 55.96, 31.69, 14.08 |
| 3ka | 8.13—6.89(m, 6H, PhH), 4.45(dd, J=7.0, 11.0 Hz, 1H, SCH), 4.22—4.16(m, 2H, OCH2), 3.13(dd, J=11.0, 13.0 Hz, 1H, SCCH), 3.09(dd, J=6.5, 13.0 Hz, 1H, SCCH), 1.29(t, J=7.0 Hz, 3H, CH3) | 170.09, 167.85, 164.85(dd, J=12.62, 253.62 Hz), 161.84(dd, J=12.25, 251.88 Hz), 152.57, 136.39, 132.21(dd, J=4.38, 14.25 Hz), 125.72, 124.78, 122.91(dd, J=4.00, 11.25 Hz), 120.12, 112.36(dd, J=3.00, 21.00 Hz), 104.63(t, J=26.19 Hz), 104.42, 61.68, 55.55, 55.51, 34.92, 34.88, 14.08 |
Table 2 1H NMR and 13C NMR data for compounds 3a—3k
| Compd. | 1H NMR(500 MHz), δ | 13C NMR(125 MHz), δ |
|---|---|---|
| 3aa | 8.07—7.11(m, 8H, PhH), 4.52(dd, J=6.0, 11.5 Hz, 1H, SCH), 3.74(s, 3H, CH3), 3.15(dd, J=11.5, 13.0 Hz, 1H, SCCH), 3.10(dd, J=6.0, 13.0 Hz, 1H, SCCH) | 170.95,167.92(d, J=1.62 Hz), 162.38, 160.39, 151.62, 137.97, 135.49, 132.59(d, J=8.88 Hz), 131.43, 130.76(d, J=3.00 Hz), 130.66, 130.51, 126.90(d, J=11.50 Hz), 125.72, 124.95, 124.66(d, J=3.25 Hz), 124.53, 121.50, 118.29, 116.68, 116.40(d, J=10.38 Hz), 115.05, 55.45, 55.41, 52.62, 34.94, 34.90, 29.72 |
| 3ba | 7.80—7.08(m, 8H, PhH), 4.36(dd, J=6.0, 12.0 Hz, 1H, SCH), 3.77(s, 3H, CH3), 3.17(dd, J=6.0, 13.5 Hz, 1H, SCCH), 3.13(dd, J=12.0, 13.5 Hz, 1H, SCCH) | 170.73, 167.90(d, J=2.50 Hz), 164.13, 162.17, 152.30, 139.80(d, J=7.12 Hz), 135.50, 130.61, 130.34(d, J=7.88 Hz), 125.65, 125.01, 123.00(d, J=2.62 Hz), 120.93, 118.25(d, J=21.38 Hz), 114.21(d, J=22.88 Hz), 55.78, 52.75, 31.56 |
| 3ca | 8.13—6.88(m, 7H, PhH), 4.48(dd, J=6.0, 12.5 Hz, 1H, SCH), 3.75(s, 3H, CH3), 3.13(dd, J=12.5, 13.0 Hz, 1H, SCCH), 3.07(dd, J=6.0, 13.0 Hz, 1H, SCCH) | 170.88, 166.66, 164.65(dd, J=12.75, 252.88 Hz), 161.72(dd, J=12.00, 251.50 Hz), 151.51, 135.52, 132.24(dd, J=4.50, 9.75 Hz), 130.57, 125.79, 124.91, 123.24(dd, J=3.62, 11.12 Hz), 121.44, 112.28(dd, J=3.00, 20.88 Hz), 104.57(dd, J=25.62, 26.75 Hz), 55.41, 55.37, 52.67, 34.81, 34.76 |
| 3da | 8.06—7.10(m, 8H, PhH), 4.48(dd, J=6.0, 12.0 Hz, 1H, SCH), 4.19(q, J=7.0 Hz, 2H, OCH2), 3.15(dd, J=12.5, 12.5 Hz, 1H, SCCH), 3.10(dd, J=6.0, 13.0 Hz, 1H, SCCH), 1.28(t, J=7.0 Hz, 1H, CH3) | 170.41, 168.02, 162.36, 160.37, 151.74, 135.51, 132.49(d, J=8.62 Hz), 130.75(d, J=2.88 Hz), 130.44, 127.05(d, J=11.5 Hz), 125.62, 124.89, 124.63(d, J=3.38 Hz), 121.59, 116.37(d, J=23.00 Hz), 61.51, 55.75, 55.71, 34.93, 34.89, 14.08 |
| 3ea | 7.80—7.10(m, 8H, PhH), 4.33(dd, J=6.5, 11.5 Hz, 1H, SCH), 4.21(q, J=7.0 Hz, 2H, OCH2), 3.16(dd, J=6.0, 13.0 Hz, 1H, SCCH), 3.12(dd, J=11.5, 13.0 Hz, 1H, SCCH), 1.30(t, J=7.0 Hz, 3H, CH3) | 170.15, 167.99(d, J=2.50 Hz), 164.13, 162.17, 152.37, 139.93(d, J=7.12 Hz), 135.51, 130.51, 130.28(d, J=7.75 Hz), 125.55, 124.94, 123.01, 121.11, 118.15(d, J=21.38 Hz), 114.21(d, J=22.75 Hz), 61.66, 56.11, 31.60, 14.08 |
| 3fa | 8.14—6.88(m, 7H, PhH), 4.45(dd, J=5.5, 12.0 Hz, 1H, SCH), 4.21—4.17(m, 2H, OCH2), 3.14(dd, J=12.0, 13.0 Hz, 1H, SCCH), 3.07(dd, J=5.5, 13.0 Hz, 1H, SCCH), 1.28(t, J=7.0 Hz, 3H, CH3) | 170.30, 166.82, 164.63(dd, J=12.25, 252.50 Hz), 161.70(dd, J=12.12, 251.75 Hz), 151.56, 135.54, 132.24(dd, J=4.62, 9.88 Hz), 130.50, 125.71, 124.85, 123.34(dd, J=3.75, 11.25 Hz), 121.57, 112.23(dd, J=3.00, 21.00 Hz), 104.74, 104.53(dd, J=25.50, 26.88 Hz), 61.56, 55.72, 55.69, 34.81, 34.76, 14.08 |
| 3ga | 8.06—7.10(m, 7H, PhH), 4.34(dd, J=5.5, 12.5 Hz, 1H, SCH), 3.77(s, 3H, CH3), 3.19(dd, J=5.5, 13.5 Hz, 1H, SCCH), 3.13(dd, J=12.5, 13.5 Hz, 1H, SCCH) | 170.76, 167.87, 165.83, 163.83, 152.45, 136.37(d, J=17.88 Hz), 133.26(d, J=2.63 Hz), 130.57, 129.65(d, J=8.75 Hz), 125.14(d, J=66.12 Hz), 120.92, 115.93(d, J=21.62 Hz), 55.70, 52.70, 31.47 |
| 3ha | 8.05—7.08(m, 7H, PhH), 4.30(dd, J=5.5, 12.5 Hz, 1H, SCH), 4.21(q, J=7.0 Hz, 2H, OCH2), 3.19(dd, J=5.5, 13.5 Hz, 1H, SCCH), 3.10(dd, J=12.5, 13.0 Hz, 1H, SCCH), 1.29(t, J=7.0 Hz, 3H, CH3) | 170.01, 168.96, 166.00, 163.99, 153.57, 136.39, 136.32, 133.73(d, J=8.75 Hz), 130.95(d, J=2.38 Hz), 129.63, 126.68(d, J=10.88 Hz), 125.24(d, J=3.00 Hz), 119.54, 117.06(d, J=22.50 Hz), 61.79, 55.85, 31.54, 14.09 |
