

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (3): 20210689.doi: 10.7503/cjcu20210689
张小玉1, 薛冬萍1, 杜宇1, 蒋粟1, 魏一帆1, 闫文付2( ), 夏会聪1, 张佳楠1(
), 夏会聪1, 张佳楠1( )
)
收稿日期:2021-09-26
出版日期:2022-03-10
发布日期:2021-11-22
通讯作者:
闫文付,张佳楠
E-mail:yanw@jlu.edu.cn;zjn@zzu.edu.cn
基金资助:
ZHANG Xiaoyu1, XUE Dongping1, DU Yu1, JIANG Su1, WEI Yifan1, YAN Wenfu2( ), XIA Huicong1, ZHANG Jianan1(
), XIA Huicong1, ZHANG Jianan1( )
)
Received:2021-09-26
Online:2022-03-10
Published:2021-11-22
Contact:
YAN Wenfu,ZHANG Jianan
E-mail:yanw@jlu.edu.cn;zjn@zzu.edu.cn
Supported by:摘要:
化石燃料的大量消耗和环境的逐渐恶化导致迫切需要开发和探索有效的能源转换和存储技术. 电化学是各种能源转换装置的基础和关键. 设计和合成具有高催化活性的非贵金属基和非金属基催化剂是最好的选择. 金属有机骨架(MOF)衍生的碳基材料具有比表面积大、 孔隙率高的特点, 可以选择性地限制不同类型的金属. 因此, MOF衍生碳作为催化剂载体使用时具有良好的限域效应, 有利于提高催化剂的活性和稳定性. 本文综合评述了MOF衍生材料在催化反应中的限域效应, 并介绍了MOF衍生碳基材料在氧还原反应(ORR)和二氧化碳还原反应(CO2RR)电催化方面的最新进展, 揭示了MOF碳基材料在电催化反应中的构效关系. 最后, 讨论了MOF衍生的碳基材料在ORR和CO2RR电催化中的挑战和机遇, 以及未来可能的解决方案.
中图分类号:
TrendMD:
张小玉, 薛冬萍, 杜宇, 蒋粟, 魏一帆, 闫文付, 夏会聪, 张佳楠. MOF衍生碳基电催化剂限域催化O2还原和CO2还原反应. 高等学校化学学报, 2022, 43(3): 20210689.
ZHANG Xiaoyu, XUE Dongping, DU Yu, JIANG Su, WEI Yifan, YAN Wenfu, XIA Huicong, ZHANG Jianan. MOF-derived Carbon-based Electrocatalysts Confinement Catalyst on O2 Reduction and CO2 Reduction Reactions. Chem. J. Chinese Universities, 2022, 43(3): 20210689.
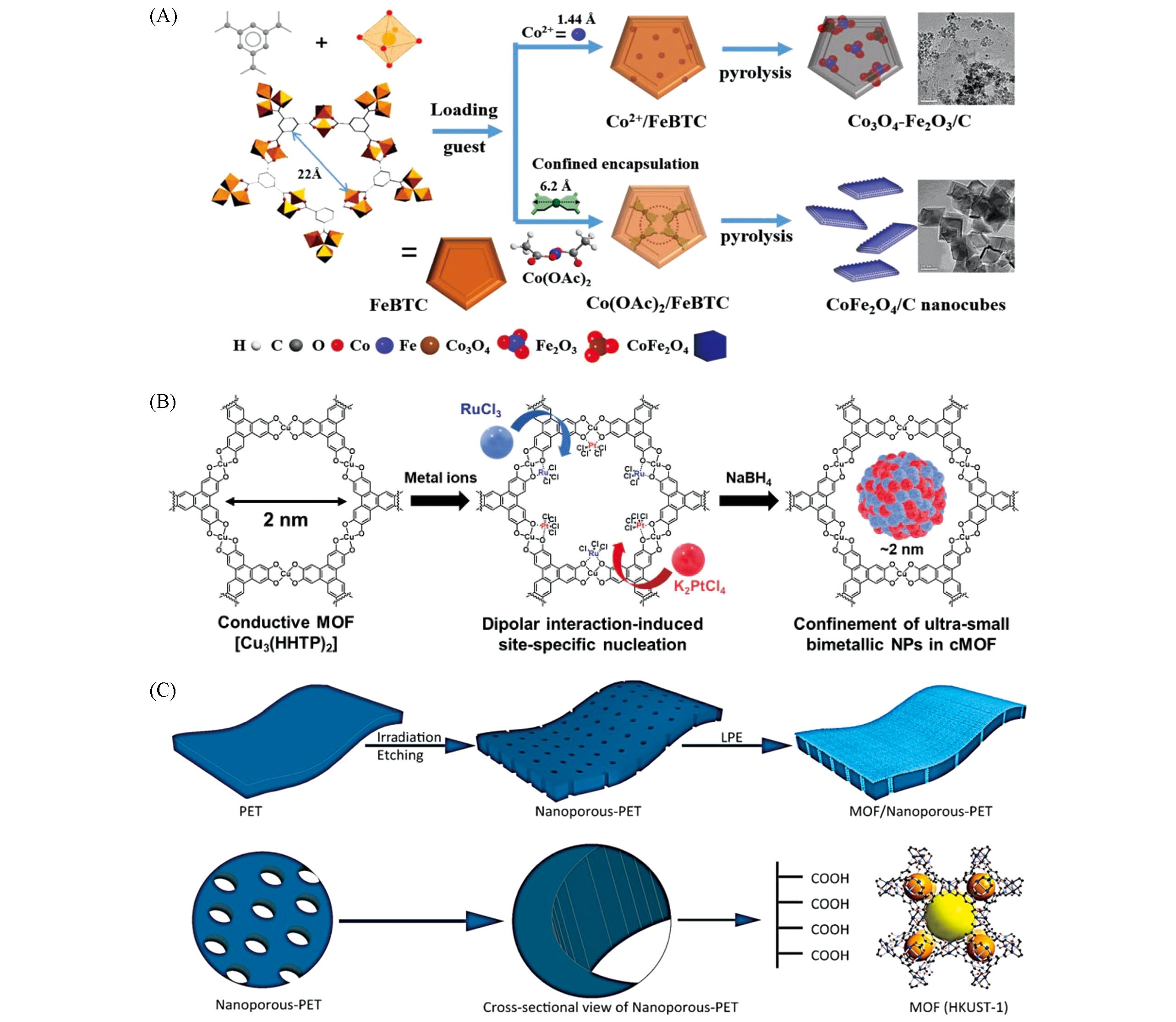
Fig.1 Confinement engneering on catalytic activity(A) Schematic illustration of assembly MOFs-derived bimetallic spinel oxides CoFe2O4 nanocubes through the combination of exchange-coordination and pyrolysis[49]. Copyright 2020, Wiley-VCH. (B) Schematic illustration for the preparation of PtRu@cMOF[53]. Copyright 2021, Wiley-VCH. (C) Schematic illustration of the formation of nanochannels in the polymer membrane and subsequent confinement of the porous HKUST-1 metal-organic framework[55]. Copyright 2020, American Chemical Society.
