

高等学校化学学报 ›› 2023, Vol. 44 ›› Issue (5): 20220717.doi: 10.7503/cjcu20220717
张潇然1, 郑建云1( ), 吕艳红1,2(
), 吕艳红1,2( ), 王双印1(
), 王双印1( )
)
收稿日期:2022-11-14
出版日期:2023-05-10
发布日期:2023-01-03
通讯作者:
郑建云,吕艳红,王双印
E-mail:jyzheng@hnu.edu.cn;lvyanhong603@163.com;shuangyinwang@hnu.edu.cn
基金资助:
ZHANG Xiaoran1, ZHENG Jianyun1( ), LYU Yanhong1,2(
), LYU Yanhong1,2( ), WANG Shuangyin1(
), WANG Shuangyin1( )
)
Received:2022-11-14
Online:2023-05-10
Published:2023-01-03
Contact:
ZHENG Jianyun, LYU Yanhong, WANG Shuangyin
E-mail:jyzheng@hnu.edu.cn;lvyanhong603@163.com;shuangyinwang@hnu.edu.cn
Supported by:摘要:
化学品的工业合成通常在高能耗的条件下进行, 加剧了能源危机和环境问题. 在可再生电力或/和太阳能的驱动下, 可以降低反应的能量屏障, 从而在更温和的条件下实现化学品的高效绿色合成. 二氧化碳和氮作为主要的小分子, 可通过电催化合成各种含碳和氮的燃料, 在缓解环境问题的同时降低能源枯竭的压力, 达到高效储能的目的. 本文综合评述了N2和CO2电化学转化的最新研究进展, 重点关注了反应条件的改进、 反应路线的调整及催化机理的研究. 最后, 对C-N电催化耦合目前面临的挑战和未来发展进行了展望. 为进一步开发N2和CO2的电化学转化提供了指导.
中图分类号:
TrendMD:
张潇然, 郑建云, 吕艳红, 王双印. 绿色路径C-N偶联合成尿素的最新研究进展. 高等学校化学学报, 2023, 44(5): 20220717.
ZHANG Xiaoran, ZHENG Jianyun, LYU Yanhong, WANG Shuangyin. Recent Advances in Green C-N Coupling for Urea Synthesis. Chem. J. Chinese Universities, 2023, 44(5): 20220717.
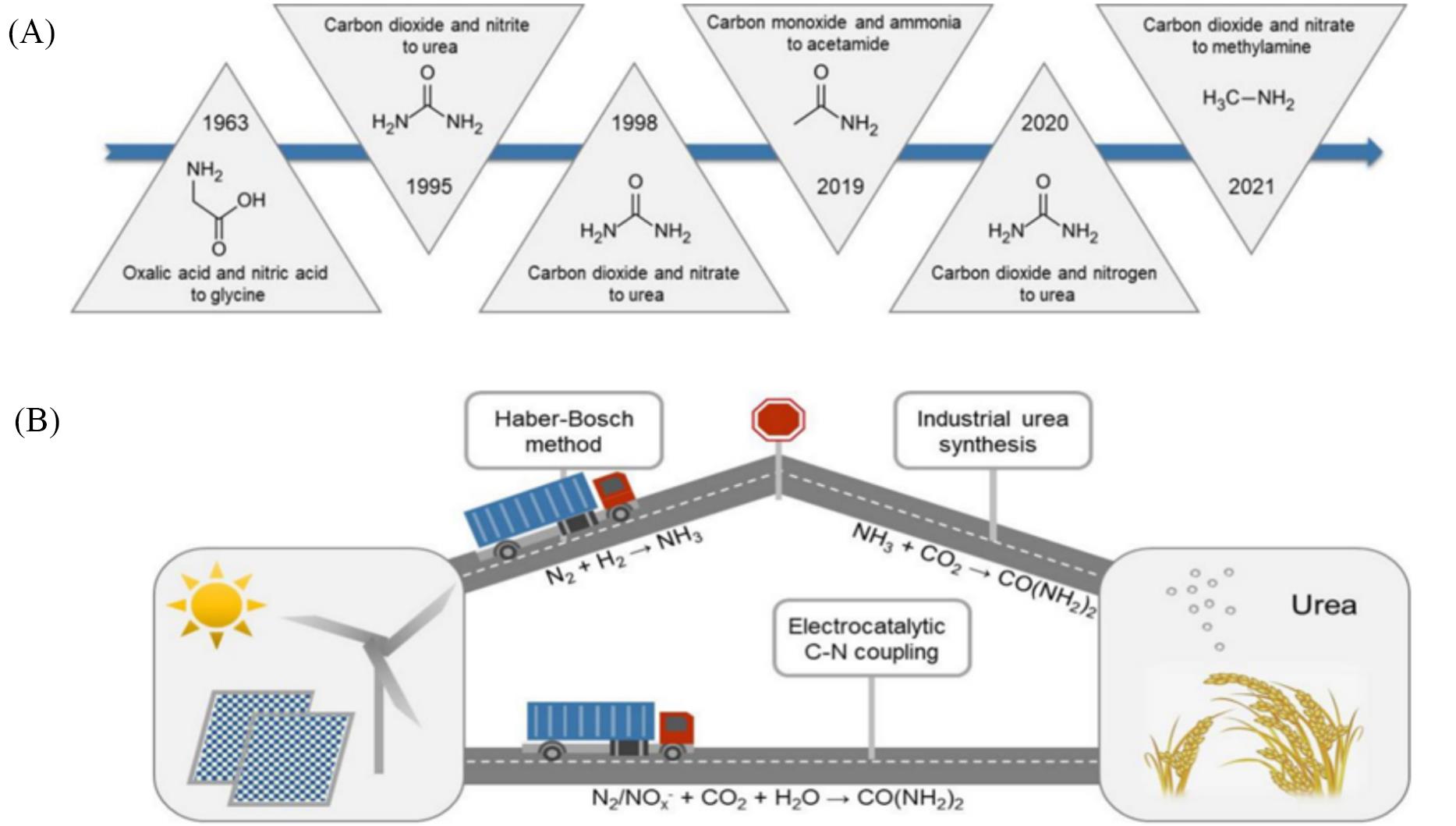
Fig.1 Progress of C⁃N coupling reactions for electrocatalytic amine synthesis(A) and pathways to urea synthesis(B)[10](A) Haber-Bosch method combined with industrial urea synthesis; (B) direct electrocatalytic C-N coupling process.
