

高等学校化学学报 ›› 2024, Vol. 45 ›› Issue (10): 20240113.doi: 10.7503/cjcu20240113
收稿日期:2024-03-08
出版日期:2024-10-10
发布日期:2024-06-17
通讯作者:
李玲
E-mail:liling@fzu.edu.cn
基金资助:
ZHANG Mengjia, ZOU Nan, LUO Jiamei, ZHONG Xionghui, LI Ling( )
)
Received:2024-03-08
Online:2024-10-10
Published:2024-06-17
Contact:
LI Ling
E-mail:liling@fzu.edu.cn
Supported by:摘要:
通过无溶剂限域封装, 将二溴对二甲苯和4,4′-联吡啶吸附至UiO-67材料微孔中, 并原位聚合成聚离子液体PBpy-Br, 得到兼具Br-, Zr-OH/Zr-OH2和N杂环3种活性中心的复合材料PBpy-Br@UiO-67. 采用X射线衍射(XRD)、 傅里叶变换红外光谱(FTIR)、 扫描电子显微镜(SEM)、 13C核磁共振波谱(13C NMR)、 比表面积分析(BET)和热重分析(TGA)等手段对材料进行了表征, 并将其应用于催化CO2与环氧氯丙烷的环加成反应中. 在单因素实验基础上, 通过响应面优化确定的最佳反应条件为反应温度100 ℃、 pCO2=0.1 MPa、 催化剂用量(质量分数) 0.22%以及反应时间22 h. 在该条件下, 环氧氯丙烷转化率达99.6%, 催化剂循环使用5次后, 转化率仅下降2.3%. 此外, 对PBpy-Br@UiO-67的普适性进行了研究, 将其用于催化其它环氧化物与CO2反应, 转化率均在50%以上. 研究结果表明, PBpy-Br@UiO-67催化剂具有反应条件温和及催化效率高的特点, 为CO2的固定与转化提供了新催化体系.