| 3ib | 7.98—7.24(m, 7H, PhH), 4.62(dd, J=6.0, 12.0 Hz, 1H, SCH), 4.13—4.07(m, 2H, OCH2), 3.16(dd, J=6.0, 13.5 Hz, 1H, SCCH), 2.94(dd, J=12.0, 13.0 Hz, 1H, SCCH), 2.50(t, J=2.0 Hz, 3H, CH3) | 170.00, 169.50, 162.19, 160.20, 153.17, 136.39, 136.32, 135.62, 133.32(d, J=2.62 Hz), 129.66(d, J=8.62 Hz), 126.72, 126.63, 125.10(d, J=66.12 Hz), 120.45, 117.15, 115.92(d, J=21.62 Hz), 61.63, 55.90, 55.87, 34.98, 34.94, 14.34 |
| 3ja | 7.79—7.08(m, 7H, PhH), 4.32(dd, J=5.5, 12.5 Hz, 1H, SCH), 4.21(q, J=7.0 Hz, 2H, OCH2), 3.17(dd, J=6.0, 13.5 Hz, 1H, SCCH), 3.10(dd, J=12.5, 13.5 Hz, 1H, SCCH), 1.30(t, J=7.0 Hz, 3H, CH3) | 169.93, 169.09(d, J=2.62 Hz), 164.13, 162.17, 153.33, 139.43(d, J=7.12 Hz), 136.44, 136.35, 130.37(d, J=8.00 Hz), 125.59, 124.88, 123.09(d, J=2.75 Hz), 119.61, 118.56(d, J=21.12 Hz), 114.29(d, J=22.75 Hz), 61.81, 55.96, 31.69, 14.08 |
| 3ka | 8.13—6.89(m, 6H, PhH), 4.45(dd, J=7.0, 11.0 Hz, 1H, SCH), 4.22—4.16(m, 2H, OCH2), 3.13(dd, J=11.0, 13.0 Hz, 1H, SCCH), 3.09(dd, J=6.5, 13.0 Hz, 1H, SCCH), 1.29(t, J=7.0 Hz, 3H, CH3) | 170.09, 167.85, 164.85(dd, J=12.62, 253.62 Hz), 161.84(dd, J=12.25, 251.88 Hz), 152.57, 136.39, 132.21(dd, J=4.38, 14.25 Hz), 125.72, 124.78, 122.91(dd, J=4.00, 11.25 Hz), 120.12, 112.36(dd, J=3.00, 21.00 Hz), 104.63(t, J=26.19 Hz), 104.42, 61.68, 55.55, 55.51, 34.92, 34.88, 14.08 |
| Compd. | Isolated yield(%) | m. p./℃ | HRMS([M+H]+), m/z | IR(KBr), |
|---|---|---|---|---|
| 4a | 34.1 | 73—74 | 300.1031(300.1030) | 1732.1, 1610.6 |
| 4b | 29.3 | 53—54 | 300.1031(300.1030) | 1736.0, 1616.4 |
| 4c | 32.0 | 57—58 | 318.0941(318.0936) | 1734.0, 1610.6 |
| 4d | 26.2 | 79—81 | 314.1190(314.1187) | 1734.1, 1610.6 |
| 4e | 31.5 | 27—29 | 314.1185(314.1187) | 1730.2, 1616.4 |
| 4f | 34.1 | 59—61 | 332.1096(332.1093) | 1736.0, 1612.5 |
| 5a | 49.1 | 71—73 | 299.1189(299.1190) | 3365.9, 1734.1, 1604.8 |
| 5b | 51.6 | 82—83 | 299.1192(299.1190) | 3362.0, 1734.1, 1616.4 |
| 5c | 50.0 | 128—130 | 317.1097(317.1096) | 3362.0, 1739.8, 1610.6 |
| 5d | 43.1 | 87—90 | 313.1350(313.1347) | 3362.0, 1732.1, 1604.8 |
| 5e | 41.3 | 97—99 | 313.1351(313.1347) | 3375.5, 1734.1, 1608.7 |
| 5f | 40.3 | 91—93 | 331.1256(331.1253) | 3364.0, 1734.1, 1610.6 |
| 6a | 49.5 | 79—81 | 318.0960(318.0958) | 3392.9, 1720.6 |
| 6b | 51.6 | 110—111 | 318.0960(318.0958) | 3387.1, 1720.6 |
| 6c | 56.3 | 96—97 | 336.0865(336.0864) | 3311.9, 1739.8 |
| 6d | 55.2 | 81—82 | 332.1112(332.1115) | 3381.3, 1716.7 |
| 6e | 52.7 | 53—54 | 332.1113(332.1115) | 3375.5, 1728.3 |
| 6f | 51.3 | 108—111 | 350.1024(350.1021) | 3379.4, 1718.6 |
| 7a | 55.4 | 258.0742(258.0747) | 1608.7 | |
| 7b | 54.8 | 258.0742(258.0747) | 1614.5 | |
| 7c | 57.4 | 276.0652(276.0653) | 1610.6 |
Table 3 Yields, melting points, HRMS and IR data for compounds 4—7
| Compd. | Isolated yield(%) | m. p./℃ | HRMS([M+H]+), m/z | IR(KBr), |
|---|---|---|---|---|
| 4a | 34.1 | 73—74 | 300.1031(300.1030) | 1732.1, 1610.6 |
| 4b | 29.3 | 53—54 | 300.1031(300.1030) | 1736.0, 1616.4 |
| 4c | 32.0 | 57—58 | 318.0941(318.0936) | 1734.0, 1610.6 |
| 4d | 26.2 | 79—81 | 314.1190(314.1187) | 1734.1, 1610.6 |
| 4e | 31.5 | 27—29 | 314.1185(314.1187) | 1730.2, 1616.4 |
| 4f | 34.1 | 59—61 | 332.1096(332.1093) | 1736.0, 1612.5 |
| 5a | 49.1 | 71—73 | 299.1189(299.1190) | 3365.9, 1734.1, 1604.8 |
| 5b | 51.6 | 82—83 | 299.1192(299.1190) | 3362.0, 1734.1, 1616.4 |
| 5c | 50.0 | 128—130 | 317.1097(317.1096) | 3362.0, 1739.8, 1610.6 |
| 5d | 43.1 | 87—90 | 313.1350(313.1347) | 3362.0, 1732.1, 1604.8 |
| 5e | 41.3 | 97—99 | 313.1351(313.1347) | 3375.5, 1734.1, 1608.7 |
| 5f | 40.3 | 91—93 | 331.1256(331.1253) | 3364.0, 1734.1, 1610.6 |
| 6a | 49.5 | 79—81 | 318.0960(318.0958) | 3392.9, 1720.6 |
| 6b | 51.6 | 110—111 | 318.0960(318.0958) | 3387.1, 1720.6 |
| 6c | 56.3 | 96—97 | 336.0865(336.0864) | 3311.9, 1739.8 |
| 6d | 55.2 | 81—82 | 332.1112(332.1115) | 3381.3, 1716.7 |
| 6e | 52.7 | 53—54 | 332.1113(332.1115) | 3375.5, 1728.3 |
| 6f | 51.3 | 108—111 | 350.1024(350.1021) | 3379.4, 1718.6 |
| 7a | 55.4 | 258.0742(258.0747) | 1608.7 | |
| 7b | 54.8 | 258.0742(258.0747) | 1614.5 | |