| Catalyst | Species of metals | Electrolyte | E1/2/V (vs. RHE) | Limiting current density/(mA· cm-2) | Eonset/V (vs. RHE) | Durability | Ref. |
|---|---|---|---|---|---|---|---|
| Co?N?GA | Nanoparticles | 0.1 mol/L KOH | — | 6 | 0.9 | 10000 s | [ |
| Fe?NC | Nanoparticles | 0.1 mol/L KOH | 0.877 | 5.82 | 0.963 | 20000 s | [ |
| 20Mn?NC?second | Single atom | 0.5 mol/L H2SO4 | 0.80 | ca. 3.9 | — | 100 h | [ |
| C?FeHZ8@g?C3N4?950 | Single atom | 0.1 mol/L HClO4 | 0.78 | ca. 5.5 | — | 8000 s | [ |
| Fe SAC/N?C | Single atom | 0.1 mol/L KOH | 0.89 | ca. 5.5 | — | 4000 s | [ |
| Fe?N?C?P/N,P?C | Single atom | 0.1 mol/L HClO4 | 0.80 | 6 | 1.06 | — | [ |
| 6%Fe?N?S CNN | Single atom | 0.1 mol/L KOH | 0.91 | ca. 5.6 | — | 12 h | [ |
| FeNi0.25?NC | Single atom | 0.1 mol/L HClO4 | 0.79 | — | — | — | [ |
| Co(mIm)?NC | Single atom | 0.5 mol/L H2SO4 | 0.82 | ca. 4 | 0.93 | 50 h | [ |
| Co SAs/N?C(900) | Single atom | 0.1 mol/L KOH | 0.881 | ca. 5.6 | 0.982 | — | [ |
| CoOx@PNC | Cluster | 0.1 mol/L KOH | 0.88 | ca. 6.5 | 0.98 | 200 h | [ |
| FeNC?S?FexC/Fe | Cluster | 0.1 mol/L HClO4 | 0.821 | 5.75 | 1.05 | — | [ |
| BTC?Co?O?Cu?BTA | Cluster | 0.1 mol/L NaOH | 0.95 | ca. 6 | 1.06 | — | [ |
| Cu@Fe?N?C | Nanoparticles | 0.1 mol/L KOH | 0.892 | ca. 5.52 | 1.01 | 20000 s | [ |
| Co?ZnO@NC/CNT?700 | Nanoparticles | 0.1 mol/L KOH | 0.86 | ca. 5.98 | 0.9 | 25000 s | [ |
Table 1 Summary of previously reported MOF-derived carbon-based catalysts and their application in ORR
| Catalyst | Species of metals | Electrolyte | E1/2/V (vs. RHE) | Limiting current density/(mA· cm-2) | Eonset/V (vs. RHE) | Durability | Ref. |
|---|---|---|---|---|---|---|---|
| Co?N?GA | Nanoparticles | 0.1 mol/L KOH | — | 6 | 0.9 | 10000 s | [ |
| Fe?NC | Nanoparticles | 0.1 mol/L KOH | 0.877 | 5.82 | 0.963 | 20000 s | [ |
| 20Mn?NC?second | Single atom | 0.5 mol/L H2SO4 | 0.80 | ca. 3.9 | — | 100 h | [ |
| C?FeHZ8@g?C3N4?950 | Single atom | 0.1 mol/L HClO4 | 0.78 | ca. 5.5 | — | 8000 s | [ |
| Fe SAC/N?C | Single atom | 0.1 mol/L KOH | 0.89 | ca. 5.5 | — | 4000 s | [ |
| Fe?N?C?P/N,P?C | Single atom | 0.1 mol/L HClO4 | 0.80 | 6 | 1.06 | — | [ |
| 6%Fe?N?S CNN | Single atom | 0.1 mol/L KOH | 0.91 | ca. 5.6 | — | 12 h | [ |
| FeNi0.25?NC | Single atom | 0.1 mol/L HClO4 | 0.79 | — | — | — | [ |
| Co(mIm)?NC | Single atom | 0.5 mol/L H2SO4 | 0.82 | ca. 4 | 0.93 | 50 h | [ |
| Co SAs/N?C(900) | Single atom | 0.1 mol/L KOH | 0.881 | ca. 5.6 | 0.982 | — | [ |
| CoOx@PNC | Cluster | 0.1 mol/L KOH | 0.88 | ca. 6.5 | 0.98 | 200 h | [ |
| FeNC?S?FexC/Fe | Cluster | 0.1 mol/L HClO4 | 0.821 | 5.75 | 1.05 | — | [ |
| BTC?Co?O?Cu?BTA | Cluster | 0.1 mol/L NaOH | 0.95 | ca. 6 | 1.06 | — | [ |
| Cu@Fe?N?C | Nanoparticles | 0.1 mol/L KOH | 0.892 | ca. 5.52 | 1.01 | 20000 s | [ |
| Co?ZnO@NC/CNT?700 | Nanoparticles | 0.1 mol/L KOH | 0.86 | ca. 5.98 | 0.9 | 25000 s | [ |
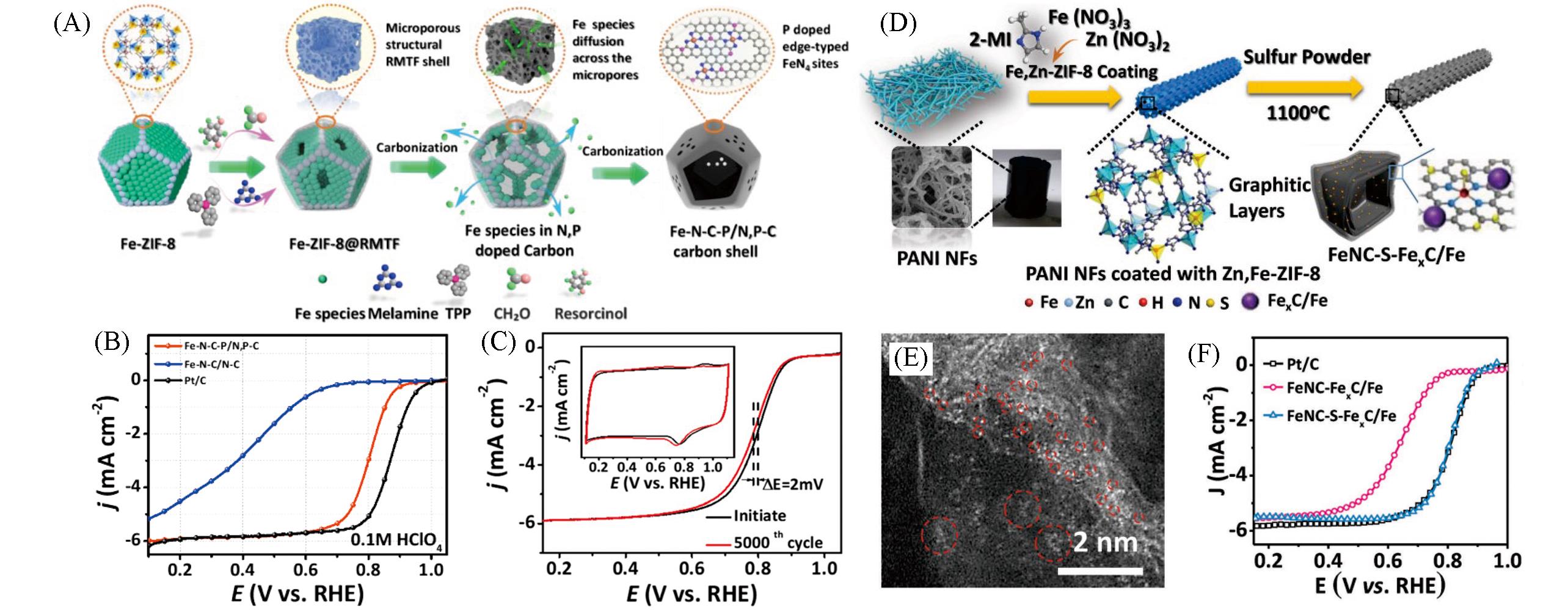
Fig.3 MOF?derived atomically dispersion metal carbon?based materials for confinement electrocatalytic ORR(A) Schematic of the preparation of the Fe-N-C-P/N,P-C; (B) LSV curves of ORR in O2-saturated 0.1 mol/L HClO4 at 1600 r/min for Fe-N-C-P/N,P-C, Fe-N-C/N-C and Pt/C; (C) ORR polarization LSV and CV curves of Fe-N-C-P/N,P-C measurement before and after 5000 potential cycles at the scan rate of 50 mV/s[62]. Copyright 2021, American Chemical Society; (D) synthesis scheme of the Fe-NC-S-Fe x C/Fe catalyst; (E) HAADF-STEM image of Fe-NC-S-Fe x C/Fe; (F) LSV curves of ORR in O2-saturated 0.1 mol/L HClO4 at 1600 r/min for different catalysts[68]. Copyright 2018, Wiley-VCH.
