

高等学校化学学报 ›› 2025, Vol. 46 ›› Issue (4): 20240490.doi: 10.7503/cjcu20240490
收稿日期:2024-10-31
出版日期:2025-04-10
发布日期:2024-12-24
通讯作者:
杨思伟
E-mail:yangsiwei@sxgkd.edu.cn;huangxr@jlu.edu.cn
作者简介:黄旭日, 男, 博士, 教授, 主要从事量子化学理论计算方面的研究. E-mail: huangxr@jlu.edu.cn
基金资助:Received:2024-10-31
Online:2025-04-10
Published:2024-12-24
Contact:
YANG Siwei
E-mail:yangsiwei@sxgkd.edu.cn;huangxr@jlu.edu.cn
Supported by:摘要:
采用密度泛函理论研究了B, N共掺杂富勒烯C70[C68B(n)N(m), n, m=1~5, 分别代表B和N取代的C位点]的氧还原反应(ORR)和氧析出反应(OER)性能. 结果表明, C68B(n)N(m)在热力学上是稳定的, 且其ΔG*OOH和ΔG*O与ΔG*OH均呈良好的线性关系. 其中, C68B(4)N(2)与C68B(5)N(2)催化剂的ORR过电位为0.45 V, 与商业Pt催化剂相当; C68B(4)N(1)的OER过电位最低(0.38 V), 优于传统RuO2催化剂(0.42 V), C68B(1)N(3)也表现出与RuO2相当的OER活性. 通过精确调控B, N共掺杂位置, 可显著降低ORR与OER过电位, 提升C70的催化性能. 根据活性趋势图, C68B(n)N(m)的最佳ORR和OER活性分别出现在ΔG*O-ΔG*OH=0.92 eV和ΔG*O-ΔG*OH=1.42 eV处. 研究结果为设计和发现新的非金属碳基电催化剂提供了线索.
中图分类号:
TrendMD:
杨思伟, 黄旭日. B, N共掺杂富勒烯C70作为氧还原和氧析出非金属电催化剂的理论研究. 高等学校化学学报, 2025, 46(4): 20240490.
YANG Siwei, HUANG Xuri. Theoretical Study of B, N Co-doped Fullerene C70 as Non-metal Electrocatalysts for Oxygen Reduction and Evolution. Chem. J. Chinese Universities, 2025, 46(4): 20240490.
| System | ΔG*OOH/eV | ΔG*O/eV | ΔG*OH/eV | System | ΔG*OOH/eV | ΔG*O/eV | ΔG*OH/eV |
|---|---|---|---|---|---|---|---|
| C68B(1)N(2) | 3.27 | -0.01 | 0.16 | C68B(3)N(4) | 4.37 | 2.00 | 1.40 |
| C68B(1)N(3) | 3.61 | 1.95 | 0.68 | C68B(3)N(5) | 4.18 | 1.25 | 1.26 |
| C68B(1)N(4) | 4.40 | 1.94 | 1.44 | C68B(4)N(1) | 3.58 | 1.97 | 0.55 |
| C68B(1)N(5) | 4.25 | 1.79 | 1.31 | C68B(4)N(2) | 3.74 | 1.57 | 0.78 |
| C68B(2)N(1) | 3.20 | 0 | 0.08 | C68B(4)N(3) | 3.74 | 1.90 | 0.73 |