中图分类号:
TrendMD:
张孟佳, 邹南, 罗佳美, 钟雄辉, 李玲. Zr-MOF固载聚离子液体对CO2环加成反应的催化性能. 高等学校化学学报, 2024, 45(10): 20240113.
ZHANG Mengjia, ZOU Nan, LUO Jiamei, ZHONG Xionghui, LI Ling. Catalytic Performance of Zr-MOF Supported Poly Ionic Liquid for CO2 Cycloaddition Reaction. Chem. J. Chinese Universities, 2024, 45(10): 20240113.
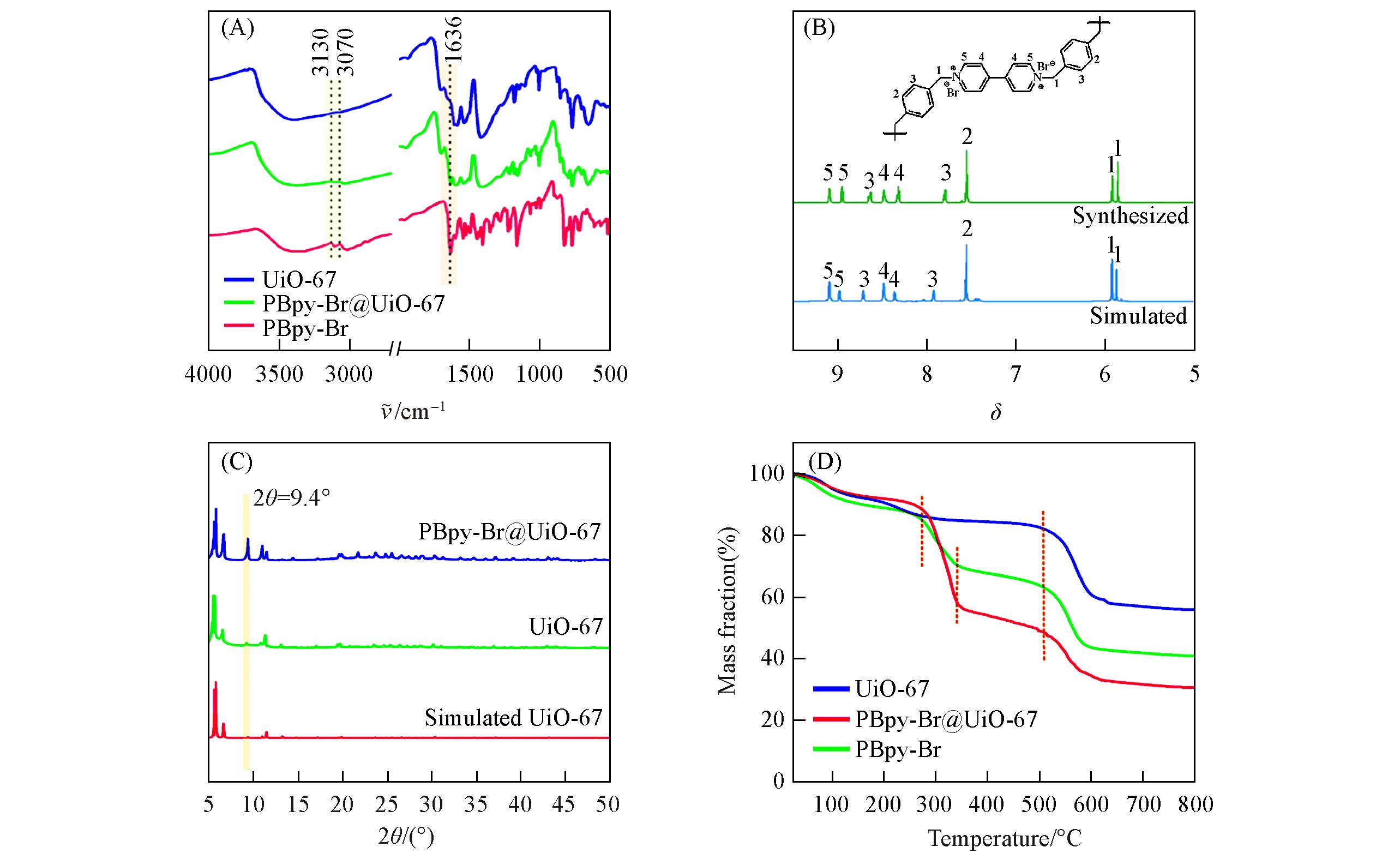
Fig.1 FTIR spectra of UiO⁃67, PBpy⁃Br and PBpy⁃Br@UiO⁃67(A), 13C NMR spectra of PBpy⁃Br(B), XRD patterns of UiO⁃67 and PBpy⁃Br@UiO⁃67(C) and TG curves of UiO⁃67, PBpy⁃Br and PBpy⁃Br@UiO⁃67(D)
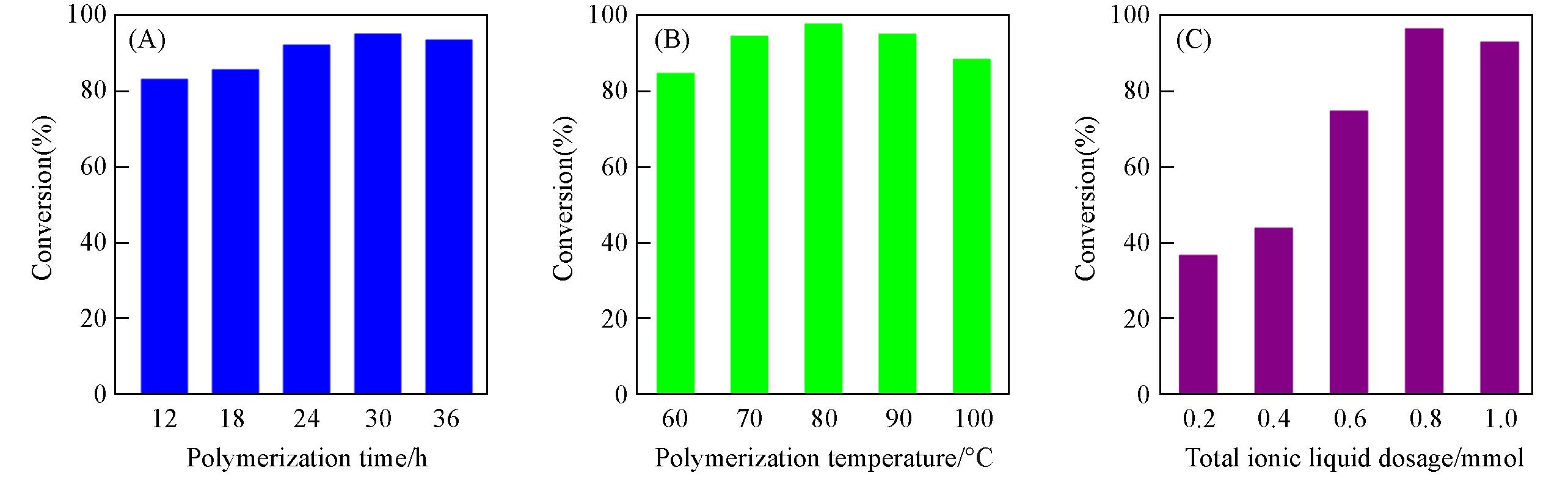
Fig.4 Effects of polymerization time(A), polymerization temperature(B) and dosage of ionic liquid(C) on catalytic performance of PBpy⁃Br@UiO⁃67Ionic liquid ECH: 1.2 g; catalyst: 0.22%; time: 22 h; temperature: 90 ℃; PCO2=105 Pa.