| 7c | 57.4 | 276.0652(276.0653) | 1610.6 |
| Compd. | 1H NMR(500 MHz), δ | 13C NMR(125 MHz), δ |
|---|---|---|
| 4aa | 8.07—6.94(m, 8H, PhH), 5.84(dd, J=3.0, 9.5 Hz, 1H, OCH), 3.69(s, 3H, OCH3), 2.88(dd, J=9.5, 15.5 Hz, 1H, OCCH), 2.64(dd, J=1.5, 15.5 Hz, 1H, OCCH) | 169.96, 162.17, 160.18, 157.65(d, J=2.88 Hz), 144.07, 133.03, 132.75(d, J=8.88 Hz), 130.53(d, J=2.88 Hz), 129.59, 127.78, 124.85(d, J=3.25 Hz), 123.61(d, J=11.75 Hz), 122.58, 116.83, 116.50(d, J=23.12 Hz), 70.05, 69.97, 52.06, 34.86 |
| 4ba | 7.81—6.94(m, 8H, PhH), 5.91(dd, J=2.5, 10.0 Hz, 1H, OCH), 3.73(s, 3H, OCH3), 2.88(dd, J=5.5, 16.0 Hz, 1H, OCCH), 2.47(dd, J=3.0, 15.5 Hz, 1H, OCCH) | 169.89, 164.18, 162.22, 157.66(d, J=2.75 Hz), 143.62, 136.87(d, J=7.25 Hz), 132.85, 130.49(d, J=8.12 Hz), 129.60, 127.88, 122.75, 122.31(d, J=2.88 Hz), 118.34(d, J=21.25 Hz), 116.88, 113.70(d, J=22.88 Hz), 68.02, 52.24, 35.30 |
| 4ca | 8.14—6.88(m, 7H, PhH), 5.80(dd, J=3.0, 10.0 Hz, 1H, OCH), 3.70(s, 3H, OCH3), 2.87(dd, J=9.5, 16.0 Hz, 1H, OCCH), 2.61(dd, J=3.0, 16.0 Hz, 1H, OCCH) | 169.87, 164.69(dd, J=12.25, 253.38 Hz), 161.51(dd, J=12.12, 252.38 Hz), 156.44, 156.41, 143.93, 132.92, 132.06(dd, J=4.62, 9.88 Hz), 129.65, 127.71, 122.65, 119.98(dd, J=3.88, 11.88 Hz), 116.87, 112.64(dd, J=3.38, 21.50 Hz), 104.68(t, J=26.12 Hz), 69.86, 69.78, 52.11, 34.78 |
| 4da | 8.06—6.93(m, 8H, PhH), 5.84(dd, J=3.0, 10.0 Hz, 1H, OCH), 4.18—4.09(m, 2H, OCH2), 2.85(dd, J=10.0, 15.5 Hz, 1H, OCCH), 2.62(dd, J=2.5, 15.5 Hz, 1H, OCCH), 1.23(t, J=7.0 Hz, 3H, CH3) | 169.52, 162.16, 160.16, 157.84(d, J=7.00 Hz), 144.15, 133.01, 132.74(d, J=8.88 Hz), 130.54(d, J=3.25 Hz), 129.57, 127.76, 124.88(d, J=3.12 Hz), 123.64(d, J=11.62 Hz), 122.54, 116.82, 116.51(d, J=23.12 Hz), 70.13, 70.05, 61.05, 35.13, 14.14 |
| 4ea | 7.84—6.96(m, 8H, PhH), 5.93(dd, J=3.5, 10.5 Hz, 1H, OCH), 4.21(m, 2H, OCH2), 2.88(dd, J=10.0, 15.5 Hz, 1H), 2.48(dd, J=3.0, 16.0 Hz, 1H, OCCH), 1.29(t, J=7.0 Hz, 3H, CH3) | 169.41, 164.17, 162.21, 157.75(d, J=2.88 Hz), 143.74, 136.95(d, J=7.25 Hz), 132.91, 130.46(d, J=7.88 Hz), 129.54, 127.87, 122.68, 122.35(d, J=2.62 Hz), 118.36, 118.28(d, J=21.62 Hz), 116.85, 113.70(d, J=22.75 Hz), 68.10, 61.24, 35.61, 14.16 |
| 4fa | 8.14—6.88(m, 7H, PhH), 5.81(dd, J=3.0, 9.5 Hz, 1H, OCH), 4.15(m, 2H, OCH2), 2.85(dd, J=10.0, 16.0 Hz, 1H, OCCH), 2.60(dd, J=1.5, 15.5 Hz, 1H, OCCH), 1.24(t, J=7.0 Hz, 3H, CH3) | 169.40, 164.67(dd, J=12.38, 253.50 Hz), 161.51(dd, J=12.25, 252.50 Hz), 156.59, 144.04, 132.95, 132.06(dd, J=5.00, 9.88 Hz), 129.61, 127.71, 122.59, 120.07(dd, J=4.00, 11.88 Hz), 116.84, 112.60(dd, J=3.25, 21.38 Hz), 104.67(t, J=26.25 Hz), 69.96, 69.88, 61.08, 35.11, |
| 5aa | 7.97—7.00(m, 8H, PhH), 4.73(dd, J=4.0, 11.5 Hz, 1H, NCH), 4.38(s, 1H, NH), 3.78(s, 3H, OCH3), 3.43(dd, J=4.0, 13.5 Hz, 1H, NCCH), 2.87(t, J=12.0 Hz, 1H, NCCH) | 173.31, 164.85, 162.41, 160.43, 138.75, 137.57, 131.71(d, J=8.50 Hz), 130.52(d, J=3.25 Hz), 128.59, 128.36(d, J=12.12 Hz), 126.91, 124.53(d, J=3.00 Hz), 121.77(d, J=41.5 Hz), 116.16(d, J=22.62 Hz), 65.90, 65.87, 52.64, 35.13, 35.08 |
| 5bb | 7.80—6.84(m, 8H, PhH), 6.04(s, 1H, NCH), 4.70(m, 1H, NH), 3.53(s, 3H, OCH3), 3.25(dd, J=8.0, 14.0 Hz, 1H, NCCH), 3.02(dd, J=4.0, 14.5 Hz, 1H, NCCH) | 172.71, 163.76, 163.42, 161.83, 142.05(d, J=7.12 Hz), 139.74, 136.74, 130.86(d, J=8.12 Hz), 130.42, 127.24, 123.41, 120.83, 119.95, 117.23(d, J=21.12 Hz), 113.64(d, J=22.62 Hz), 64.31, 52.21, 33.28 |
| 5ca | 8.00—6.90(m, 7H, PhH), 4.70(dd, J=4.0, 11.5 Hz, 1H, NCH), 4.36(s, 1H, NH), 3.79(s, 3H, OCH3), 3.40(dd, J=3.0, 13.5 Hz, 1H, NCCH), 2.85(t, J=13.0 Hz, 1H, NCCH) | 173.15, 164.09(dd, J=12.12, 263.12 Hz), 161.64(dd, J=11.75, 250.25 Hz), 138.60, 137.51, 131.88(dd, J=5.12, 9.75 Hz), 128.57, 126.98, 124.68(dd, J=3.62, 12.00 Hz), 122.00, 121.60, 112.00(dd, J=3.12, 21.38 Hz), 104.34(t, J=25.88 Hz), 65.83, 52.65, 35.03, 34.98 |