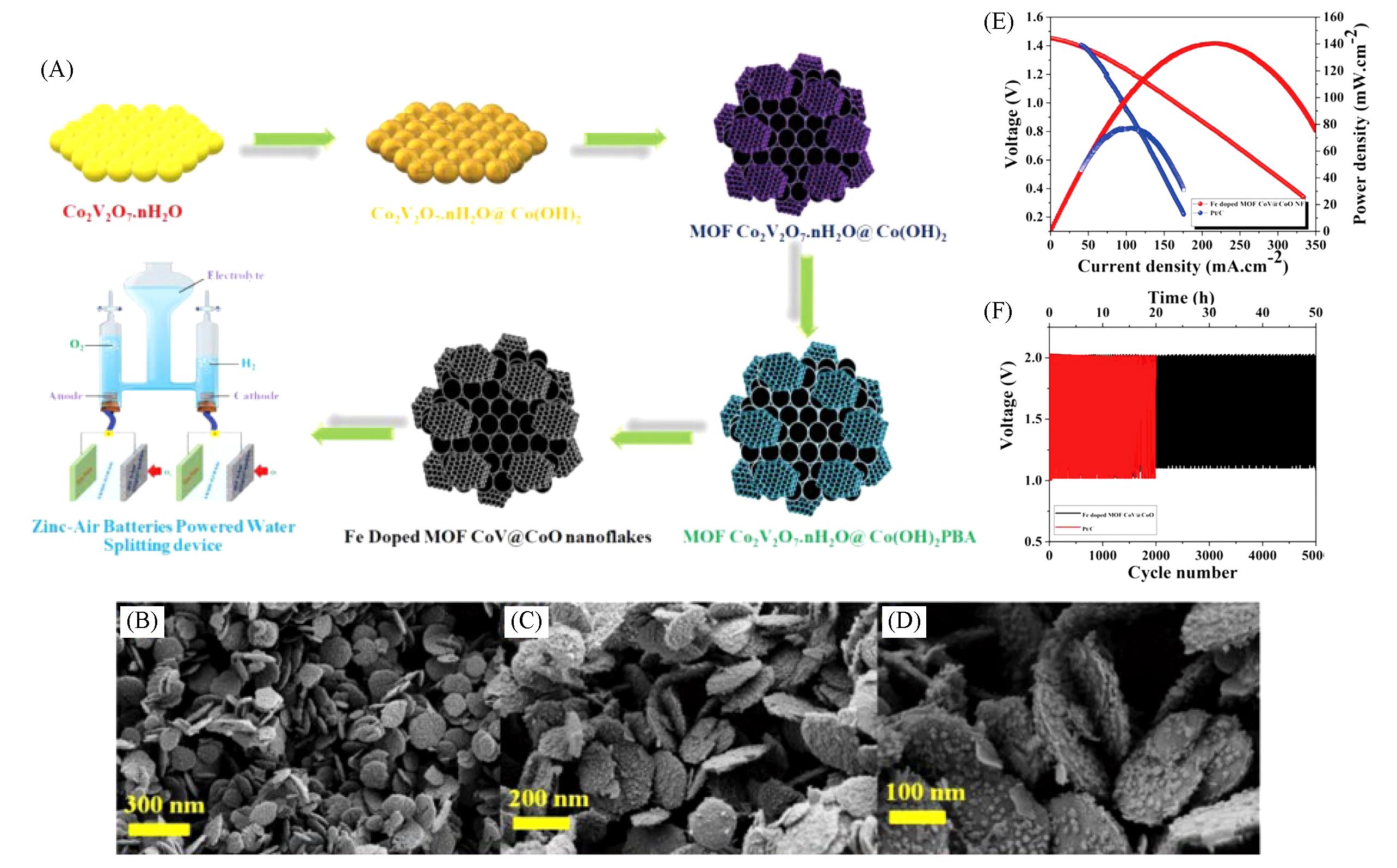
Fig.4 MOF?derived metal nanoparticles carbon?based materials for confinement electrocatalytic ORR[82](A) The schematic illustration of synthetic strategy of Fe doped MOF CoV@CoO nanoflakes and self-powered zinc-air battery water splitting applications; (B—D) different magnifications FESEM images of Fe doped MOF CoV@CoO nanoflakes; (E) discharge pola-rization curves and related power densities of Fe doped MOF CoV@CoO nanoflakes and Pt/C/IrO2 catalyst; (F) galvanostatic charge and discharge cycling curve at 10 mA/cm2 for Fe doped MOF CoV@CoO nanoflakes and commercial Pt/C/IrO2 catalyst. Copyright 2021, Elsevier.
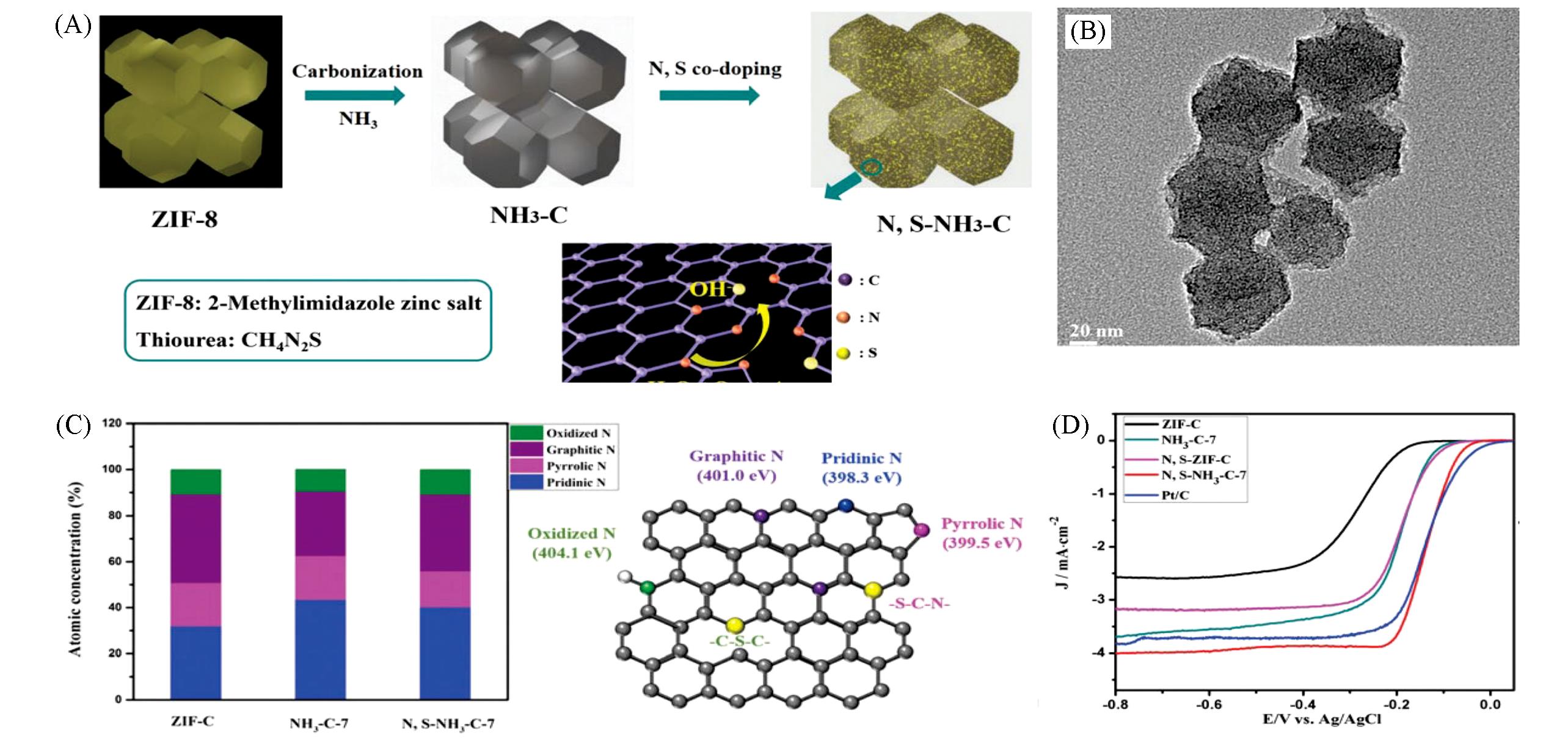
Fig.5 MOF?derived nonmetallic carbon?based materials for confinement electrocatalytic ORR[84](A) Schematic illustration of the fabrication of the N,S-co-doped nanocarbon as the electrocatalyst toward ORR; (B) TEM image of N,S-NH3-C-7; (C) bar diagrams representing the atomic concentration of four kinds of nitrogen species(left); atomic structure of the N,S-doped nanocarbon with chemical bonding configurations of nitrogen and sulfur dopants(right); (D) linear sweep voltammograms(LSVs) of ZIF-C(black), NH3-C-7(blue), N,S-NH3-C-7(red). Copyright 2017, Royal Society of Chemistry.