| Variable | Symbol | Code value | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| Temperature/℃ | X1 | 60 | 90 | 120 |
| Reaction time | X2 | 8.0 | 16 | 24 |
| Catalyst amount(mass fraction, %) | X3 | 0.05 | 0.17 | 0.30 |
Table 1 Factors and levels of RSM (Response surface methodology)
| Variable | Symbol | Code value | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| Temperature/℃ | X1 | 60 | 90 | 120 |
| Reaction time | X2 | 8.0 | 16 | 24 |
| Catalyst amount(mass fraction, %) | X3 | 0.05 | 0.17 | 0.30 |
| Entry | Value | Experimental value | Predictive value | |||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Average | Standard deviation | ||
| 1 | 0 | -1 | 1 | 85.99 | 0.021 | 90.09 |
| 2 | 0 | 0 | 0 | 32.89 | 0.34 | 33.14 |
| 3 | 0 | 0 | 0 | 90.49 | 1.1 | 90.09 |
| 4 | 1 | 0 | 1 | 88.05 | 0.20 | 88.32 |
| 5 | 0 | 0 | 0 | 50.48 | 1.6 | 51.84 |
| 6 | 0 | 1 | 1 | 81.55 | 0.57 | 81.54 |
| 7 | 1 | 0 | -1 | 69.95 | 0.49 | 65.58 |
| 8 | 0 | 1 | -1 | 93.69 | 0.30 | 90.09 |
| 9 | -1 | 0 | 1 | 68.98 | 0.10 | 70.36 |
| 10 | 0 | 0 | 0 | 55.37 | 0.42 | 57.00 |
| 11 | 0 | 0 | 0 | 92.16 | 0.10 | 90.09 |
| 12 | -1 | 1 | 0 | 89.33 | 0.10 | 89.08 |
| 13 | 1 | -1 | 0 | 23.81 | 0.21 | 23.82 |
| 14 | -1 | 0 | -1 | 57.24 | 0.16 | 55.88 |
| 15 | -1 | -1 | 0 | 56.37 | 0.71 | 54.74 |
| 16 | 1 | 1 | 0 | 88.12 | 0.82 | 90.09 |
| 17 | 0 | -1 | -1 | 39.91 | 0.93 | 34.64 |
Table 2 Predictive and experimental values of conversion(%) of Box-Behnken design
| Entry | Value | Experimental value | Predictive value | |||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Average | Standard deviation | ||
| 1 | 0 | -1 | 1 | 85.99 | 0.021 | 90.09 |
| 2 | 0 | 0 | 0 | 32.89 | 0.34 | 33.14 |
| 3 | 0 | 0 | 0 | 90.49 | 1.1 | 90.09 |
| 4 | 1 | 0 | 1 | 88.05 | 0.20 | 88.32 |
| 5 | 0 | 0 | 0 | 50.48 | 1.6 | 51.84 |
| 6 | 0 | 1 | 1 | 81.55 | 0.57 | 81.54 |
| 7 | 1 | 0 | -1 | 69.95 | 0.49 | 65.58 |
| 8 | 0 | 1 | -1 | 93.69 | 0.30 | 90.09 |
| 9 | -1 | 0 | 1 | 68.98 | 0.10 | 70.36 |
| 10 | 0 | 0 | 0 | 55.37 | 0.42 | 57.00 |
| 11 | 0 | 0 | 0 | 92.16 | 0.10 | 90.09 |
| 12 | -1 | 1 | 0 | 89.33 | 0.10 | 89.08 |