| 5da | 7.94—6.98(m, 8H, PhH), 4.68(dd, J=4.0, 11.5 Hz, 1H, NCH), 4.30(s, 1H, NH), 4.23—4.19(m, 2H, OCH2), 3.41(dd, J=4.0, 13.5 Hz, 1H, NCCH), 2.85(dd, J=11.5, 13.5 Hz, 1H, NCCH), 1.27(t, J=7.0 Hz, 3H, CH3) | 172.81, 165.02, 162.40, 160.42, 138.72, 137.67, 131.71(d, J=8.50 Hz), 130.54(d, J=3.25 Hz), 128.60, 128.41(d, J=12.00 Hz), 126.91, 124.54(d, J=3.00 Hz), 121.74(d, J=33.62 Hz), 116.17(d, J=22.38 Hz), 66.02, 61.79, 35.17, 35.12, 14.19 |
| 5ea | 7.79—6.96(m, 8H, PhH), 4.53(dd, J=4.0, 11.0 Hz, 1H, NCH), 4.34(s, 1H, NH), 4.24(q, J=7.0 Hz, 2H, OCH2), 3.45(dd, J=4.0, 13.5 Hz, 1H, NCCH), 2.86(dd, J=11.0, 13.5 Hz, 1H, NCCH), 1.31(t, J=7.0 Hz, 3H, CH3) | 172.47, 164.49(d, J=2.38 Hz), 164.11, 162.16, 141.14, 141.06(d, J=7.00 Hz), 138.95, 137.38, 130.14, 130.05(d, J=8.00 Hz), 128.85, 126.94, 122.70(d, J=2.62 Hz), 122.06, 121.47, 117.35(d, J=21.38 Hz), 114.10, 113.95(d, J=22.62 Hz), 66.12, 62.04, 32.03, 14.29 |
| 5fa | 7.99—6.87(m, 7H, PhH), 4.65(dd, J=4.0, 11.5 Hz, 1H, NCH), 4.36(s, 1H, NH), 4.23—4.19(m, 2H, OCH2), 3.37(dd, J=4.0, 13.5 Hz, 1H, NCCH), 2.83(dd, J=11.5, 13.5 Hz, 1H, NCCH), 1.28(t, J=7.5 Hz, 3H, CH3) | 172.67, 164.08(dd, J=12.12, 251.25 Hz), 161.64(dd, J=12.25, 250.88 Hz), 138.55, 137.61, 131.90(dd, J=4.88, 9.75 Hz), 128.57, 127.00, 124.71(dd, J=3.75, 11.88 Hz), 121.95, 121.61, 112.01(dd, J=3.25, 21.25 Hz), 104.35(t, J=26.00 Hz), 65.95, 61.83, 35.06, 35.01, 14.19 |
| 6ab | 7.78—6.70(m, 8H, PhH), 5.46(d, J=3.0 Hz, 1H, NH), 5.27—5.25(m, 1H, NCH), 4.11(dd, J=4.5, 9.5 Hz, 1H,SCH ), 3.62(s, 3H,OCH3 ), 2.51—2.46(m, 1H, SCCH), 2.23—2.17(m, 1H, SCCH) | 171.52, 160.85, 158.90, 150.15, 132.94, 129.95(d, J=13.38 Hz), 129.78(d, J=8.25 Hz), 129.65(d, J=3.75 Hz), 128.97, 125.01(d, J=2.88 Hz), 120.44(d, J=20.5 Hz), 119.20, 115.89(d, J=21.88 Hz), 52.79, 50.49, 43.80, 37.55 |
| 6bc | 7.45—6.77(m, 8H, PhH), 5.13—5.10(m, 1H, NCH), 4.85(s, 1H, NH), 4.07(dd, J=4.5, 9.5 Hz, 1H, SCH), 3.67(s, 3H, OCH3), 2.50—2.45(m, 1H, SCCH), 2.41—2.36(m, 1H, SCCH) | 171.24, 163.87, 161.93, 149.58, 146.41(d, J=7.00 Hz), 132.86, 130.41(d, J=8.12 Hz), 128.69, 123.03(d, J=2.75 Hz), 120.64, 120.01, 119.89, 114.14(d, J=18.12 Hz), 113.97(d, J=19.00 Hz), 56.98, 51.74, 43.12, 38.68 |
| 6cc | 7.94—6.78(m, 7H, PhH), 5.40—5.38(m, 1H, NCH), 4.87(s, 1H, NH), 4.08(t, J=10.0 Hz, 1H, SCH), 3.67(s, 3H, OCH3), 2.57—2.52(m, 1H, SCCH), 2.36—2.31(m, 1H, SCCH) | 171.16, 162.24(dd, J=12.12, 245.00 Hz), 160.02(dd, J=12.12, 246.62 Hz), 149.54, 132.82, 130.43(dd, J=6.00, 9.75 Hz), 128.70, 126.27(dd, J=3.62, 13.88 Hz), 120.73, 119.98, 119.87, 111.26(dd, J=3.38, 21.00 Hz), 103.60(t, J=26.00 Hz), 51.73, 50.26, 43.19, 36.93 |
| 6dc | 7.86—6.79(m, 8H, PhH), 5.48—5.47(m, 1H, NCH), 4.81(s, 1H, NH), 4.16—4.13(m, 3H, OCH2, SCH), 2.60—2.54(m, 1H, SCCH), 2.39—2.34(m, 1H, SCCH), 1.23(t, J=7.0 Hz, 3H, CH3) | 170.60, 160.85, 158.90, 149.66, 132.72, 129.96(d, J=13.50 Hz), 129.30(d, J=8.62 Hz), 129.02(d, J=4.25 Hz), 128.53, 124.54(d, J=3.50 Hz), 120.60, 120.04, 119.98, 115.39(d, J=22.00 Hz), 60.89, 50.70, 43.81, 37.55, 13.55 |
| 6ec | 7.46—6.78(m, 8H, PhH), 5.12—5.10(m, 1H, NCH), 4.84(s, 1H, NH), 4.17—4.07(m, 2H, OCH2), 4.04(dd, J=4.5, 10.0 Hz, 1H, SCH), 2.50—2.44(m, 1H, SCCH), 2.41—2.36(m, 1H, SCCH), 1.21(t, J=7.0 Hz, 3H, CH3) | 170.67, 163.87, 161.93, 149.66, 146.50(d, J=7.00 Hz), 132.92, 130.40(d, J=8.25 Hz), 128.65, 123,02(d, J=2.62 Hz), 120.62, 120.02, 114.11(d, J=17.25 Hz), 113.94(d, J=18.12 Hz), 60.84, 57.01, 43.28, 38.74, 13.53 |
| 6fb | 7.84—6.71(m, 7H, PhH), 5.48(d, J=3.0 Hz, 1H, NH), 5.18(d, J=9.0 Hz, 1H, NCH), 4.01—4.04(m, 2H, OCH2), 4.02(dd, J=4.5, 9.5 Hz, 1H, SCH), 2.45(dd, J=9.0, 9.5 Hz, 1H, SCCH), 2.21—2.15(m, 1H, SCCH) | 170.96, 162.04(dd, J=12.75, 244.38 Hz), 159.96(dd, J=12.62, 246.50 Hz), 133.13, 130.96(dd, J=6.00, 9.75 Hz), 129.06, 126.46(dd, J=3.38, 13.62 Hz), 120.62, 120.37, 119.31, 111.89(dd, J=3.00, 20.75 Hz), 104.35(t, J=25.88 Hz), 61.42, 50.10, 43.68, 37.23, 14.40 |
| 7aa | 8.07—7.08(m, 8H, PhH), 3.68(t, J=6.75 Hz, 2H, SCH2), 2.94(t, J=6.75 Hz, 2H, SCCH2) | 169.73, 162.35, 160.36, 152.18, 134.77, 132.22(d, J=8.88 Hz), 130.79(d, J=3.25 Hz), 129.56, 127.32(d, J=11.62 Hz), 125.16(d, J=25.25 Hz), 124.54(d, J=3.25 Hz), 116.32(d, J=22.88 Hz), 41.19, 30.60 |