| C68B(2)N(3) | 3.85 | 1.28 | 0.90 | C68B(4)N(5) | 2.98 | 0.22 | -0.01 |
| C68B(2)N(4) | 4.52 | 1.97 | 1.59 | C68B(5)N(1) | 3.65 | 1.44 | 0.71 |
| C68B(2)N(5) | 4.32 | 1.99 | 1.35 | C68B(5)N(2) | 3.73 | 1.85 | 0.78 |
| C68B(3)N(1) | 4.07 | 1.53 | 1.10 | C68B(5)N(3) | 4.30 | 1.62 | 1.22 |
| C68B(3)N(2) | 3.79 | 1.13 | 0.78 | C68B(5)N(4) | 4.19 | 1.44 | 1.24 |
Table 1 Adsorption free energies of *OOH, *O and *OH
| System | ΔG*OOH/eV | ΔG*O/eV | ΔG*OH/eV | System | ΔG*OOH/eV | ΔG*O/eV | ΔG*OH/eV |
|---|---|---|---|---|---|---|---|
| C68B(1)N(2) | 3.27 | -0.01 | 0.16 | C68B(3)N(4) | 4.37 | 2.00 | 1.40 |
| C68B(1)N(3) | 3.61 | 1.95 | 0.68 | C68B(3)N(5) | 4.18 | 1.25 | 1.26 |
| C68B(1)N(4) | 4.40 | 1.94 | 1.44 | C68B(4)N(1) | 3.58 | 1.97 | 0.55 |
| C68B(1)N(5) | 4.25 | 1.79 | 1.31 | C68B(4)N(2) | 3.74 | 1.57 | 0.78 |
| C68B(2)N(1) | 3.20 | 0 | 0.08 | C68B(4)N(3) | 3.74 | 1.90 | 0.73 |
| C68B(2)N(3) | 3.85 | 1.28 | 0.90 | C68B(4)N(5) | 2.98 | 0.22 | -0.01 |
| C68B(2)N(4) | 4.52 | 1.97 | 1.59 | C68B(5)N(1) | 3.65 | 1.44 | 0.71 |
| C68B(2)N(5) | 4.32 | 1.99 | 1.35 | C68B(5)N(2) | 3.73 | 1.85 | 0.78 |
| C68B(3)N(1) | 4.07 | 1.53 | 1.10 | C68B(5)N(3) | 4.30 | 1.62 | 1.22 |
| C68B(3)N(2) | 3.79 | 1.13 | 0.78 | C68B(5)N(4) | 4.19 | 1.44 | 1.24 |
| System | ΔG1/eV | ΔG2/eV | ΔG3/eV | ΔG4/eV | ηORR/V | ηOER/V | U | U |
|---|---|---|---|---|---|---|---|---|
| C68B(1)N(2) | -1.65 | -3.28 | 0.17 | -0.16 | 1.40 | 2.05 | -0.17 | 3.28 |
| C68B(1)N(3) | -1.31 | -1.66 | -1.27 | -0.68 | 0.55 | 0.43 | 0.68 | 1.66 |
| C68B(1)N(4) | -0.52 | -2.46 | -0.50 | -1.44 | 0.73 | 1.23 | 0.50 | 2.46 |