| 13 | 1 | -1 | 0 | 23.81 | 0.21 | 23.82 |
| 14 | -1 | 0 | -1 | 57.24 | 0.16 | 55.88 |
| 15 | -1 | -1 | 0 | 56.37 | 0.71 | 54.74 |
| 16 | 1 | 1 | 0 | 88.12 | 0.82 | 90.09 |
| 17 | 0 | -1 | -1 | 39.91 | 0.93 | 34.64 |
| Source | Variance | Free degree | Mean square | F | P(Prob>F) |
|---|---|---|---|---|---|
| Standard deviation=2.7 | R2=0.9940 | Adj R2=0.9863 | Pred R2=0.9685 | Mean=68.20 | Adeq precision=31.96 |
| Model | 8469.36 | 9 | 941.04 | 128.76 | <0.0001 |
| X1⁃temperature | 1798.20 | 1 | 1798.20 | 246.04 | <0.0001 |
| X2⁃time | 1346.55 | 1 | 1346.55 | 184.24 | <0.0001 |
| X3⁃catalyst | 1537.63 | 1 | 1537.63 | 210.38 | <0.0001 |
| X1X2 | 52.56 | 1 | 52.56 | 7.19 | 0.0315 |
| X1X3 | 10.18 | 1 | 10.18 | 1.39 | 0.2766 |
| X2X3 | 63.76 | 1 | 63.76 | 8.72 | 0.0213 |
| X12 | 2019.64 | 1 | 2019.64 | 276.33 | <0.0001 |
| X22 | 482.40 | 1 | 482.40 | 66.00 | <0.0001 |
| X32 | 815.13 | 1 | 815.13 | 111.53 | <0.0001 |
| Deviation | 51.16 | 7 | 7.31 | — | — |
| Lack of fit | 13.07 | 3 | 4.36 | 0.46 | 0.7268 |
| Pure error | 38.10 | 4 | 9.52 | — | — |
Table 3 Analysis of variance and statistical criteria
| Source | Variance | Free degree | Mean square | F | P(Prob>F) |
|---|---|---|---|---|---|
| Standard deviation=2.7 | R2=0.9940 | Adj R2=0.9863 | Pred R2=0.9685 | Mean=68.20 | Adeq precision=31.96 |
| Model | 8469.36 | 9 | 941.04 | 128.76 | <0.0001 |
| X1⁃temperature | 1798.20 | 1 | 1798.20 | 246.04 | <0.0001 |
| X2⁃time | 1346.55 | 1 | 1346.55 | 184.24 | <0.0001 |
| X3⁃catalyst | 1537.63 | 1 | 1537.63 | 210.38 | <0.0001 |
| X1X2 | 52.56 | 1 | 52.56 | 7.19 | 0.0315 |
| X1X3 | 10.18 | 1 | 10.18 | 1.39 | 0.2766 |
| X2X3 | 63.76 | 1 | 63.76 | 8.72 | 0.0213 |
| X12 | 2019.64 | 1 | 2019.64 | 276.33 | <0.0001 |
| X22 | 482.40 | 1 | 482.40 | 66.00 | <0.0001 |
| X32 | 815.13 | 1 | 815.13 | 111.53 | <0.0001 |
| Deviation | 51.16 | 7 | 7.31 | — | — |
| Lack of fit | 13.07 | 3 | 4.36 | 0.46 | 0.7268 |
| Pure error | 38.10 | 4 | 9.52 | — | — |
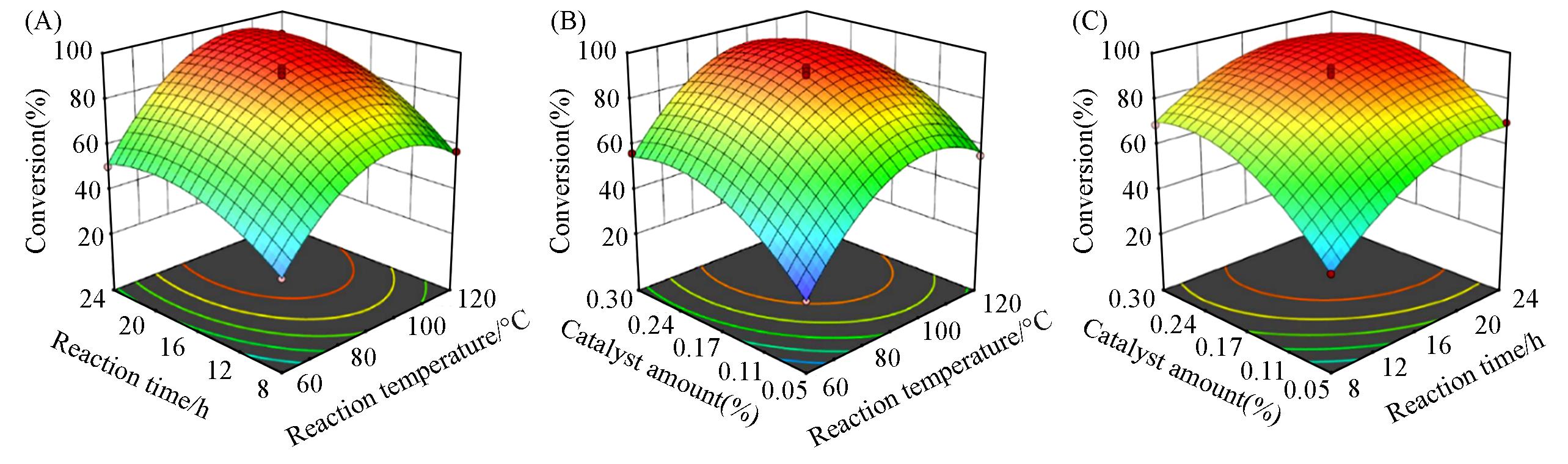
Fig.5 Response surface graphs for conversion of epichlorohydrin(A) Time and temperature; (B) catalyst amount and temperature; (C) time and catalyst amount.