| Catalysis | Species of metals | Electrolyte | Product and FE (%) | Current density/(mA·cm-2) | E/V (vs. RHE) | Durability/h | Ref. |
|---|---|---|---|---|---|---|---|
| DHPC | Single atom | 0.5 mol/L KHCO3 | CO@99.5 | jCOca. -5 | -0.5 | — | [ |
| DPC?NH3?950 | Single atom | 0.1 mol/L KHCO3 | CO@95.2 | 2.84 | -0.5 | 24 | [ |
| Ni SAs/N?C | Single atom | 0.5 mol/L KHCO3 | CO@71.9 | 10.48 | -1.0 | 60 | [ |
| Co?N2 | Single atom | 0.5 mol/L KHCO3 | CO@94 | 18.1 | -0.63 | — | [ |
| Ni1?N?C | Single atom | 0.5 mol/L KHCO3 | CO@96.8 | jCOca. 27 | -0.8 | 10 | [ |
| Ni/Fe?NC | Single atom | 0.5 mol/L KHCO3 | CO@98 | 9.5 | -0.70 | >30 | [ |
| InCuO?0.92 | Nanoparticles | 0.5 mol/L KHCO3 | CO@92.1 | 11.2 | -0.8 | 24 | [ |
| PcCu?O8?Zn/CNT | Nanoparticles | 0.1 mol/L KHCO3 | CO@88 | — | -0.7 | >10 | [ |
| m?Cu NPs | Nanoparticles | 0.1 mol/L KHCO3 | CH4@>50 | 10.9 | -1.4 | — | [ |
Table 2 Summary of previously reported MOF-derived carbon-based catalysts and their application in CO2RR
| Catalysis | Species of metals | Electrolyte | Product and FE (%) | Current density/(mA·cm-2) | E/V (vs. RHE) | Durability/h | Ref. |
|---|---|---|---|---|---|---|---|
| DHPC | Single atom | 0.5 mol/L KHCO3 | CO@99.5 | jCOca. -5 | -0.5 | — | [ |
| DPC?NH3?950 | Single atom | 0.1 mol/L KHCO3 | CO@95.2 | 2.84 | -0.5 | 24 | [ |
| Ni SAs/N?C | Single atom | 0.5 mol/L KHCO3 | CO@71.9 | 10.48 | -1.0 | 60 | [ |
| Co?N2 | Single atom | 0.5 mol/L KHCO3 | CO@94 | 18.1 | -0.63 | — | [ |
| Ni1?N?C | Single atom | 0.5 mol/L KHCO3 | CO@96.8 | jCOca. 27 | -0.8 | 10 | [ |
| Ni/Fe?NC | Single atom | 0.5 mol/L KHCO3 | CO@98 | 9.5 | -0.70 | >30 | [ |
| InCuO?0.92 | Nanoparticles | 0.5 mol/L KHCO3 | CO@92.1 | 11.2 | -0.8 | 24 | [ |
| PcCu?O8?Zn/CNT | Nanoparticles | 0.1 mol/L KHCO3 | CO@88 | — | -0.7 | >10 | [ |
| m?Cu NPs | Nanoparticles | 0.1 mol/L KHCO3 | CH4@>50 | 10.9 | -1.4 | — | [ |
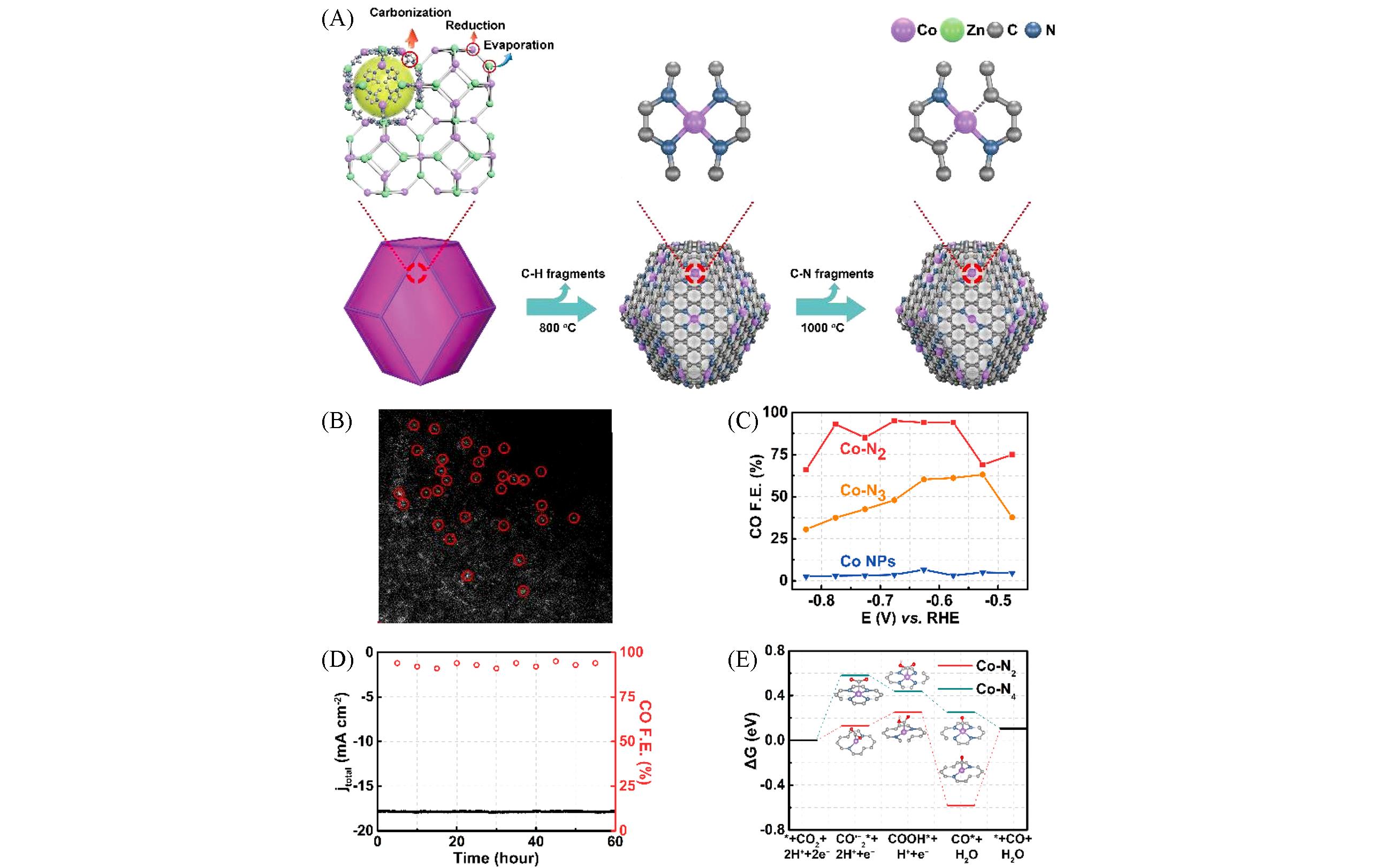
Fig.7 MOF?derived atomically dispersion metal carbon?based materials for confinement electrocatalytic CO2RR[96](A) Schematic formation process of Co-N4 and Co-N2; (B) magnified HAADF-STEM images of Co-N2 shows the atomic dispersion of Co atoms; (C) CO Faradaic efficiencies at different applied potentials; (D) catalytic stability test at -0.63 V for 60 h; (E) calculated Gibbs free energy diagrams for CO2 electroreduction to CO on Co-N2 and Co-N4. Copyright 2018, Wiley-VCH.
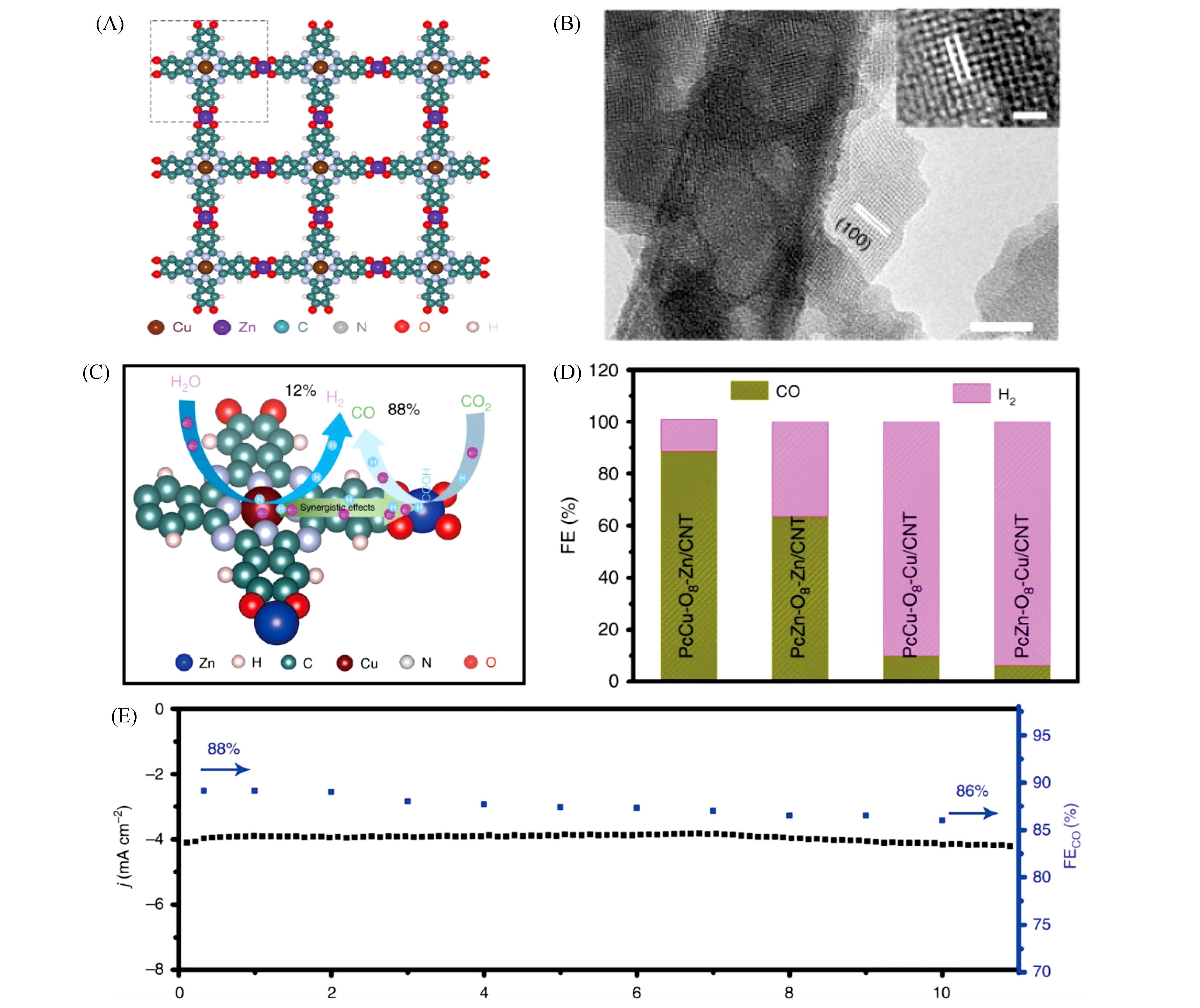
Fig.8 MOF?derived metal nanoparticles carbon?based materials for confinement electrocatalytic CO2RR[100](A) Schematic structure of PcCu-O8-Zn (the dashed rectangular indicates the unit cell); (B) HRTEM image of PcCu-O8-Zn sample. Scale bar: 20?nm (inset: 5?nm); (C) schematic HER and CO2RR reaction process of PcCu-O8-Zn; (D) Faradaic efficiency of CO and H2 for PcCu-O8-Zn/CNT, PcCu-O8-Cu/CNT, PcZn-O8-Zn/CNT and PcZn-O8-Cu/CNT at -0.7?V(vs. RHE); (E) amperometry (i?t) stability and the according Faradaic efficiency for CO of PcCu-O8-Zn/CNT at -0.7?V(vs. RHE) in CO2-saturated 0.1 mol/L KHCO3.Copyright 2020, Springer Nature.