| C68B(1)N(5) | -0.67 | -2.46 | -0.48 | -1.31 | 0.75 | 1.23 | 0.48 | 2.46 |
| C68B(2)N(1) | -1.72 | -3.20 | 0.08 | -0.08 | 1.31 | 1.97 | -0.08 | 3.20 |
| C68B(2)N(3) | -1.07 | -2.57 | -0.38 | -0.90 | 0.85 | 1.34 | 0.38 | 2.57 |
| C68B(2)N(4) | -0.40 | -2.55 | -0.38 | -1.59 | 0.85 | 1.32 | 0.38 | 2.55 |
| C68B(2)N(5) | -0.60 | -2.33 | -0.64 | -1.35 | 0.63 | 1.10 | 0.60 | 2.33 |
| C68B(3)N(1) | -0.85 | -2.54 | -0.43 | -1.10 | 0.80 | 1.31 | 0.43 | 2.54 |
| C68B(3)N(2) | -1.13 | -2.66 | -0.35 | -0.78 | 0.88 | 1.43 | 0.35 | 2.66 |
| C68B(3)N(4) | -0.55 | -2.37 | -0.60 | -1.40 | 0.68 | 1.14 | 0.55 | 2.37 |
| C68B(3)N(5) | -0.74 | -2.93 | 0.01 | -1.26 | 1.24 | 1.70 | -0.01 | 2.93 |
| C68B(4)N(1) | -1.34 | -1.61 | -1.42 | -0.55 | 0.68 | 0.38 | 0.55 | 1.61 |
| C68B(4)N(2) | -1.18 | -2.17 | -0.79 | -0.78 | 0.45 | 0.94 | 0.78 | 2.17 |
| C68B(4)N(3) | -1.18 | -1.84 | -1.17 | -0.73 | 0.50 | 0.61 | 0.73 | 1.84 |
| C68B(4)N(5) | -1.94 | -2.76 | -0.23 | 0.01 | 1.24 | 1.53 | -0.01 | 2.76 |
| C68B(5)N(1) | -1.27 | -2.21 | -0.73 | -0.71 | 0.52 | 0.98 | 0.71 | 2.21 |
| C68B(5)N(2) | -1.19 | -1.88 | -1.07 | -0.78 | 0.45 | 0.65 | 0.78 | 1.88 |
| C68B(5)N(3) | -0.62 | -2.68 | -0.40 | -1.22 | 0.83 | 1.45 | 0.40 | 2.68 |
| C68B(5)N(4) | -0.73 | -2.75 | -0.20 | -1.24 | 1.03 | 1.52 | 0.20 | 2.75 |
Table 2 Free energy changes of each elementary step(ΔG1, ΔG2, ΔG3 and ΔG4) in ORR on C68B(n)N(m)and the overpotential of ORR and OER (ηORR and ηOER)
| System | ΔG1/eV | ΔG2/eV | ΔG3/eV | ΔG4/eV | ηORR/V | ηOER/V | U | U |
|---|---|---|---|---|---|---|---|---|
| C68B(1)N(2) | -1.65 | -3.28 | 0.17 | -0.16 | 1.40 | 2.05 | -0.17 | 3.28 |