| Factor | Time/h | Catalyst(%) | Temperature/℃ | pCO2/MPa | Conversion(%) |
|---|---|---|---|---|---|
| Predictive value | 20.79 | 0.22 | 101.3 | 0.1 | 99.5 |
| Experimental value | 22.00 | 0.22 | 100.0 | 0.1 | 99.6 |
Table 4 Conversion of PO at optimum predictive and experimental factors
| Factor | Time/h | Catalyst(%) | Temperature/℃ | pCO2/MPa | Conversion(%) |
|---|---|---|---|---|---|
| Predictive value | 20.79 | 0.22 | 101.3 | 0.1 | 99.5 |
| Experimental value | 22.00 | 0.22 | 100.0 | 0.1 | 99.6 |
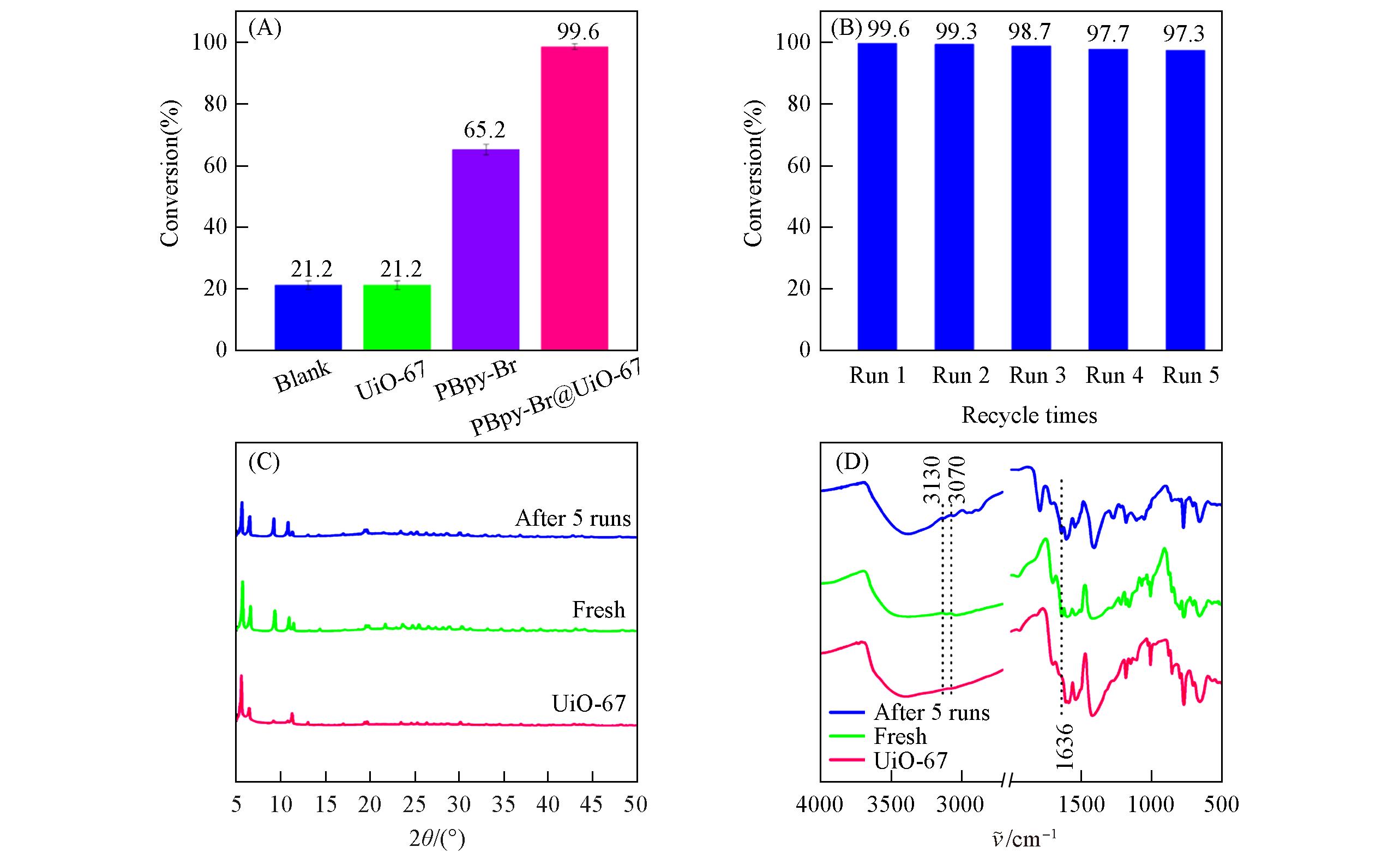
Fig.6 Activity and reuse performance of PBpy⁃Br@UiO⁃67(A) Catalyst activity; (B) relationship between conversion and recycle times; (C) XRD patterns of PBpy-Br@UiO-67 before and after 5 runs; (D) FTIR spectra of PBpy-Br@UiO-67 before and after 5 runs.