| 7ba | 7.72—7.00(m, 8H, PhH), 3.57(t, J=7.0 Hz, 2H, SCH2), 2.88(t, J=7.0 Hz, 2H, SCCH2) | 169.71, 164.13, 162.17,152.75, 140.23(d, J=7.12 Hz), 134.83, 130.21(d, J=8.00 Hz), 129.66, 125.16(d, J=14.38 Hz), 123.35, 122.93(d, J=2.62 Hz), 117.89(d, J=21.50 Hz), 114.15(d, J=22.62 Hz), 41.65, 29.74 |
| 7ca | 8.13—6.88(m, 7H, PhH), 3.67(t, J=6.75 Hz, 2H, SCH2), 2.91(t, J=6.75 Hz, 2H, SCCH2) | 168.49, 164.47(dd, J=12.25, 251.88 Hz), 161.68(dd, J=12.25, 251.75 Hz), 152.00, 134.79, 132.22(dd, J=4.75, 77.00 Hz), 129.62, 125.34, 125.01, 123.59(dd, J=3.62, 11.62 Hz), 112.08(dd, J=3.12, 21.00 Hz), 104.46(t, J=26.12 Hz), 41.17, 32.91 |
Table 4 1H NMR and 13C NMR data for compounds 4—7
| Compd. | 1H NMR(500 MHz), δ | 13C NMR(125 MHz), δ |
|---|---|---|
| 4aa | 8.07—6.94(m, 8H, PhH), 5.84(dd, J=3.0, 9.5 Hz, 1H, OCH), 3.69(s, 3H, OCH3), 2.88(dd, J=9.5, 15.5 Hz, 1H, OCCH), 2.64(dd, J=1.5, 15.5 Hz, 1H, OCCH) | 169.96, 162.17, 160.18, 157.65(d, J=2.88 Hz), 144.07, 133.03, 132.75(d, J=8.88 Hz), 130.53(d, J=2.88 Hz), 129.59, 127.78, 124.85(d, J=3.25 Hz), 123.61(d, J=11.75 Hz), 122.58, 116.83, 116.50(d, J=23.12 Hz), 70.05, 69.97, 52.06, 34.86 |
| 4ba | 7.81—6.94(m, 8H, PhH), 5.91(dd, J=2.5, 10.0 Hz, 1H, OCH), 3.73(s, 3H, OCH3), 2.88(dd, J=5.5, 16.0 Hz, 1H, OCCH), 2.47(dd, J=3.0, 15.5 Hz, 1H, OCCH) | 169.89, 164.18, 162.22, 157.66(d, J=2.75 Hz), 143.62, 136.87(d, J=7.25 Hz), 132.85, 130.49(d, J=8.12 Hz), 129.60, 127.88, 122.75, 122.31(d, J=2.88 Hz), 118.34(d, J=21.25 Hz), 116.88, 113.70(d, J=22.88 Hz), 68.02, 52.24, 35.30 |
| 4ca | 8.14—6.88(m, 7H, PhH), 5.80(dd, J=3.0, 10.0 Hz, 1H, OCH), 3.70(s, 3H, OCH3), 2.87(dd, J=9.5, 16.0 Hz, 1H, OCCH), 2.61(dd, J=3.0, 16.0 Hz, 1H, OCCH) | 169.87, 164.69(dd, J=12.25, 253.38 Hz), 161.51(dd, J=12.12, 252.38 Hz), 156.44, 156.41, 143.93, 132.92, 132.06(dd, J=4.62, 9.88 Hz), 129.65, 127.71, 122.65, 119.98(dd, J=3.88, 11.88 Hz), 116.87, 112.64(dd, J=3.38, 21.50 Hz), 104.68(t, J=26.12 Hz), 69.86, 69.78, 52.11, 34.78 |
| 4da | 8.06—6.93(m, 8H, PhH), 5.84(dd, J=3.0, 10.0 Hz, 1H, OCH), 4.18—4.09(m, 2H, OCH2), 2.85(dd, J=10.0, 15.5 Hz, 1H, OCCH), 2.62(dd, J=2.5, 15.5 Hz, 1H, OCCH), 1.23(t, J=7.0 Hz, 3H, CH3) | 169.52, 162.16, 160.16, 157.84(d, J=7.00 Hz), 144.15, 133.01, 132.74(d, J=8.88 Hz), 130.54(d, J=3.25 Hz), 129.57, 127.76, 124.88(d, J=3.12 Hz), 123.64(d, J=11.62 Hz), 122.54, 116.82, 116.51(d, J=23.12 Hz), 70.13, 70.05, 61.05, 35.13, 14.14 |
| 4ea | 7.84—6.96(m, 8H, PhH), 5.93(dd, J=3.5, 10.5 Hz, 1H, OCH), 4.21(m, 2H, OCH2), 2.88(dd, J=10.0, 15.5 Hz, 1H), 2.48(dd, J=3.0, 16.0 Hz, 1H, OCCH), 1.29(t, J=7.0 Hz, 3H, CH3) | 169.41, 164.17, 162.21, 157.75(d, J=2.88 Hz), 143.74, 136.95(d, J=7.25 Hz), 132.91, 130.46(d, J=7.88 Hz), 129.54, 127.87, 122.68, 122.35(d, J=2.62 Hz), 118.36, 118.28(d, J=21.62 Hz), 116.85, 113.70(d, J=22.75 Hz), 68.10, 61.24, 35.61, 14.16 |
| 4fa | 8.14—6.88(m, 7H, PhH), 5.81(dd, J=3.0, 9.5 Hz, 1H, OCH), 4.15(m, 2H, OCH2), 2.85(dd, J=10.0, 16.0 Hz, 1H, OCCH), 2.60(dd, J=1.5, 15.5 Hz, 1H, OCCH), 1.24(t, J=7.0 Hz, 3H, CH3) | 169.40, 164.67(dd, J=12.38, 253.50 Hz), 161.51(dd, J=12.25, 252.50 Hz), 156.59, 144.04, 132.95, 132.06(dd, J=5.00, 9.88 Hz), 129.61, 127.71, 122.59, 120.07(dd, J=4.00, 11.88 Hz), 116.84, 112.60(dd, J=3.25, 21.38 Hz), 104.67(t, J=26.25 Hz), 69.96, 69.88, 61.08, 35.11, |
| 5aa | 7.97—7.00(m, 8H, PhH), 4.73(dd, J=4.0, 11.5 Hz, 1H, NCH), 4.38(s, 1H, NH), 3.78(s, 3H, OCH3), 3.43(dd, J=4.0, 13.5 Hz, 1H, NCCH), 2.87(t, J=12.0 Hz, 1H, NCCH) | 173.31, 164.85, 162.41, 160.43, 138.75, 137.57, 131.71(d, J=8.50 Hz), 130.52(d, J=3.25 Hz), 128.59, 128.36(d, J=12.12 Hz), 126.91, 124.53(d, J=3.00 Hz), 121.77(d, J=41.5 Hz), 116.16(d, J=22.62 Hz), 65.90, 65.87, 52.64, 35.13, 35.08 |
| 5bb | 7.80—6.84(m, 8H, PhH), 6.04(s, 1H, NCH), 4.70(m, 1H, NH), 3.53(s, 3H, OCH3), 3.25(dd, J=8.0, 14.0 Hz, 1H, NCCH), 3.02(dd, J=4.0, 14.5 Hz, 1H, NCCH) | 172.71, 163.76, 163.42, 161.83, 142.05(d, J=7.12 Hz), 139.74, 136.74, 130.86(d, J=8.12 Hz), 130.42, 127.24, 123.41, 120.83, 119.95, 117.23(d, J=21.12 Hz), 113.64(d, J=22.62 Hz), 64.31, 52.21, 33.28 |