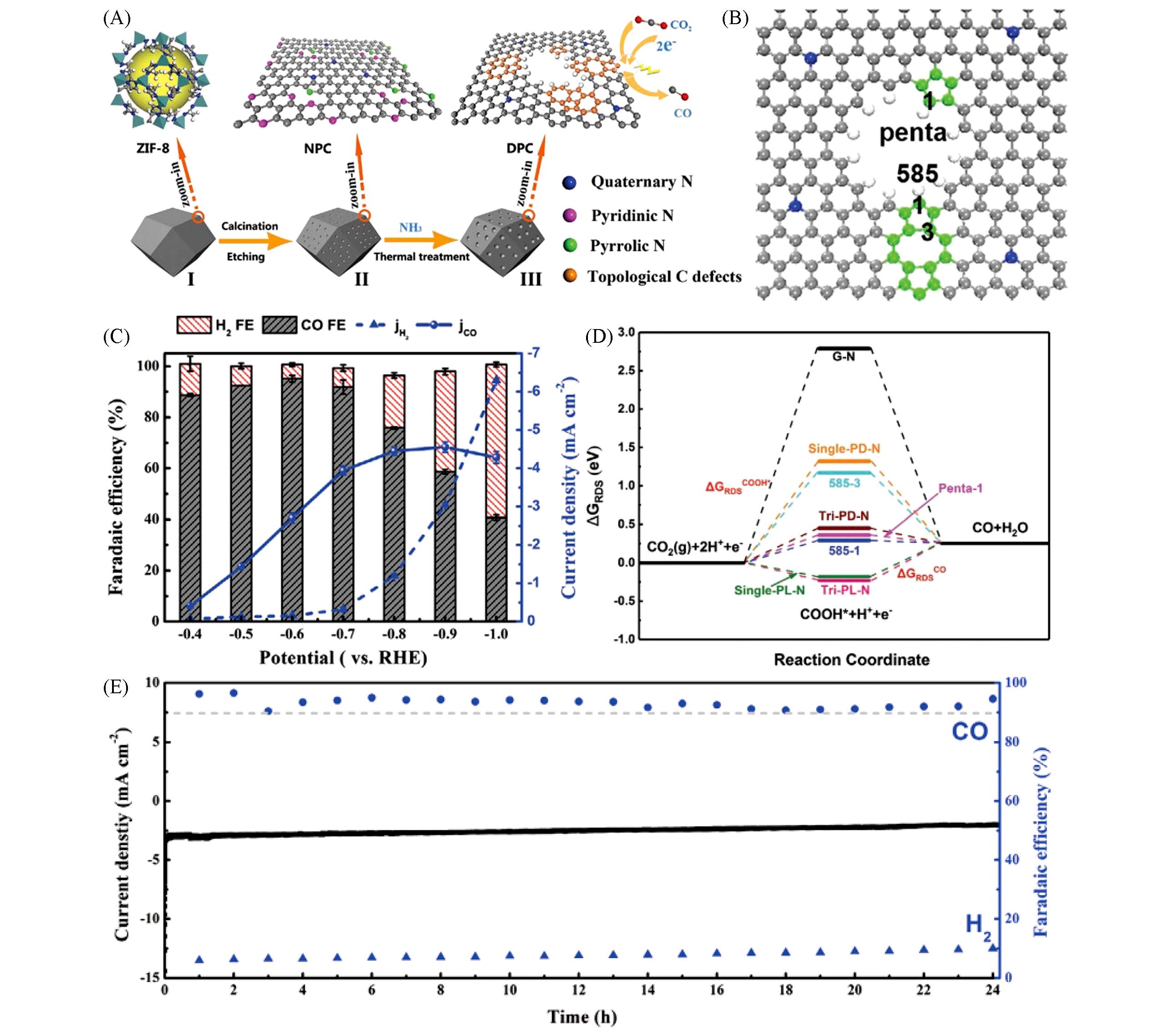
Fig.9 MOF?derived nonmetallic carbon?based materials for confinement electrocatalytic CO2RR[94](A) Schematic illustration of the synthetic route and the corresponding models of ZIF-8 precursor(I), 3D N-enriched porous carbon particles(II), and 3D topologically defected porous carbon particles(III); (B) the partial charge distribution at defect sites to illustrate their high activitie, C, O, N, and H atoms are represented by gray, red, blue, and white spheres, the green atoms emphasized the penta and 585 defects; (C) Faradaic efficiencies of CO(gray) and H2(red) and the partial current of CO on DPC-NH3-950 under a range of applied potentials; (D) the calculated free-energy diagram for CO2RR at N-doped sites, penta-hole, and 585-1(3) sites, G-N, single/tri-PD-N, and single/tri-PL-N refer to graphite-N, and single or triple pyridinic-N and pyrrolic-N, respectively; (E) the CO2RR stability test of DPC-NH3-950 under the potential of -0.6 V(vs, RHE) for 24 h. Copyright 2020, Wiley-VCH.