| C68B(1)N(3) | -1.31 | -1.66 | -1.27 | -0.68 | 0.55 | 0.43 | 0.68 | 1.66 |
| C68B(1)N(4) | -0.52 | -2.46 | -0.50 | -1.44 | 0.73 | 1.23 | 0.50 | 2.46 |
| C68B(1)N(5) | -0.67 | -2.46 | -0.48 | -1.31 | 0.75 | 1.23 | 0.48 | 2.46 |
| C68B(2)N(1) | -1.72 | -3.20 | 0.08 | -0.08 | 1.31 | 1.97 | -0.08 | 3.20 |
| C68B(2)N(3) | -1.07 | -2.57 | -0.38 | -0.90 | 0.85 | 1.34 | 0.38 | 2.57 |
| C68B(2)N(4) | -0.40 | -2.55 | -0.38 | -1.59 | 0.85 | 1.32 | 0.38 | 2.55 |
| C68B(2)N(5) | -0.60 | -2.33 | -0.64 | -1.35 | 0.63 | 1.10 | 0.60 | 2.33 |
| C68B(3)N(1) | -0.85 | -2.54 | -0.43 | -1.10 | 0.80 | 1.31 | 0.43 | 2.54 |
| C68B(3)N(2) | -1.13 | -2.66 | -0.35 | -0.78 | 0.88 | 1.43 | 0.35 | 2.66 |
| C68B(3)N(4) | -0.55 | -2.37 | -0.60 | -1.40 | 0.68 | 1.14 | 0.55 | 2.37 |
| C68B(3)N(5) | -0.74 | -2.93 | 0.01 | -1.26 | 1.24 | 1.70 | -0.01 | 2.93 |
| C68B(4)N(1) | -1.34 | -1.61 | -1.42 | -0.55 | 0.68 | 0.38 | 0.55 | 1.61 |
| C68B(4)N(2) | -1.18 | -2.17 | -0.79 | -0.78 | 0.45 | 0.94 | 0.78 | 2.17 |
| C68B(4)N(3) | -1.18 | -1.84 | -1.17 | -0.73 | 0.50 | 0.61 | 0.73 | 1.84 |
| C68B(4)N(5) | -1.94 | -2.76 | -0.23 | 0.01 | 1.24 | 1.53 | -0.01 | 2.76 |
| C68B(5)N(1) | -1.27 | -2.21 | -0.73 | -0.71 | 0.52 | 0.98 | 0.71 | 2.21 |
| C68B(5)N(2) | -1.19 | -1.88 | -1.07 | -0.78 | 0.45 | 0.65 | 0.78 | 1.88 |
| C68B(5)N(3) | -0.62 | -2.68 | -0.40 | -1.22 | 0.83 | 1.45 | 0.40 | 2.68 |
| C68B(5)N(4) | -0.73 | -2.75 | -0.20 | -1.24 | 1.03 | 1.52 | 0.20 | 2.75 |
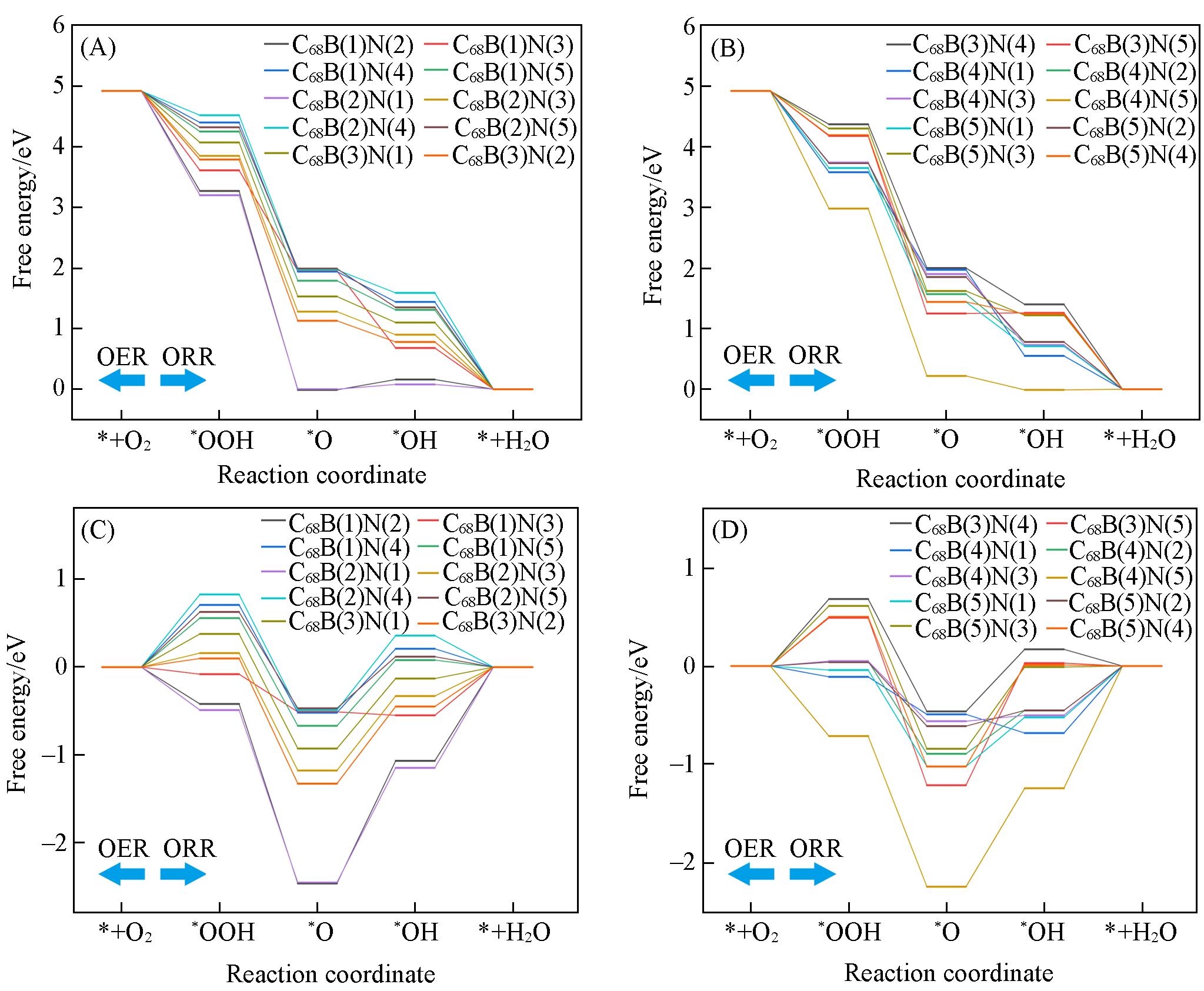
Fig.5 ORR and OER free energy diagrams of C68B(n)N(m)(A, B) 0 V; (C, D) 1.23 V. The ORR progress is from left to right, while the OER progress is contrary.