| Epoxide | Conversion(%) | Selectivity(%) | Epoxide | Conversion(%) | Selectivity(%) |
|---|---|---|---|---|---|
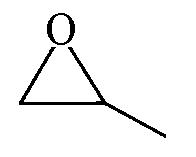 | 97.1 | >99 | 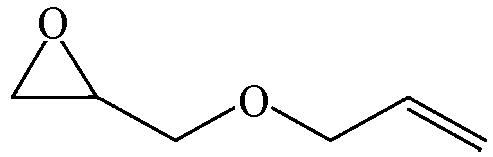 | 87.9 | 99 |
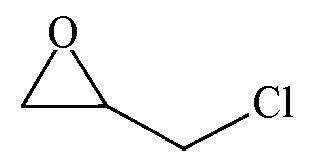 | 99.6 | >99 | 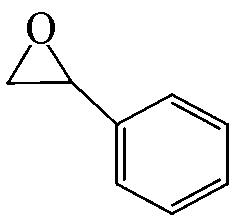 | 57.2 | 98 |
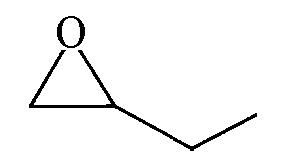 | 90.0 | 99 | 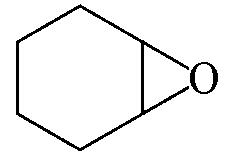 | 50.1 | 98 |
Table 5 Results of CO2 cycloaddition with different epoxides catalyzed by PBpy-Br@UiO-67
| Epoxide | Conversion(%) | Selectivity(%) | Epoxide | Conversion(%) | Selectivity(%) |
|---|---|---|---|---|---|
 | 97.1 | >99 |  | 87.9 | 99 |
 | 99.6 | >99 |  | 57.2 | 98 |
 | 90.0 | 99 |  | 50.1 | 98 |
| Catalyst | Dosage(molar ratio) | Time/h | Pressure/MPa | Temperature/℃ | Conversion(%) | Ref. |
|---|---|---|---|---|---|---|
| UiO⁃67⁃IL | 1.5 | 8 | 0.1 | 90 | 95.0 | [ |
| IL⁃ZIF⁃90 | 0.49 | 3 | 1.0 | 120 | 94.0 | [ |
| Meim⁃UiO⁃66 | 0.745 | 24 | 0.1 | 120 | 93.0 | [ |
| PBpy⁃Br@UiO⁃67 | 0.72 | 22 | 0.1 | 100 | 99.6 | This work |
Table 6 Summary of the reported CO2 cycloaddition of epichlorohydrin catalyzed by IL-MOFs catalytic systems
| Catalyst | Dosage(molar ratio) | Time/h | Pressure/MPa | Temperature/℃ | Conversion(%) | Ref. |
|---|---|---|---|---|---|---|
| UiO⁃67⁃IL | 1.5 | 8 | 0.1 | 90 | 95.0 | [ |
| IL⁃ZIF⁃90 | 0.49 | 3 | 1.0 | 120 | 94.0 | [ |
| Meim⁃UiO⁃66 | 0.745 | 24 | 0.1 | 120 | 93.0 | [ |
| PBpy⁃Br@UiO⁃67 | 0.72 | 22 | 0.1 | 100 | 99.6 | This work |
| 1 | Sanz⁃Pérez E. S., Murdock C. R., Didas S. A., Jones C. W., Chem. Rev., 2016, 116(19), 11840—11876 |
| 2 | Zhang Z., Yao Z. Z., Xiang S., Chen B., Energy Environ. Sci., 2014, 7(9),2868—2899 |
| 3 | Trickett C. A., Helal A., Al⁃Maythalony B. A., Yamani Z. H., Cordova K. E., Yaghi O. M., Nat. Rev. Mater., 2017, 2, 17045 |
| 4 | Wang W., Wang S., Ma X., Gong J. X., Chem. Soc. Rev., 2011, 40(7), 3703—3727 |
| 5 | Maina J. W., Pozo-Gonzalo C., Kong L., Schütz J., Hill M., Dumée. L. F., Mater. Horiz., 2017, 4(3), 345—361 |
| 6 | He H., Perman J. A., Zhu G., Ma S., Small, 2016, 12(46), 6309—6324 |
| 7 | Ding M., Jiang H. L., ACS Catal., 2018, 8(4), 3194—3201 |
| 8 | Liu F., Duan X., Liu M., Du J., Ma J., Liu F., Ind. Eng. Chem. Res., 2021, 60(42), 15027—15036 |
| 9 | Ke S. C., Luo T. T., Chang G. G., Huang K. X., Li J. X., Ma X. C., Wu J., Chen J., Yang X. Y., Inorg. Chem., 2020, 59(3), 1736—1745 |
| 10 | Deng L., Su Q., Tan X., Wang Y., Dong L., He H., Li Z., Cheng W., Mol. Catal., 2022, 519, 112153 |
| 11 | Zhang W., Dai J., Wu Y. C., Chen J. X., Shan S. Y., Cai Z., Zhu J. B., ACS Macro Lett., 2022, 11(2), 173—178 |
| 12 | Gonzalez A. C. S., Felgueiras A. P., Aroso R. T., Carrilho R. M. B., Pereira M. M., J. Organomet. Chem., 2021, 950, 121979 |