| 5ca | 8.00—6.90(m, 7H, PhH), 4.70(dd, J=4.0, 11.5 Hz, 1H, NCH), 4.36(s, 1H, NH), 3.79(s, 3H, OCH3), 3.40(dd, J=3.0, 13.5 Hz, 1H, NCCH), 2.85(t, J=13.0 Hz, 1H, NCCH) | 173.15, 164.09(dd, J=12.12, 263.12 Hz), 161.64(dd, J=11.75, 250.25 Hz), 138.60, 137.51, 131.88(dd, J=5.12, 9.75 Hz), 128.57, 126.98, 124.68(dd, J=3.62, 12.00 Hz), 122.00, 121.60, 112.00(dd, J=3.12, 21.38 Hz), 104.34(t, J=25.88 Hz), 65.83, 52.65, 35.03, 34.98 |
| 5da | 7.94—6.98(m, 8H, PhH), 4.68(dd, J=4.0, 11.5 Hz, 1H, NCH), 4.30(s, 1H, NH), 4.23—4.19(m, 2H, OCH2), 3.41(dd, J=4.0, 13.5 Hz, 1H, NCCH), 2.85(dd, J=11.5, 13.5 Hz, 1H, NCCH), 1.27(t, J=7.0 Hz, 3H, CH3) | 172.81, 165.02, 162.40, 160.42, 138.72, 137.67, 131.71(d, J=8.50 Hz), 130.54(d, J=3.25 Hz), 128.60, 128.41(d, J=12.00 Hz), 126.91, 124.54(d, J=3.00 Hz), 121.74(d, J=33.62 Hz), 116.17(d, J=22.38 Hz), 66.02, 61.79, 35.17, 35.12, 14.19 |
| 5ea | 7.79—6.96(m, 8H, PhH), 4.53(dd, J=4.0, 11.0 Hz, 1H, NCH), 4.34(s, 1H, NH), 4.24(q, J=7.0 Hz, 2H, OCH2), 3.45(dd, J=4.0, 13.5 Hz, 1H, NCCH), 2.86(dd, J=11.0, 13.5 Hz, 1H, NCCH), 1.31(t, J=7.0 Hz, 3H, CH3) | 172.47, 164.49(d, J=2.38 Hz), 164.11, 162.16, 141.14, 141.06(d, J=7.00 Hz), 138.95, 137.38, 130.14, 130.05(d, J=8.00 Hz), 128.85, 126.94, 122.70(d, J=2.62 Hz), 122.06, 121.47, 117.35(d, J=21.38 Hz), 114.10, 113.95(d, J=22.62 Hz), 66.12, 62.04, 32.03, 14.29 |
| 5fa | 7.99—6.87(m, 7H, PhH), 4.65(dd, J=4.0, 11.5 Hz, 1H, NCH), 4.36(s, 1H, NH), 4.23—4.19(m, 2H, OCH2), 3.37(dd, J=4.0, 13.5 Hz, 1H, NCCH), 2.83(dd, J=11.5, 13.5 Hz, 1H, NCCH), 1.28(t, J=7.5 Hz, 3H, CH3) | 172.67, 164.08(dd, J=12.12, 251.25 Hz), 161.64(dd, J=12.25, 250.88 Hz), 138.55, 137.61, 131.90(dd, J=4.88, 9.75 Hz), 128.57, 127.00, 124.71(dd, J=3.75, 11.88 Hz), 121.95, 121.61, 112.01(dd, J=3.25, 21.25 Hz), 104.35(t, J=26.00 Hz), 65.95, 61.83, 35.06, 35.01, 14.19 |
| 6ab | 7.78—6.70(m, 8H, PhH), 5.46(d, J=3.0 Hz, 1H, NH), 5.27—5.25(m, 1H, NCH), 4.11(dd, J=4.5, 9.5 Hz, 1H,SCH ), 3.62(s, 3H,OCH3 ), 2.51—2.46(m, 1H, SCCH), 2.23—2.17(m, 1H, SCCH) | 171.52, 160.85, 158.90, 150.15, 132.94, 129.95(d, J=13.38 Hz), 129.78(d, J=8.25 Hz), 129.65(d, J=3.75 Hz), 128.97, 125.01(d, J=2.88 Hz), 120.44(d, J=20.5 Hz), 119.20, 115.89(d, J=21.88 Hz), 52.79, 50.49, 43.80, 37.55 |
| 6bc | 7.45—6.77(m, 8H, PhH), 5.13—5.10(m, 1H, NCH), 4.85(s, 1H, NH), 4.07(dd, J=4.5, 9.5 Hz, 1H, SCH), 3.67(s, 3H, OCH3), 2.50—2.45(m, 1H, SCCH), 2.41—2.36(m, 1H, SCCH) | 171.24, 163.87, 161.93, 149.58, 146.41(d, J=7.00 Hz), 132.86, 130.41(d, J=8.12 Hz), 128.69, 123.03(d, J=2.75 Hz), 120.64, 120.01, 119.89, 114.14(d, J=18.12 Hz), 113.97(d, J=19.00 Hz), 56.98, 51.74, 43.12, 38.68 |
| 6cc | 7.94—6.78(m, 7H, PhH), 5.40—5.38(m, 1H, NCH), 4.87(s, 1H, NH), 4.08(t, J=10.0 Hz, 1H, SCH), 3.67(s, 3H, OCH3), 2.57—2.52(m, 1H, SCCH), 2.36—2.31(m, 1H, SCCH) | 171.16, 162.24(dd, J=12.12, 245.00 Hz), 160.02(dd, J=12.12, 246.62 Hz), 149.54, 132.82, 130.43(dd, J=6.00, 9.75 Hz), 128.70, 126.27(dd, J=3.62, 13.88 Hz), 120.73, 119.98, 119.87, 111.26(dd, J=3.38, 21.00 Hz), 103.60(t, J=26.00 Hz), 51.73, 50.26, 43.19, 36.93 |
| 6dc | 7.86—6.79(m, 8H, PhH), 5.48—5.47(m, 1H, NCH), 4.81(s, 1H, NH), 4.16—4.13(m, 3H, OCH2, SCH), 2.60—2.54(m, 1H, SCCH), 2.39—2.34(m, 1H, SCCH), 1.23(t, J=7.0 Hz, 3H, CH3) | 170.60, 160.85, 158.90, 149.66, 132.72, 129.96(d, J=13.50 Hz), 129.30(d, J=8.62 Hz), 129.02(d, J=4.25 Hz), 128.53, 124.54(d, J=3.50 Hz), 120.60, 120.04, 119.98, 115.39(d, J=22.00 Hz), 60.89, 50.70, 43.81, 37.55, 13.55 |
| 6ec | 7.46—6.78(m, 8H, PhH), 5.12—5.10(m, 1H, NCH), 4.84(s, 1H, NH), 4.17—4.07(m, 2H, OCH2), 4.04(dd, J=4.5, 10.0 Hz, 1H, SCH), 2.50—2.44(m, 1H, SCCH), 2.41—2.36(m, 1H, SCCH), 1.21(t, J=7.0 Hz, 3H, CH3) | 170.67, 163.87, 161.93, 149.66, 146.50(d, J=7.00 Hz), 132.92, 130.40(d, J=8.25 Hz), 128.65, 123,02(d, J=2.62 Hz), 120.62, 120.02, 114.11(d, J=17.25 Hz), 113.94(d, J=18.12 Hz), 60.84, 57.01, 43.28, 38.74, 13.53 |
| 6fb | 7.84—6.71(m, 7H, PhH), 5.48(d, J=3.0 Hz, 1H, NH), 5.18(d, J=9.0 Hz, 1H, NCH), 4.01—4.04(m, 2H, OCH2), 4.02(dd, J=4.5, 9.5 Hz, 1H, SCH), 2.45(dd, J=9.0, 9.5 Hz, 1H, SCCH), 2.21—2.15(m, 1H, SCCH) | 170.96, 162.04(dd, J=12.75, 244.38 Hz), 159.96(dd, J=12.62, 246.50 Hz), 133.13, 130.96(dd, J=6.00, 9.75 Hz), 129.06, 126.46(dd, J=3.38, 13.62 Hz), 120.62, 120.37, 119.31, 111.89(dd, J=3.00, 20.75 Hz), 104.35(t, J=25.88 Hz), 61.42, 50.10, 43.68, 37.23, 14.40 |