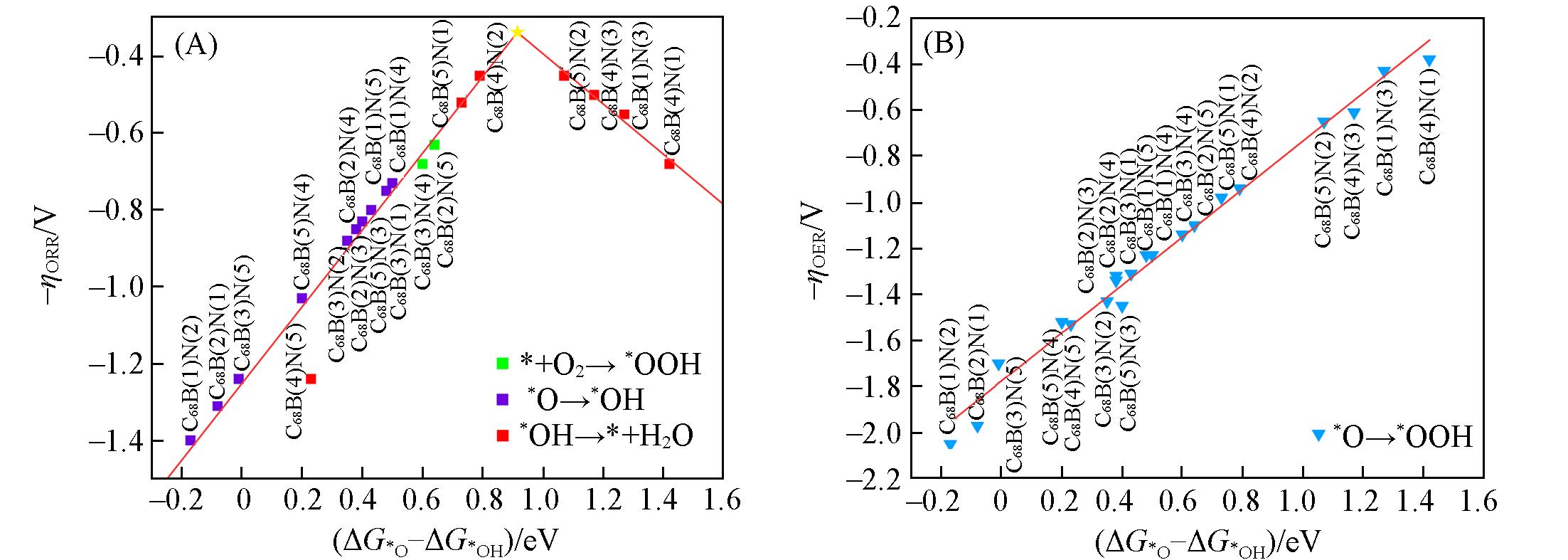
Fig.7 Activity trends towards oxygen reduction(A) and evolution(B) reactions for C68B(n)N(m)The potential-determining steps for ORR and OER are flagged out.