| 13 | Liu A. H., He L. N., Peng S. Y., Pan Z. D., Wang J. L., Gao J., Sci. China Chem., 2010, 53(7), 1578—1585 |
| 14 | Cai S., Zhu D., Zou Y., Zhao J., Nanoscale Res. Lett., 2016, 11(1), 321 |
| 15 | Motokucho S., Takenouchi Y., Satoh R., Morikawa H., Nakatani H., ACS Sustainable Chem. Eng., 2020, 8(11), 4337—4340 |
| 16 | Zhao Z., Kong X., Yuan Q., Xie H., Yang D., Zhao J., Fan H., Jiang L., Phys. Chem. Chem. Phys., 2018, 20(29), 19314—19320 |
| 17 | Xu Y., Yuan D., Wang Y., Yao Y., Dalton Trans., 2017, 46(18),5848—5855 |
| 18 | Bousquet B., Martinez A., Dufaud V., ChemCatChem, 2018, 10(4), 843—848 |
| 19 | Sharma N., Dhankhar S. S., Nagaraj C. M., Microporous Mesoporous Mater., 2019, 280, 372—378 |
| 20 | Noh J., Kim Y., Park H., Lee J., Yoon M., Park M. H., Kim Y., Kim M., J. Ind. Eng. Chem., 2018, 64, 478—483 |
| 21 | Abdelsayed V., Gardner T. H., Kababji A. H., Fan Y., Appl. Catal. A: Gen., 2019, 586, 117225 |
| 22 | Zhang L., Yuan S., Fan W., Pang J., Li F., Guo B., Zhang P., Sun D., Zhou H. C., ACS Appl. Mater. Interfaces, 2019, 11(25), 22390—22397 |
| 23 | Wu Y., Song. X., Xu S., Chen Y., Oderinde O., Gao L., Wei R., Xiao G., Dalton Trans., 2020, 49(2), 312—321 |
| 24 | Alves M., Grignard B., Boyaval A., Méreau R, De Winter J., Gerbaux P., Detrembleur C., Tassaing T., Jérôme C., ChemSusChem, 2017, 10(6),1128—1138 |
| 25 | Shen Y. M., Duan W. L., Shi M., Eur. J. Org. Chem., 2004, 2004(14), 3080—3089 |
| 26 | Sun Y., Huang H., Vardhan H., Aguila B., Zhong C., Perman J. A., Al⁃Eniz A. M., Nafady A., Ma S., ACS Appl. Mater. Interfaces, 2018, 10(32), 27124—27130 |
| 27 | Yin M., Wang L., Tang S., ACS Appl. Mater. Interaces, 2022, 14(50), 55674—55685 |
| 28 | Du Y. R., Yang X., Wang Y. F., Guan P. X., Wang R., Xu B. H., Mol. Catal., 2022, 520, 112164 |
| 29 | Li Y., Zhang X., Lai S., Dong H., Chen X., Wang X., Nie Y., Sheng Y., Zhang S., Fuel, 2012, 94, 617—619 |
| 30 | Chen X., Yuan S., Abdeltawab A. A., Al-Deyab S. S., Zhang J., Yu L., Yu G., Sep. Purif. Technol., 2014, 133, 187—193 |
| 31 | Ding L. G., Yao B. J., Jiang W. L., Li J. T., Fu Q. J., Li Y. A., Liu Z. H., Ma J. P., Dong Y. B., Inorg. Chem., 2017, 56(4), 2337—2344 |
| 32 | Bui M., Adjiman C. S., Bardow A. Anthony E. J., Boston A., Brown S., Fennell P. S., Fuss S., Galindo A., Hackett L. A., Hallett J. P., Herzog. H. J., Jackson G., Kemper J., Krevor S., Maitland G. C., Matuszewski M., Metcalfe L. S., Petit C., Puxty G., Reimer J., Reiner D. M., Rubin E. S., Scott S. A., Shah N., Smit B., Trusler J. P. M., Webley P., Wilcox J., Mac Dowell N., Energy Environ. Sci., 2018, 11(5), 1062—1176 |
| 33 | Ding M., Flaig R. W., Jiang H. L., Yaghi O. M., Chem. Soc. Rev., 2019, 48(10), 2783—2828 |