| 46 | Ji S., Chen Y., Wang X., Zhang Z., Wang D., Li Y., Chem. Rev., 2020, 120(21), 11900—11955 |
| 47 | Liu J., Yang D., Zhou Y., Zhang G., Xing G., Liu Y., Ma Y., Terasaki O., Yang S., Chen L., Angew. Chem. Int. Ed., 2021, 60(26), 14473—14479 |
| 48 | He T., Chen S., Ni B., Gong Y., Wu Z., Song L., Gu L., Hu W., Wang X., Angew. Chem. Int. Ed., 2018, 57(13), 3493—3498 |
| 49 | Qin L., Xu Z., Zheng Y., Li C., Mao J., Zhang G., Adv. Funct. Mater., 2020, 30(14), 1910257 |
| 50 | Yi J. D., Si D. H., Xie R., Yin Q., Zhang M. D., Wu Q., Chai G. L., Huang Y. B., Cao R., Angew. Chem. Int. Ed., 2021, 60(31), 17108—17114 |
| 51 | Dang Q., Huang H., Li L., Lyu X., Zhong S., Yu Y., Xu D., Chem. Mater., 2021, 33(14), 5690—5699 |
| 52 | Chen C., Chen H. Z., Qiao H. Y., Wang K., Xu L., Zhang N., Chem. J. Chinese Universities, 2016, 37(4), 723—727(陈超, 陈恒泽, 乔慧颖, 王凯, 徐力, 张宁. 高等学校化学学报, 2016, 37(4), 723—727) |
| 53 | Park C., Koo W. T., Chong S., Shin H., Kim Y. H., Cho H. J., Jang J. S., Kim D. H., Lee J., Park S., Ko J., Kim J., Kim I. D., Adv. Mater., 2021, 2101216 |
| 54 | Wang T. T., Kou Z. K., Mu S. C., Liu J. P., He D. P., Amiinu I. S., Meng W., Zhou K., Lou Z. X., Cheamchuen S., Verpoort F., Adv. Funct. Mater., 2018, 28(5), 1705048 |
| 55 | Usman M., Ali M., Al⁃Maythalony B. A., Ghanem A. S., Saadi O. W., Ali M., Jafar Mazumder M. A., Abdel⁃Azeim S., Habib M. A., Yamani Z. H., Ensinger W., ACS Appl. Mater. Inter., 2020, 12(44), 49992—50001 |
| 56 | Wroblowa H. S., Yen Chi P., Razumney G., J. Electroanal. Chem. Interfacial Electrochem., 1976, 69(2), 195—201 |
| 57 | Choi C. H., Lim H. K., Chung M. W., Chon G., Ranjbar Sahraie N., Altin A., Sougrati M. T., Stievano L., Oh H. S., Park E. S., Luo F., Strasser P., Dražić G., Mayrhofer K. J. J., Kim H., Jaouen F., Energy Environ. Sci., 2018, 11(11), 3176—3182 |
| 58 | Wang H. F., Chen L., Pang H., Kaskel S., Xu Q., Chem. Soc. Rev., 2020, 49(5), 1414—1448 |
| 59 | Ren Q., Wang H., Lu X. F., Tong Y. X., Li G. R., Adv. Sci., (Weinh), 2018, 5(3), 1700515 |
| 60 | Deng Y., Chi B., Tian X., Cui Z., Liu E., Jia Q., Fan W., Wang G., Dang D., Li M., Zang K., Luo J., Hu Y., Liao S., Sun X., Mukerjee S., J. Mater. Chem. A, 2019, 7(9), 5020—5030 |
| 61 | Lin Y., Liu P., Velasco E., Yao G., Tian Z., Zhang L., Chen L., Adv. Mater., 2019, 31(18), 1—9 |
| 62 | Yin H., Yuan P., Lu B. A., Xia H., Guo K., Yang G., Qu G., Xue D., Hu Y., Cheng J., Mu S., Zhang J. N., ACS Catal., 2021, 11(20), 12754—12762 |
| 63 | Jin H., Zhou H., He D., Wang Z., Wu Q., Liang Q., Liu S., Mu S., Appl. Catal. B: Environ., 2019, 250, 143—149 |
| 64 | Liu J., Fan C., Liu G., Jiang L., Appl. Surf. Sci., 2021, 538, 148017 |
| 65 | Xie X., He C., Li B., He Y., Cullen D. A., Wegener E. C., Kropf A. J., Martinez U., Cheng Y., Engelhard M. H., Bowden M. E., Song M., Lemmon T., Li X. S., Nie Z., Liu J., Myers D. J., Zelenay P., Wang G., Wu G., Ramani V., Shao Y., Nat. Catal., 2020, 3(12), 1044—1054 |
| 66 | Yin P., Yao T., Wu Y., Zheng L., Lin Y., Liu W., Ju H., Zhu J., Hong X., Deng Z., Zhou G., Wei S., Li Y., Angew. Chem. Int. Ed., 2016, 55(36), 10800—10805 |
| 67 | Tan Y., Zhu W., Zhang Z., Wu W., Chen R., Mu S., Lv H., Cheng N., Nano Energy, 2021, 83, 105813 |
| 68 | Qiao Y., Yuan P., Hu Y., Zhang J., Mu S., Zhou J., Li H., Xia H., He J., Xu Q., Adv. Mater., 2018, 30(46), 1804504 |
| 69 | Sanad M. F., Puente Santiago A. R., Tolba S. A., Ahsan M. A., Fernandez⁃Delgado O., Shawky Adly M., Hashem E. M., Mahrous Abodouh M., El⁃Shall M. S., Sreenivasan S. T., Allam N. K., Echegoyen L., J. Am. Chem. Soc., 2021, 143(10), 4064—4073 |
| 70 | Wang Z., Jin H., Meng T., Liao K., Meng W., Yang J., He D., Xiong Y., Mu S., Adv. Funct. Mater., 2018, 28(39), 1802596 |
| 71 | Zhao X., He X., Chen B., Yin F., Li G., Appl. Surf. Sci., 2019, 487, 1049—1057 |
| 72 | Fu X., Zamani P., Choi J. Y., Hassan F. M., Jiang G., Higgins D. C., Zhang Y., Hoque M. A., Chen Z., Adv. Mater., 2017, 29(7), 1604456 |
| 73 | Huynh M., Ozel T., Liu C., Lau E. C., Nocera D. G., Chem. Sci., 2017, 8(7), 4779—4794 |
| 74 | Zhang H., Chung H. T., Cullen D. A., Wagner S., Kramm U. I., More K. L., Zelenay P., Wu G., Energy Environ. Sci., 2019, 12(8), 2548—2558 |
| 75 | Martinez U., Komini Babu S., Holby E. F., Chung H. T., Yin X., Zelenay P., Adv. Mater., 2019, 31(31), 1—20 |
| 76 | Holby E. F., Wang G., Zelenay P., ACS Catal., 2020, 10(24), 14527—14539 |
| 77 | Chen Z., Liao X., Sun C., Zhao K., Ye D., Li J., Wu G., Fang J., Zhao H., Zhang J., Appl. Catal. B: Environ., 2021, 288, 120021 |
| 78 | Wang Y., Gan R., Liu H., Dirican M., Wei C., Ma C., Shi J., Zhang X., J. Mater. Chem. A, 2021, 9(5), 2764—2774 |
| 79 | Shi Q., Liu Q., Ma Y., Fang Z., Liang Z., Shao G., Tang B., Yang W., Qin L., Fang X., Adv. Energy Mater., 2020, 10(10), 1903854 |
| 80 | Liu X., Amiinu I. S., Liu S., Cheng K., Mu S., Nanoscale, 2016, 8(27), 13311—13320 |
| 81 | Morris R. H., Coord. Chem. Rev., 2017, 350, 105—116 |
| 82 | Muthurasu A., Tiwari A. P., Chhetri K., Dahal B., Kim H. Y., Nano Energy, 2021, 88, 106238 |
| 83 | Zhu Y., Zhang Z., Li W., Lei Z., Cheng N., Tan Y., Mu S., Sun X., ACS Sustain. Chem. Eng., 2019, 7(21), 17855—17862 |
| 84 | Song Z., Liu W., Cheng N., Norouzi Banis M., Li X., Sun Q., Xiao B., Liu Y., Lushington A., Li R., Liu L., Sun X., Mater. Horizons, 2017, 4(5), 900—907 |
| 85 | Yang Q., Xiao Z., Kong D., Zhang T., Duan X., Zhou S., Niu Y., Shen Y., Sun H., Wang S., Zhi L., Nano Energy, 2019, 66, 104096 |
| 86 | Zheng T., Jiang J., Wang J., Hu S., Ding W., Wei Z., Acta Phys. Chim. Sin., 2021, 37(11), 101—113 |
| 87 | Zhang Z., Ma C., Tu Y., Si R., Wei J., Zhang S., Wang Z., Li J. F., Wang Y., Deng D., Nano Research, 2019, 12(9), 2313—2317 |
| 88 | Lee S., Kim D., Lee J., Angew. Chem. Int. Ed., 2015, 54(49), 14701—14705 |