| 7aa | 8.07—7.08(m, 8H, PhH), 3.68(t, J=6.75 Hz, 2H, SCH2), 2.94(t, J=6.75 Hz, 2H, SCCH2) | 169.73, 162.35, 160.36, 152.18, 134.77, 132.22(d, J=8.88 Hz), 130.79(d, J=3.25 Hz), 129.56, 127.32(d, J=11.62 Hz), 125.16(d, J=25.25 Hz), 124.54(d, J=3.25 Hz), 116.32(d, J=22.88 Hz), 41.19, 30.60 |
| 7ba | 7.72—7.00(m, 8H, PhH), 3.57(t, J=7.0 Hz, 2H, SCH2), 2.88(t, J=7.0 Hz, 2H, SCCH2) | 169.71, 164.13, 162.17,152.75, 140.23(d, J=7.12 Hz), 134.83, 130.21(d, J=8.00 Hz), 129.66, 125.16(d, J=14.38 Hz), 123.35, 122.93(d, J=2.62 Hz), 117.89(d, J=21.50 Hz), 114.15(d, J=22.62 Hz), 41.65, 29.74 |
| 7ca | 8.13—6.88(m, 7H, PhH), 3.67(t, J=6.75 Hz, 2H, SCH2), 2.91(t, J=6.75 Hz, 2H, SCCH2) | 168.49, 164.47(dd, J=12.25, 251.88 Hz), 161.68(dd, J=12.25, 251.75 Hz), 152.00, 134.79, 132.22(dd, J=4.75, 77.00 Hz), 129.62, 125.34, 125.01, 123.59(dd, J=3.62, 11.62 Hz), 112.08(dd, J=3.12, 21.00 Hz), 104.46(t, J=26.12 Hz), 41.17, 32.91 |
| Compd. | C.albicans ATCC10231 | C.Neofonmans | Compd. | C.albicans ATCC10231 | C.Neofonmans | ||
|---|---|---|---|---|---|---|---|
| ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | ||||
| 3a | 9.8 | 22.3 | 24.8 | 3h | 6.0 | 6.0 | 15.7 |
| 3b | 10.3 | 20.4 | 24.5 | 3i | 6.0 | 6.0 | 6.0 |
| 3c | 6.0 | 15.5 | 18.5 | 3j | 6.0 | 6.0 | 12.2 |
| 3d | 10.4 | 22.5 | 21.1 | 3k | 6.0 | 6.0 | 6.0 |
| 3e | 6.0 | 20.3 | 23.4 | Controlb | 6.0 | 6.0 | 6.0 |
| 3f | 6.0 | 26.3 | 30.8 | A | 12.1 | 27.0 | 30.5 |
| 3g | 6.0 | 6.0 | 18.4 | ||||
Table 5 Zone of growth inhibition of compounds 3a—3k(mm)a
| Compd. | C.albicans ATCC10231 | C.Neofonmans | Compd. | C.albicans ATCC10231 | C.Neofonmans | ||
|---|---|---|---|---|---|---|---|
| ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | ||||
| 3a | 9.8 | 22.3 | 24.8 | 3h | 6.0 | 6.0 | 15.7 |
| 3b | 10.3 | 20.4 | 24.5 | 3i | 6.0 | 6.0 | 6.0 |
| 3c | 6.0 | 15.5 | 18.5 | 3j | 6.0 | 6.0 | 12.2 |
| 3d | 10.4 | 22.5 | 21.1 | 3k | 6.0 | 6.0 | 6.0 |
| 3e | 6.0 | 20.3 | 23.4 | Controlb | 6.0 | 6.0 | 6.0 |
| 3f | 6.0 | 26.3 | 30.8 | A | 12.1 | 27.0 | 30.5 |
| 3g | 6.0 | 6.0 | 18.4 | ||||
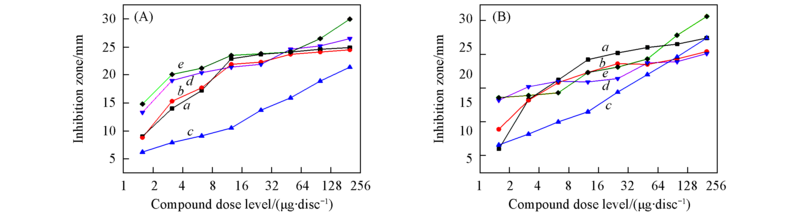
Fig.2 Inhibition zones at different dose levels for compounds 3a(a), 3b(b), 3d—3f(c—e) against C. neoformans(ATCC34874)(A) and C. neoformans(clinical isolate)(B)
| Compd. | MIC | MIC80 | MFC | |||
|---|---|---|---|---|---|---|
| 1a | 2a | 1a | 2a | 1a | 2a | |
| 3a | 3.0 | 3.0 | 2.0 | 2.0 | 12.0 | 12.0 |
| 3b | 4.0 | 3.0 | 2.0 | 2.0 | 12.0 | 14.0 |
| 3d | 2.0 | 1.5 | —b | —b | 8.0 | 8.0 |
| 3e | 2.0 | 2.0 | 1.0 | 1.0 | 10.0 | 8.0 |
| 3f | 1.0 | 0.5 | 0.5 | 0.5 | 6.0 | 6.0 |
| Fluconazole | >128.0 | >128.0 | 2.0 | 3.0 | >128.0 | >128.0 |
Table 6 MIC, MIC80 and MFC values for compounds 3a, 3b, 3d—3f and fluconazole(μg/mL)
| Compd. | MIC | MIC80 | MFC | |||
|---|---|---|---|---|---|---|
| 1a | 2a | 1a | 2a | 1a | 2a | |
| 3a | 3.0 | 3.0 | 2.0 | 2.0 | 12.0 | 12.0 |
| 3b | 4.0 | 3.0 | 2.0 | 2.0 | 12.0 | 14.0 |
| 3d | 2.0 | 1.5 | —b | —b | 8.0 | 8.0 |
| 3e | 2.0 | 2.0 | 1.0 | 1.0 | 10.0 | 8.0 |
| 3f | 1.0 | 0.5 | 0.5 | 0.5 | 6.0 | 6.0 |
| Fluconazole | >128.0 | >128.0 | 2.0 | 3.0 | >128.0 | >128.0 |
| Compd. | C. Neofonmans | Compd. | C. Neofonmans | ||
|---|---|---|---|---|---|
| ATCC34874 | Clinical isolate | ATCC34874 | Clinical isolate | ||
| 4a,4b,4d | 6.0 | 6.0 | 5a | 13.5 | 13.8 |
| 4c | 14.1 | 13.4 | 5b—5f | 6.0 | 6.0 |
| 4e | 11.2 | 12.7 | 6a—6f | 6.0 | 6.0 |
| 4f | 15.5 | 13.1 | 7a—7c | 6.0 | 6.0 |
| A | 27.0 | 30.5 | |||
Table 7 Zone of growth inhibition of compounds 4—7(mm)*
| Compd. | C. Neofonmans | Compd. | C. Neofonmans | ||
|---|---|---|---|---|---|
| ATCC34874 | Clinical isolate | ATCC34874 | Clinical isolate | ||
| 4a,4b,4d | 6.0 | 6.0 | 5a | 13.5 | 13.8 |
| 4c | 14.1 | 13.4 | 5b—5f | 6.0 | 6.0 |
| 4e | 11.2 | 12.7 | 6a—6f | 6.0 | 6.0 |
| 4f | 15.5 | 13.1 | 7a—7c | 6.0 | 6.0 |