| 1 | Tang T. M., Bai X., Wang Z. L., Guan J. Q., Chem. Sci., 2024, 15(14), 5082—5112 |
| 2 | Chen Y. J., Cui H., Jiang Q., Bai X., Shan P. Y., Jia Z. P., Lu S., Song P., Feng R., Kang Q., Liang Z. Y., Yuan H. K., ACS Appl. Nano Mater., 2023, 6(9), 7694—7703 |
| 3 | Liu X. M., Cui X. Y., Dastafkan K., Wang H. F., Tang C., Zhao C., Chen A. B., He C. X., Han M. H., Zhang Q., J. Energy Chem., 2021, 53, 290—302 |
| 4 | Chang J. W., Zhang Q., Yu J. K., Jing W., Wang S. Y., Yin G. C., Waterhouse G. I. N., Lu S. Y., Adv. Sci., 2023, 10(22), 2301656 |
| 5 | Li Y. W., Zhang W. J., Li J., Ma H. Y., Du H. M., Li D. C., Wang S. N., Zhao J. S., Dou J. M., Xu L. Q., ACS Appl. Mater. Interfaces, 2020, 12(40), 44710—44719 |
| 6 | Hu Y. J., Zhao X., Yang Y., Xiao W. J., Zhou X., Wang D. G., Wang G., Bi J. S., Luo Z. J., Liu X. F., Appl. Surf. Sci., 2023, 614, 156256 |
| 7 | Niu H., Wan X. H., Wang X. T., Shao C., Robertson J., Zhang Z. F., Guo Y. Z., ACS Sustainable Chem. Eng., 2021, 9(9), 3590—3599 |
| 8 | Li D. Y., Zhang A. D., Feng Z. Z., Wang W. T., ACS Appl. Mater. Interfaces, 2024, 16(5), 5779—5791 |
| 9 | Ren M. Q., Lei J. C., Zhang J. B., Yakobson B. I., Tour J. M., ACS Appl. Mater. Interfaces, 2021, 13(36), 42715—42723 |
| 10 | Zhang W. J., Li J., Wei Z. D., Chin. J. Catal., 2023, 48, 15—31 |
| 11 | Wang J., Kong H., Zhang J. Y., Hao Y., Shao Z. P., Ciucci F., Prog. Mater. Sci., 2021, 116, 100717 |
| 12 | Hu C. G., Paul R., Dai Q. B., Dai L. M., Chem. Soc. Rev., 2021, 50(21), 11785—11843 |
| 13 | Zhao S. L., Wang D. W., Amal R., Dai L. M., Adv. Mater., 2019, 31(9), 1801526 |
| 14 | Guo K., Li N., Bao L. P., Lu X., Green Energy Environ., 2024, 9(1), 7—27 |
| 15 | Sinha S., Kim H., Robertson A. W., Mater. Today Adv., 2021, 12, 100169 |
| 16 | Kothandam G., Singh G., Guan X. W., Lee J. M., Ramadass K., Joseph S., Benzigar M., Karakoti A., Yi J. B., Kumar P., Vinu A., Adv. Sci., 2023, 10(18), 2301045 |
| 17 | Munawar T., Sardar S., Mukhtar F., Nadeem M. S., Manzoor S., Ashiq M. N., Khan S. A., Koc M., Iqbal F., Phys. Chem. Chem. Phys., 2023, 25(9), 7010—7027 |
| 18 | Park C., Lee E., Kim S. H., Han J. G., Hwang C., Joo S. H., Baek K., Kang S. J., Kwak S. K., Song H. K., Choi N. S., J. Power Sources, 2022, 521, 230923 |