| 89 | Birdja Y. Y., Pérez⁃Gallent E., Figueiredo M. C., Göttle A. J., Calle⁃Vallejo F., Koper M. T. M., Nat. Energy, 2019, 4(9), 732—745 |
| 90 | Benson E. E., Kubiak C. P., Sathrum A. J., Smieja J. M., Chem. Soc. Rev., 2009, 38(1), 89—99 |
| 91 | Zhang Y. J., Sethuraman V., Michalsky R., Peterson A. A., ACS Catal., 2014, 4(10), 3742—3748 |
| 92 | Hori Y., Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry, Ed.: Vayenas C. G., White R. E., Gamboa⁃Aldeco M. E., Springer, New York, 2008, 89—189 |
| 93 | Wu Q., Gao J., Feng J., Liu Q., Zhou Y., Zhang S., Nie M., Liu Y., Zhao J., Liu F., Zhong J., Kang Z., J. Mater. Chem. A, 2020, 8(3), 1205—1211 |
| 94 | Dong Y., Zhang Q., Tian Z., Li B., Yan W., Wang S., Jiang K., Su J., Oloman C. W., Gyenge E. L., Ge R., Lu Z., Ji X., Chen L., Adv. Mater., 2020, 32(28), 1—10 |
| 95 | Zhao C., Dai X., Yao T., Chen W., Wang X., Wang J., Yang J., Wei S., Wu Y., Li Y., J. Am. Chem. Soc., 2017, 139(24), 8078—8081 |
| 96 | Wang X., Chen Z., Zhao X., Yao T., Chen W., You R., Zhao C., Wu G., Wang J., Huang W., Yang J., Hong X., Wei S., Wu Y., Li Y., Angew. Chem. Int. Ed., 2018, 57(7), 1944—1948 |
| 97 | Jiao L., Yang W., Wan G., Zhang R., Zheng X., Zhou H., Yu S. H., Jiang H. L., Angew. Chem. Int. Ed., 2020, 59(46), 20589—20595 |
| 98 | Ren W., Tan X., Yang W., Jia C., Xu S., Wang K., Smith S. C., Zhao C., Angew. Chem. Int. Ed., 2019, 58(21), 6972—6976 |
| 99 | Guo W., Sun X., Chen C., Yang D., Lu L., Yang Y., Han B., Green Chem., 2019, 21(3), 503—508 |
| 100 | Zhong H., Ghorbani⁃Asl M., Ly K. H., Zhang J., Ge J., Wang M., Liao Z., Makarov D., Zschech E., Brunner E., Weidinger I. M., Zhang J., Krasheninnikov A. V., Kaskel S., Dong R., Feng X., Nat. Commun., 2020, 11(1), 1409 |
| 101 | Kim M. K., Kim H. J., Lim H., Kwon Y., Jeong H. M., Electrochim. Acta, 2019, 306, 28—34 |
| 102 | Wang A., Li J., Zhang T., Nature Reviews Chemistry, 2018, 2(6), 65—81 |
| 103 | Yang X. F., Wang A., Qiao B., Li J., Liu J., Zhang T., Acc. Chem. Res., 2013, 46(8), 1740—1748 |
| 104 | Liu L., Corma A., Chem. Rev., 2018, 118(10), 4981—5079 |
| 105 | Qin R., Liu P., Fu G., Zheng N., Small Methods, 2018, 2(1), 1700286 |
| 106 | Chen Y., Ji S., Zhao S., Chen W., Dong J., Cheong W. C., Shen R., Wen X., Zheng L., Rykov A. I., Cai S., Tang H., Zhuang Z., Chen C., Peng Q., Wang D., Li Y., Nat. Commun., 2018, 9(1), 5422 |
| 107 | Wei S., Wang Y., Chen W., Li Z., Cheong W. C., Zhang Q., Gong Y., Gu L., Chen C., Wang D., Peng Q., Li Y., Chem. Sci., 2019, 11(3), 786—790 |
| 108 | Yoo M., Yu Y. S., Ha H., Lee S., Choi J. S., Oh S., Kang E., Choi H., An H., Lee K. S., Park J. Y., Celestre R., Marcus M. A., Nowrouzi K., Taube D., Shapiro D. A., Jung W., Kim C., Kim H. Y., Energy Environ. Sci., 2020, 13(4), 1231—1239 |
| 109 | Mohd Adli N., Shan W., Hwang S., Samarakoon W., Karakalos S., Li Y., Cullen D. A., Su D., Feng Z., Wang G., Wu G., Angew. Chem. Int. Ed., 2021, 60(2), 1022—1032 |
| 110 | Nam D. H., De Luna P., Rosas⁃Hernández A., Thevenon A., Li F., Agapie T., Peters J. C., Shekhah O., Eddaoudi M., Sargent E. H., Nat. Mater., 2020, 19(3), 266—276 |
| 111 | Jampaiah D., Damma D., Chalkidis A., Venkataswamy P., Bhargava S. K., Reddy B. M., Catal. Today, 2020, 356, 519—526 |
| 112 | Zhang X., Xie X. L., Xiong L. K., Peng Y., Chem. J. Chinese Universities, 2021, 42(9), 2824—2831(张想, 谢旭岚, 熊力堃, 彭扬. 高等学校化学学报, 2021, 42(9), 2824—2831) |
| 113 | Dong H., Zhang L., Li L., Deng W., Hu C., Zhao Z. J., Gong J., Small, 2019, 15(17), 1900289 |
| 114 | Jiao Y., Zheng Y., Jaroniec M., Qiao S. Z., J. Am. Chem. Soc., 2014, 136(11), 4394—4403 |
| 115 | Zhang L., Niu J., Li M., Xia Z., J. Phys. Chem. C, 2014, 118(7), 3545—3553 |
| 1 | Li W., Wang D., Zhang Y., Tao L., Wang T., Zou Y., Wang Y., Chen R., Wang S., Adv. Mater., 2020, 32(19), 1—20 |
| 2 | Tang L., Meng X., Deng D., Bao X., Adv. Mater., 2019, 31(50), 1—16 |
| 116 | Pei Z., Li H., Huang Y., Xue Q., Huang Y., Zhu M., Wang Z., Zhi C., Energy Environ. Sci., 2017, 10(3), 742—749 |
| 117 | Pei Z., Meng Q., Wei L., Fan J., Chen Y., Zhi C., Energy Storage Mater., 2020, 28, 55—63 |
| 118 | Zhao J., Lai H., Lyu Z., Jiang Y., Xie K., Wang X., Wu Q., Yang L., Jin Z., Ma Y., Liu J., Hu Z., Adv. Mater., 2015, 27(23), 3541—3545 |
| 3 | Banham D., Kishimoto T., Zhou Y., Sato T., Bai K., Ozaki J. I., Imashiro Y., Ye S., Sci. Adv., 2018, 4(3), 1—8 |
| 4 | Lin L., Liu T., Xiao J., Li H., Wei P., Gao D., Nan B., Si R., Wang G., Bao X., Angew. Chem. Int. Ed., 2020, 59(50), 22408—22413 |
| 119 | Jia Y., Zhang L., Zhuang L., Liu H., Yan X., Wang X., Liu J., Wang J., Zheng Y., Xiao Z., Taran E., Chen J., Yang D., Zhu Z., Wang S., Dai L., Yao X., Nat. Catal., 2019, 2(8), 688—695 |
| 120 | Wang Q., Lei Y., Wang D., Li Y., Energy Environ. Sci., 2019, 12(6), 1730—1750 |
| 5 | Lee J. W., Torres Pineda I., Lee J. H., Kang Y. T., Appl. Energy, 2016, 178, 164—176 |
| 6 | Jiao L., Seow J. Y. R., Skinner W. S., Wang Z. U., Jiang H. L., Mater. Today, 2019, 27, 43—68 |
| 7 | Nam D. H., Bushuyev O. S., Li J., De Luna P., Seifitokaldani A., Dinh C. T., Garcia de Arquer F. P., Wang Y., Liang Z., Proppe A. H., Tan C. S., Todorovic P., Shekhah O., Gabardo C. M., Jo J. W., Choi J., Choi M. J., Baek S. W., Kim J., Sinton D., Kelley S. O., Eddaoudi M., Sargent E. H., J. Am. Chem. Soc., 2018, 140(36), 11378—11386 |
| 8 | Li X., You S., Du J., Dai Y., Chen H., Cai Z., Ren N., Zou J., J. Mater. Chem. A, 2019, 7(45), 25853—25864 |
| 9 | Song Z., Zhang L., Doyle‐Davis K., Fu X., Luo J. L., Sun X., Adv. Energy Mater., 2020, 10(38), 2001561 |
| 10 | Srinivas K., Lu Y., Chen Y., Zhang W., Yang D., ACS Sustain. Chem. Eng., 2020, 8(9), 3820—3831 |
| 11 | Sankar S. S., Ede S. R., Anantharaj S., Karthick K., Sangeetha K., Kundu S., Catal. Sci. Technol., 2019, 9(8), 1847—1856 |