| A | 27.0 | 30.5 | |||
| [1] | Nagao T., Sato M., Iwasawa Y., Takada T., Ishida R., Jpn. J. Pharmacol., 1972, 22, 467—478 |
| [2] | Nagao T., Sato M., Nakajima H., Kiyomoto A., Chem. Pharm. Bull., 1973, 21(1), 92—97 |
| [3] | Yamada K., Shimamura T., Nakajima H., Jpn. J. Pharmacol., 1973, 23, 321—328 |
| [4] | Yamamoto H., Asai H., Chem. Pharm. Bull., 1986, 34(9), 3844—3853 |
| [5] | Asano T., Okumura T., Hirano K., Adachi T., Sugiura M., Chem. Pharm. Bull., 1986, 34(10), 4238—4243 |
| [6] | Ambrogi V., Furlani A., Grandolini G., Papaioannou A., Perioli L., Scarcia V., Tuttobello L., Eur. J. Med. Chem., 1993, 28, 659—667 |
| [7] | Desai M. D., Desai K. K., Asian J. Chem., 2002, 14(2), 974—978 |
| [8] | Kang W., Du X. Q., Wang L. Z., Hu L. J., Dong Y. H., Bian Y. Q., Li Y., Chin. J. Chem., 2013, 31, 1305—1314 |
| [9] | Qing F. X., Chin. J. Org. Chem., 2012, 32, 815—824 |
| (卿凤翔. 有机化学, 2012, 32, 815—824) | |
| [10] | Wang J., Liu H., Chin. J. Org. Chem., 2011, 31, 1785—1798 |
| (王江, 柳红. 有机化学, 2011, 31, 1785—1798 | |
| [11] | Guo Q., Liu B. Q., Guo H. Y., Chin. J. Pharm., 2008, 39(4), 297—303 |
| (郭强, 刘秉全, 郭慧元. 中国医药工业杂志, 2008, 39(4), 297—303) | |
| [12] | Sang P., Zou J. W., Xu L., Huang G. D., Chem., 2009, 11, 973—978 |
| (桑鹏, 邹建卫, 许林, 黄光东. 化学通报, 2009, 11, 973—978) | |
| [13] | Zhang J., Jin C. F., Zhang Y. J., Chin. J. Org. Chem., 2014, 34, 662—680 |
| (张霁, 金传飞, 张英俊. 有机化学, 2014, 34, 662—680) | |
| [14] | Song Y., Wang J. C., Xu H., Zhang G. Z., Selim H. A., Li G. S., Wang Q., Gao Z. L., Chem. Res. Chinese Universities, 2013, 29(2), 263—269 |
| [15] | Zhang W.S., Li A. L., Medicinal Chemistry, Higher Education Press, Beijing, 1999, 545—553 |
| (仉文升, 李安良. 药物化学, 北京:高等教育出版社, 1999, 545—553) | |
| [16] | Jiang X. L., Cui Y. B., Cao S. H., Chin. J. Antibiot., 2011, 36(4), 255—263 |
| (蒋晓磊, 崔玉彬, 曹胜华. 中国抗生素杂志, 2011, 36(4), 255—263) | |
| [17] | Wan K., Zhang Y. Y., Zhou C. H., Zhou X. D., Geng R. X., Ji Q. G., Chin. J. Antibiot., 2012, 37(1), 8—15 |
| (万昆, 张奕奕, 周成合, 周向东, 耿蓉霞, 吉庆刚. 中国抗生素杂志, 2012, 37(1), 8—15) | |
| [18] | Wang A. P., Li R. Y., Chin. J. Mycol., 2010, 5(1), 44—47 |
| (王爱平, 李若瑜. 中国真菌学杂志, 2010, 5(1), 44—47) | |
| [19] | Tang L. S., J. Univ. Sci. Technol. Qingdao(Natural Science Edition), 2008, 29(2), 98—100 |
| (唐林生. 青岛科技大学学报(自然科学版), 2008, 29(2), 98—100) | |
| [20] | Li W. H., Liu X. Y., Zhang B., Li Y., Chin. J. Org. Chem., 2013, 33, 1503—1508 |
| (李文红, 刘喜莹, 张博, 李媛. 有机化学, 2013, 33, 1503—1508) | |
| [21] | Kang W., Bu H. J., Li W. H., Li Y., Chem. J. Chinese Universities, 2014, 35(4), 766—775 |
| (康旺, 卜辉娟, 李文红, 李媛. 高等学校化学学报, 2014, 35(4), 766—775) |
| [1] | 赵盈喆, 张建玲. 金属-有机框架基材料在二氧化碳光催化转化中的应用[J]. 高等学校化学学报, 2022, 43(7): 20220223. |
| [2] | 杨丹, 刘旭, 戴翼虎, 祝艳, 杨艳辉. 金团簇电催化二氧化碳还原反应的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220198. |
| [3] | 左怀龙, 雷思敏, 张锐, 李玉新, 陈伟. 新型异喹啉衍生物的设计合成及抑菌活性[J]. 高等学校化学学报, 2021, 42(9): 2766. |
| [4] | 胡皓程, 李文利, 张嘉宁, 刘宇博. 黑木耳寡糖的提取、 结构表征及生物活性[J]. 高等学校化学学报, 2021, 42(8): 2465. |
| [5] | 孙海珠, 杨国夺, 杨柏. 碳点的设计合成、 结构调控及应用[J]. 高等学校化学学报, 2021, 42(2): 349. |
| [6] | 侯华, 王宝山. 六氟化硫替代气体绝缘强度的官能团加和理论方法[J]. 高等学校化学学报, 2021, 42(12): 3709. |
| [7] | 叶晓栋, 齐国栋, 徐君, 邓风. Au负载SBA-15分子筛上葡萄糖氧化反应[J]. 高等学校化学学报, 2020, 41(5): 960. |
| [8] | 李普, 陈英, 夏榕娇, 郭涛, 张敏, 仕春, 汤旭, 贺鸣, 薛伟. 含喹喔啉杨梅素衍生物的合成及生物活性[J]. 高等学校化学学报, 2019, 40(5): 909. |
| [9] | 李冰, 王学敏, 白凤英, 刘淑清. 稀土氮杂环配合物的合成、 结构及抑菌活性[J]. 高等学校化学学报, 2019, 40(4): 632. |
| [10] | 常俊朋, 赵佳瑞, 陈思佳, 孟凯, 石微妮, 李瑞芳. 抗菌肽SAMP1及其类似肽的构效关系[J]. 高等学校化学学报, 2019, 40(4): 705. |
| [11] | 万金林, 巫受群, 甘宜远, 孟娇, 王贞超, 欧阳贵平. 含1,3,4-噻二唑结构的查尔酮缩氨基脲类化合物的合成及抗细菌活性[J]. 高等学校化学学报, 2018, 39(8): 1683. |
| [12] | 余敏, 黄晶晶, 马敏, 付瑞燕, 鄢嫣, 张福生, 殷俊峰, 谢宁宁. 三肽的锌螯合活性及定量构效关系分析[J]. 高等学校化学学报, 2018, 39(2): 234. |
| [13] | 侯华, 余小娟, 周文俊, 罗运柏, 王宝山. 绝缘气体介电强度的构效关系[J]. 高等学校化学学报, 2018, 39(11): 2477. |
| [14] | 王磊, 郑国钧, 季奇, 陈博, 巩龙龙, 高聪敏, 杜镇建, 张兴民. PI3K/mTOR抑制剂的合成及生物活性[J]. 高等学校化学学报, 2017, 38(9): 1590. |
| [15] | 谭英, 肖梦武, 叶姣, 胡艾希, 曾子清, 欧晓明. (Z)-3,3-二甲基-1-(1H-1,2,4-三唑-1-基)-2-丁酮肟(5-芳基-1,3,4-噁二唑-2-基)甲基醚的合成和抑菌活性[J]. 高等学校化学学报, 2017, 38(8): 1375. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||