| 19 | Nguyen N. N., Lee H. C., Yoo M. S., Lee E., Lee H., Lee S. B., Cho K., Adv. Sci., 2020, 7(6), 1902315 |
| 20 | Benzigar M. R., Joseph S., Ilbeygi H., Park D. H., Sarkar S., Chandra G., Umapathy S., Srinivasan S., Talapaneni S. N., Vinu A., Angew. Chem. Int. Ed., 2018, 57(2), 569—573 |
| 21 | Benzigar M. R., Joseph S., Baskar A. V., Park D. H., Chandra G., Umapathy S., Talapaneni S. N., Vinu A., Adv. Funct. Mater., 2018, 28(35), 1803701 |
| 22 | Gao R., Dai Q. B., Du F., Yan D. P., Dai L. M., J. Am. Chem. Soc., 2019, 141(29), 11658—11666 |
| 23 | Ahsan M. A., He T. W., Eid K., Abdullah A. M., Curry M. L., Du A. J., Puente Santiago A. R. P., Echegoyen L., Noveron J. C., J. Am. Chem. Soc., 2021, 143(2), 1203—1215 |
| 24 | Zhai Q. F., Pan Y., Dai L. M., Acc. Mater. Res., 2021, 2(12), 1239—1250 |
| 25 | Wu X., Tang C. J., Cheng Y., Min X. B., Jiang S. P., Wang S. Y., Chem. ⁃Eur. J., 2020, 26(18), 3906—3929 |
| 26 | Jaryal V. B., Villa A., Gupta N., ACS Sustainable Chem. Eng., 2023, 11(41), 14841—14865 |
| 27 | Dai L. M., Xue Y. H., Qu L. T., Choi H. J., Baek J. B., Chem. Rev., 2015, 115(11), 4823—4892 |
| 28 | Gao S. Y., Wei X. J., Fan H., Li L. Y., Geng K. R., Wang J. J., Nano Energy, 2015, 13, 518—526 |
| 29 | Li Q. Z., Zheng J. J., Dang J. S., Zhao X., ChemPhysChem, 2015, 16(2), 390—395 |
| 30 | Wang Y., Jiao M. G., Song W., Wu Z. J., Carbon, 2017, 114, 393—401 |
| 31 | Yang S. W., Cheng Y. X., Liu H. L., Huang X. R., Diamond Relat. Mater., 2022, 124, 108954 |
| 32 | Chen X. H., Ye P. C., Wang H. Y., Huang H., Zhong Y. J., Hu Y., Adv. Funct. Mater., 2023, 33(12), 2212915 |
| 33 | Lu Y. Y., Li Z. W., Bai Z. Y., Mi H. Y., Ji C. C., Pang H., Yu C., Qiu J. S., Nano Energy, 2019, 66, 104132 |
| 34 | Fu Y. Y., Cao C. C., Song W. R., Li B., Sun X. Z., Wang Z. X., Fan L. Q., Chen J., Chem. ⁃Eur. J., 2024, 30(28), e202400252 |
| 35 | Kang Y. M., Wang W., Li J. M., Imhanria S., Hao Y. X., Lei Z. Q., J. Power Sources, 2021, 493, 229665 |
| 36 | Lu Z. Y., Wang J., Huang S. F., Hou Y. L., Li Y. G., Zhao Y. P., Mu S. C., Zhang J. J., Zhao Y. F., Nano Energy, 2017, 42, 334—340 |
| 37 | Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Keith T., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision D.01, Gaussian Inc., Wallingford CT, 2013 |
| 38 | Becke A. D., J. Chem. Phys., 1993, 98(7), 5648—5652 |