| 34 | Ji H., Naveen K., Lee W., Kim T. S., Kim D., Cho D. H., ACS Appl. Mater. Interfaces, 2020, 12(22), 24868—24876 |
| 35 | Wu Y., Yang X., Yuan H., Zhang Z., Shi S., Wei R., Gao L., Xiao G., Microporous Mesoporous Mater., 2021, 310, 110578 |
| 36 | Katz M. J., Brown Z. J., Colón Y. J., Siu P. W., Scheidt K. A., Snurr R. Q., Hupp J. T., Farha O. K., Chem. Commun., 2013, 49(82), 9449—9451 |
| 37 | Mazille F., Fei Z., Kuang D., Zhao D., Zakeeruddin S. M., Grätzel M., Dyson P. J., Inorg. Chem., 2006, 45(4), 1585—1590 |
| 38 | Ambroz F., Macdonald T. J.,Martis V., Parkin V. I., Small Methods, 2018, 2(11), 1800173 |
| 39 | Wang X., Lyu Q., Tong T., Sun K., Lin L. C., Tang C. Y., Yang F., Guiver M. D., Quan X., Dong Y., Nat. Commun., 2022, 13, 266 |
| 40 | Kathalikkattil A. C., Babu R., Roshan R. K., Lee H., Kim H., Tharun J., Suresh E., Park D. W., J. Mater. Chem. A, 2015, 3(45), 22636—22647 |
| 41 | Tharun J., Bhin K. M., Roshan R., Kim D. W., Kathalikkattil A. C., Babu R., Ahn H. Y., Won Y. S., Park D. W., Green Chem., 2016, 18(8), 2479—2487 |
| 42 | Boz E., Tüzün N. Ş., J. Organomet. Chem., 2013, 724, 167—176 |
| 43 | Liang J., Chen R. P., Wang X. Y., Liu T. T., Wang X. S., Huang Y. B., Cao R., Chem. Sci., 2017, 8(2), 1570—1575 |
| [1] | 龚文朋, 周林楠. 3,5-二硝基苯甲酸功能化的金属有机框架CAU-10-NH-DNBA荧光检测硫化氢[J]. 高等学校化学学报, 2024, 45(8): 20240069. |
| [2] | 唐海燕, 自丽梦, 张冰, 付昱. 用于光热转化的CuBDC-NH2/PmPD混合基质膜的制备与应用[J]. 高等学校化学学报, 2024, 45(2): 20230379. |
| [3] | 陈晓萍, 王旭潭, 刘宁, 汪庆祥, 倪建聪, 杨伟强, 林振宇. MOFs基微流控电化学芯片对多种重金属离子的实时在线检测[J]. 高等学校化学学报, 2024, 45(2): 20230395. |
| [4] | 杨思卿, 许神剑, 唐卿涵, 缪涵, 杨溪, 林绍梁. 木质素基聚离子液体膜的制备与应用[J]. 高等学校化学学报, 2024, 45(10): 130. |
| [5] | 李欣, 周颖, 王鼎南, 裴勇, 武斌, 张宜明. 基于硼亲和分子印迹策略的MOF/MIPs对沙丁胺醇的选择性吸附和计算模拟[J]. 高等学校化学学报, 2024, 45(1): 20230348. |
| [6] | 郭文娟, 张颖, 于洁, 代昭, 侯伟钊. Cu-NIC-TMED富集黄芩中黄芩苷的规律及机理[J]. 高等学校化学学报, 2023, 44(2): 20220511. |
| [7] | 郭昊天, 鲁新环, 孙凡棋, 陶艺元, 段金贵, 张望, 周丹, 夏清华. 纳米球型Mo-MOF材料的调控合成及催化硫醚选择性氧化[J]. 高等学校化学学报, 2023, 44(12): 20230408. |
| [8] | 路雨, 王铁. 中空金属有机框架材料的研究进展[J]. 高等学校化学学报, 2023, 44(1): 20220662. |
| [9] | 姜宏斌, 代文臣, 张娆, 徐晓晨, 陈捷, 杨光, 杨凤林. Co3O4/UiO-66@α-Al2O3陶瓷膜对VOCs废气的分离催化性能[J]. 高等学校化学学报, 2022, 43(6): 20220025. |
| [10] | 张小玉, 薛冬萍, 杜宇, 蒋粟, 魏一帆, 闫文付, 夏会聪, 张佳楠. MOF衍生碳基电催化剂限域催化O2还原和CO2还原反应[J]. 高等学校化学学报, 2022, 43(3): 20210689. |
| [11] | 李华, 杨科, 黄俊峰, 陈凤娟. UiO-66-NH2/wood的设计构筑及高效去除水中微量重金属离子性能[J]. 高等学校化学学报, 2022, 43(3): 20210701. |
| [12] | 柳雪广, 杨晓珊, 马菁菁, 刘伟生. 铕基金属有机框架材料从混合染料中选择性分离亚甲基蓝[J]. 高等学校化学学报, 2022, 43(1): 20210715. |
| [13] | 王婕, 霍海燕, 王洋, 张仲, 刘术侠. 铜箔上原位合成NENU-n系列多酸基MOFs的通用策略[J]. 高等学校化学学报, 2022, 43(1): 20210557. |
| [14] | 莫宗文, 张学文, 周浩龙, 周东东, 张杰鹏. 一种多孔配位聚合物的氢键协同客体响应[J]. 高等学校化学学报, 2022, 43(1): 20210576. |
| [15] | 常书晴, 辛旭, 黄雅琦, 张信聪, 傅仰河, 朱伟东, 张富民, 李晓娜. Zr基金属有机框架材料的冷热驱动热释电催化性能[J]. 高等学校化学学报, 2021, 42(8): 2558. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||