| 12 | Xing Z., Wang D., Meng T., Yang X., ACS Appl. Mater. Interfaces, 2020, 12(35), 39163—39169 |
| 13 | Cui X., Ren P., Ma C., Zhao J., Chen R., Chen S., Rajan N. P., Li H., Yu L., Tian Z., Deng D., Adv. Mater., 2020, 32(25), 1—7 |
| 14 | Yang M., Zhou Y. N., Cao Y. N., Tong Z., Dong B., Chai Y. M., Appl. Mater. Today, 2020, 20, 100692 |
| 15 | Zhang J., Liu C., Zhang B., Small Methods, 2019, 3(9), 1800481 |
| 16 | Zhu Y., Murali S., Cai W., Li X., Suk J. W., Potts J. R., Ruoff R. S., Adv. Mater., 2010, 22(35), 3906—3924 |
| 17 | Tang T., Jiang W. J., Liu X. Z., Deng J., Niu S., Wang B., Jin S. F., Zhang Q., Gu L., Hu J. S., Wan L. J., J. Am. Chem. Soc., 2020, 142(15), 7116—7127 |
| 18 | He C., Zhang Y., Zhang Y., Zhao L., Yuan L. P., Zhang J., Ma J., Hu J. S., Angew. Chem. Int. Ed., 2020, 59(12), 4914—4919 |
| 19 | Zhang J., Jiang W. J., Niu S., Zhang H., Liu J., Li H., Huang G. F., Jiang L., Huang W. Q., Hu J. S., Hu W., Adv. Mater., 2020, 32(11), 1—15 |
| 20 | Choi I., Jung Y. E., Yoo S. J., Kim J. Y., Kim H. J., Lee C. Y., Jang J. H., J. Electrochem. Sci. Te., 2017, 8(1), 61—68 |
| 21 | Furukawa H., Cordova K. E., O'Keeffe M., Yaghi O. M., Science, 2013, 341(6149), 1230444 |
| 22 | Deng H., Grunder S., Cordova K. E., Valente C., Furukawa H., Hmadeh M., Gandara F., Whalley A. C., Liu Z., Asahina S., Kazumori H., O'Keeffe M., Terasaki O., Stoddart J. F., Yaghi O. M., Science, 2012, 336(6084), 1018—1023 |
| 23 | Gascon J., Corma A., Kapteijn F., Llabrés i Xamena F. X., ACS Catal., 2013, 4(2), 361—378 |
| 24 | Vilhelmsen L. B., Walton K. S., Sholl D. S., J. Am. Chem. Soc., 2012, 134(30), 12807—12816 |
| 25 | Ma Y., Peng H., Liu J., Wang Y., Hao X., Feng X., Khan S. U., Tan H., Li Y., Inorg. Chem., 2018, 57(7), 4109—4116 |
| 26 | Zhu Q. L., Xu Q., Chem. Soc. Rev., 2014, 43(16), 5468—5512 |
| 27 | Jiang J., Yaghi O. M., Chem. Rev., 2015, 115(14), 6966—6997 |
| 28 | Kobayashi H., Mitsuka Y., Kitagawa H., Inorg. Chem., 2016, 55(15), 7301—7310 |
| 29 | Yang Q., Xu Q., Jiang H. L., Chem. Soc. Rev., 2017, 46(15), 4774—4808 |
| 30 | McCarthy B. D., Beiler A. M., Johnson B. A., Liseev T., Castner A. T., Ott S., Coord. Chem. Rev., 2020, 406, 213137 |
| 31 | Wen J., Li Y., Gao J., Chem. Res. Chinese Universities, 2020, 36(4), 662—679 |
| 32 | Lei J., Zeng M., Fu L., Chem. Res. Chinese Universities, 2020, 36(4), 504—510 |
| 33 | Shi X. F., Zhu J., Bai T. Y., Fu Z. X., Zhang J. J., Bu X. H., Chem. J. Chinese Universities, 2022, 43(1), 20210613(史潇凡, 朱 剑, 白田宇, 付子萱, 张冀杰, 卜显和. 高等学校化学学报, 2022, 43(1), 20210613) |
| 34 | Jin E., Song K. X., Cui L. L., Chem. J. Chinese Universities, 2020, 41(6), 1362—1369(金娥, 宋开绪, 崔丽莉. 高等学校化学学报,2020, 41(6), 1362—1369) |
| 35 | Han X., Ling X., Wang Y., Ma T., Zhong C., Hu W., Deng Y., Angew. Chem. Int. Ed., 2019, 58(16), 5359—5364 |
| 36 | He Y., Shi Q., Shan W., Li X., Kropf A. J., Wegener E. C., Wright J., Karakalos S., Su D., Cullen D. A., Wang G., Myers D. J., Wu G., Angew. Chem. Int. Ed., 2021, 60(17), 9516—9526 |
| 37 | Fang S., Zhu X., Liu X., Gu J., Liu W., Wang D., Zhang W., Lin Y., Lu J., Wei S., Li Y., Yao T., Nat. Commun., 2020, 11(1), 1029 |
| 38 | Zhou H., He D., Saana A. I., Yang J., Wang Z., Zhang J., Liang Q., Yuan S., Zhu J., Mu S., Nanoscale, 2018, 10(13), 6147—6154 |
| 39 | Zhu Z., Yang Y., Guan Y., Xue J., Cui L., J. Mater. Chem. A, 2016, 4(40), 15536—15545 |
| 40 | Wang Y., Pan Y., Zhu L., Yu H., Duan B., Wang R., Zhang Z., Qiu S., Carbon, 2019, 146, 671—679 |
| 41 | Jia Y., Chen J., Yao X., Mater. Chem. Front., 2018, 2(7), 1250—1268 |
| 42 | Jia Y., Zhang L., Du A., Gao G., Chen J., Yan X., Brown C. L., Yao X., Adv. Mater., 2016, 28(43), 9532—9538 |
| 43 | Wang J., Huang Z., Liu W., Chang C., Tang H., Li Z., Chen W., Jia C., Yao T., Wei S., Wu Y., Li Y., J. Am. Chem. Soc., 2017, 139(48), 17281—17284 |
| 44 | Chen Y., Ji S., Wang Y., Dong J., Chen W., Li Z., Shen R., Zheng L., Zhuang Z., Wang D., Li Y., Angew. Chem. Int. Ed., 2017, 56(24), 6937—6941 |
| 45 | Li J., Chen M., Cullen D. A., Hwang S., Wang M., Li B., Liu K., Karakalos S., Lucero M., Zhang H., Lei C., Xu H., Sterbinsky G. E., Feng Z., Su D., More K. L., Wang G., Wang Z., Wu G., Nat. Catal., 2018, 1(12), 935—945 |
| [1] | 范建玲, 唐灏, 秦凤娟, 许文静, 谷鸿飞, 裴加景, 陈文星. 氮掺杂超薄碳纳米片复合铂钌单原子合金催化剂的电化学析氢性能[J]. 高等学校化学学报, 2022, 43(9): 20220366. |
| [2] | 程前, 杨博龙, 吴文依, 向中华. S掺杂Fe-N-C高活性氧还原反应催化剂[J]. 高等学校化学学报, 2022, 43(9): 20220341. |
| [3] | 楚宇逸, 兰畅, 罗二桂, 刘长鹏, 葛君杰, 邢巍. 单原子铈对弱芬顿效应活性位点氧还原稳定性的提升[J]. 高等学校化学学报, 2022, 43(9): 20220294. |
| [4] | 汪思聪, 庞贝贝, 刘潇康, 丁韬, 姚涛. XAFS技术在单原子电催化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220487. |
| [5] | 姜宏斌, 代文臣, 张娆, 徐晓晨, 陈捷, 杨光, 杨凤林. Co3O4/UiO-66@α-Al2O3陶瓷膜对VOCs废气的分离催化性能[J]. 高等学校化学学报, 2022, 43(6): 20220025. |
| [6] | 李加富, 张凯, 王宁, 孙启明. 分子筛限域单原子金属催化剂的研究进展[J]. 高等学校化学学报, 2022, 43(5): 20220032. |
| [7] | 谷雨, 奚宝娟, 李江潇, 熊胜林. 单原子催化剂在氧还原反应中的分子级调控[J]. 高等学校化学学报, 2022, 43(5): 20220036. |
| [8] | 黄汉浩, 卢湫阳, 孙明子, 黄勃龙. 石墨炔原子催化剂的崭新道路:基于自验证机器学习方法的筛选策略[J]. 高等学校化学学报, 2022, 43(5): 20220042. |
| [9] | 李华, 杨科, 黄俊峰, 陈凤娟. UiO-66-NH2/wood的设计构筑及高效去除水中微量重金属离子性能[J]. 高等学校化学学报, 2022, 43(3): 20210701. |
| [10] | 周颖, 贺培楠, 丰海松, 张欣. 双原子位点M-N-C电催化剂在CO2还原反应中活性位点的最佳分布[J]. 高等学校化学学报, 2022, 43(2): 20210640. |
| [11] | 丁钦, 张梓轩, 徐培程, 李晓宇, 段莉梅, 王寅, 刘景海. Cu, Ni, Co掺杂对Fe碳纳米管的结构及电催化性能的影响[J]. 高等学校化学学报, 2022, 43(11): 20220421. |
| [12] | 何宇婧, 李佳乐, 王东洋, 王福玲, 肖作旭, 陈艳丽. 锌活化Fe/Co/N掺杂的生物质碳基高效氧还原催化剂[J]. 高等学校化学学报, 2022, 43(11): 20220475. |
| [13] | 王婕, 霍海燕, 王洋, 张仲, 刘术侠. 铜箔上原位合成NENU-n系列多酸基MOFs的通用策略[J]. 高等学校化学学报, 2022, 43(1): 20210557. |
| [14] | 柳雪广, 杨晓珊, 马菁菁, 刘伟生. 铕基金属有机框架材料从混合染料中选择性分离亚甲基蓝[J]. 高等学校化学学报, 2022, 43(1): 20210715. |
| [15] | 莫宗文, 张学文, 周浩龙, 周东东, 张杰鹏. 一种多孔配位聚合物的氢键协同客体响应[J]. 高等学校化学学报, 2022, 43(1): 20210576. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||