| 39 | Lee C., Yang W. T., Parr R. G., Phys. Rev. B, 1988, 37(2), 785—789 |
| 40 | Grimme S., Antony J., Ehrlich S., Krieg H., J. Chem. Phys., 2010, 132(15), 154104 |
| 41 | Nørskov J. K., Rossmeisl J., Logadottir A., Lindqvist L., J. Phys. Chem. B, 2004, 108, 17886—17892 |
| 42 | Chen X., Chang J. B., Ke Q., Carbon, 2018, 126, 53—57 |
| 43 | Rossmeisl J., Logadottir A., Nørskov J. K., Chem. Phys., 2005, 319, 178—184 |
| 44 | Man I. C., Su H. Y., Calle⁃Vallejo F., Hansen H. A., Martínez J. I., Inoglu N. G., Kitchin J., Jaramillo T. F., Nørskov J. K., Rossmeisl J., ChemCatChem, 2011, 3(7), 1159—1165 |
| 45 | Medford A. J., Vojvodic A., Hummelshøj J. S., Voss J., Abild⁃Pedersen F., Studt F., Bligaard T., Nilsson A., Nørskov J. K., J. Catal., 2015, 328, 36—42 |
| [1] | 黄智瑶, 李丽, 徐华卿, 杨一凡, 韦瑶瑶, 刘国魁, 夏其英. N掺杂石墨烯缺陷材料催化OER/ORR的第一性原理研究[J]. 高等学校化学学报, 2025, 46(2): 20240430. |
| [2] | 何军, 朱傲阳, 魏雨晨, 朱怡全, 蒋莉, 何孝军. 三维氮掺杂分级多孔碳纳米片的制备及储锌性能[J]. 高等学校化学学报, 2024, 45(7): 20240099. |
| [3] | 陈俊杰, 张瑞丹, 陈越. 单层GeTe在锂/钠/钾离子电池中潜在应用的第一性原理研究[J]. 高等学校化学学报, 2024, 45(7): 20240148. |
| [4] | 张硕, 赵刘洋, 黄昊, 吴爱民, 李爱魁. 基于第一性原理高价元素Mo稳定层状富锂锰基材料的氧框架机制[J]. 高等学校化学学报, 2024, 45(5): 20240035. |
| [5] | 陈荣, 温良英, 岳东, 杨仲卿. Cl2和O2在TiC(100)表面共吸附行为的密度泛函理论分析[J]. 高等学校化学学报, 2024, 45(4): 20230497. |
| [6] | 陈晴晴, 李江涛, 黄欣蓉, 顾芳, 王海军. 氢键流体中Janus粒子的过量熵[J]. 高等学校化学学报, 2024, 45(2): 20230443. |
| [7] | 王鑫, 祁金阳, 杨瑞杰, 宋志国, 王敏. 基于苯磺酸配体构筑的Cu(II)配合物的合成、 表征及催化性能[J]. 高等学校化学学报, 2024, 45(10): 20240297. |
| [8] | 富忠恒, 陈翔, 姚楠, 余乐耕, 沈馨, 张睿, 张强. 固态电解质锂离子输运机制研究进展[J]. 高等学校化学学报, 2023, 44(5): 20220703. |
| [9] | 王军, 杜石谦, 陶李. 高温聚合物电解质膜燃料电池催化剂的研究进展[J]. 高等学校化学学报, 2023, 44(5): 20220722. |
| [10] | 李瑞松, 苗政培, 李静, 田新龙. 中空贵金属纳米材料氧还原催化的研究进展[J]. 高等学校化学学报, 2023, 44(5): 20220730. |
| [11] | 李轩, 亓帅, 周伟良, 李小杰, 景玲胭, 冯超, 蒋兴星, 杨恒攀, 胡琪, 何传新. 纤维基氧化还原电催化剂的研究进展[J]. 高等学校化学学报, 2023, 44(5): 20220770. |
| [12] | 鲍春竹, 向中华. 非热解共价有机聚合物基氧还原电催化材料[J]. 高等学校化学学报, 2023, 44(5): 20220715. |
| [13] | 彭辛哲, 葛娇阳, 王访丽, 余国静, 冉雪芹, 周栋, 杨磊, 解令海. 基于苯并噻吩平面格的张力与重组能的理论研究[J]. 高等学校化学学报, 2023, 44(2): 20220313. |
| [14] | 张海平, 孔雪, 夏文生, 张庆红, 万惠霖. C18环基过渡金属(Os, Ir)单原子对甲烷C—H的活化[J]. 高等学校化学学报, 2023, 44(11): 20230259. |
| [15] | 冯林雁, 胡晓波, 闫苗, 苗常青, 陈瑞, 郭谨昌, 王迎进. 平面十二配位MB8C4(M=Ca, Sr, Ba)分子轮团簇的理论研究[J]. 高等学校化学学报, 2023, 44(10): 20230281. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