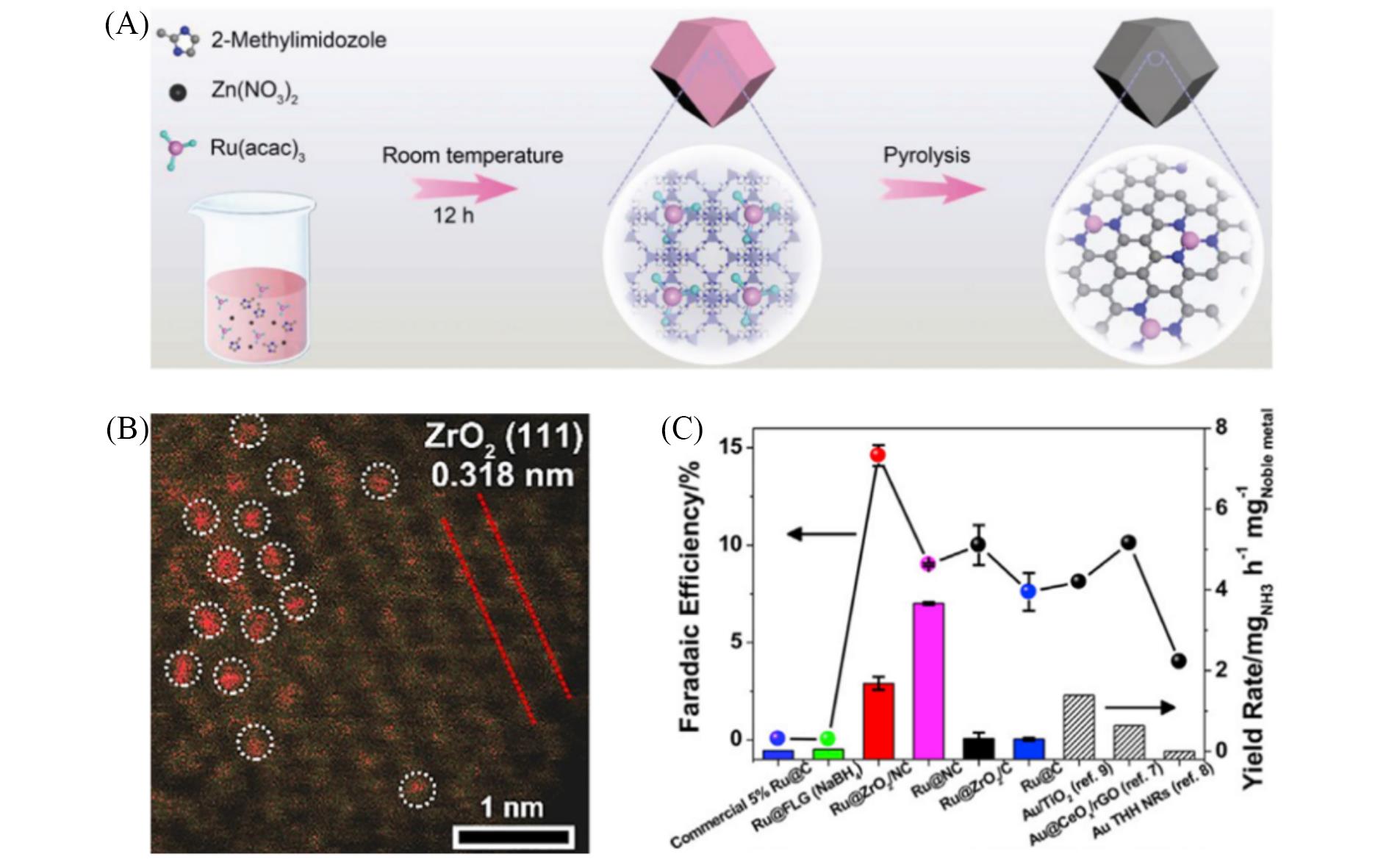
Fig.3 Synthesis schematic diagram of single⁃atom ruthenium catalyst(A)[44], scanning transmission electron microscope photo of ruthenium single⁃atom catalyst containing zirconia(B)[61], electrocatalytic nitrogen reduction performance of single⁃atom ruthenium catalyst containing zirconia and performance comparison with other catalysts(C)[62](A) Copyright 2018, Wiley-VCH; (B) Copyright 2019, Elsevier; (C) Copyright 2019, Springer Nature.
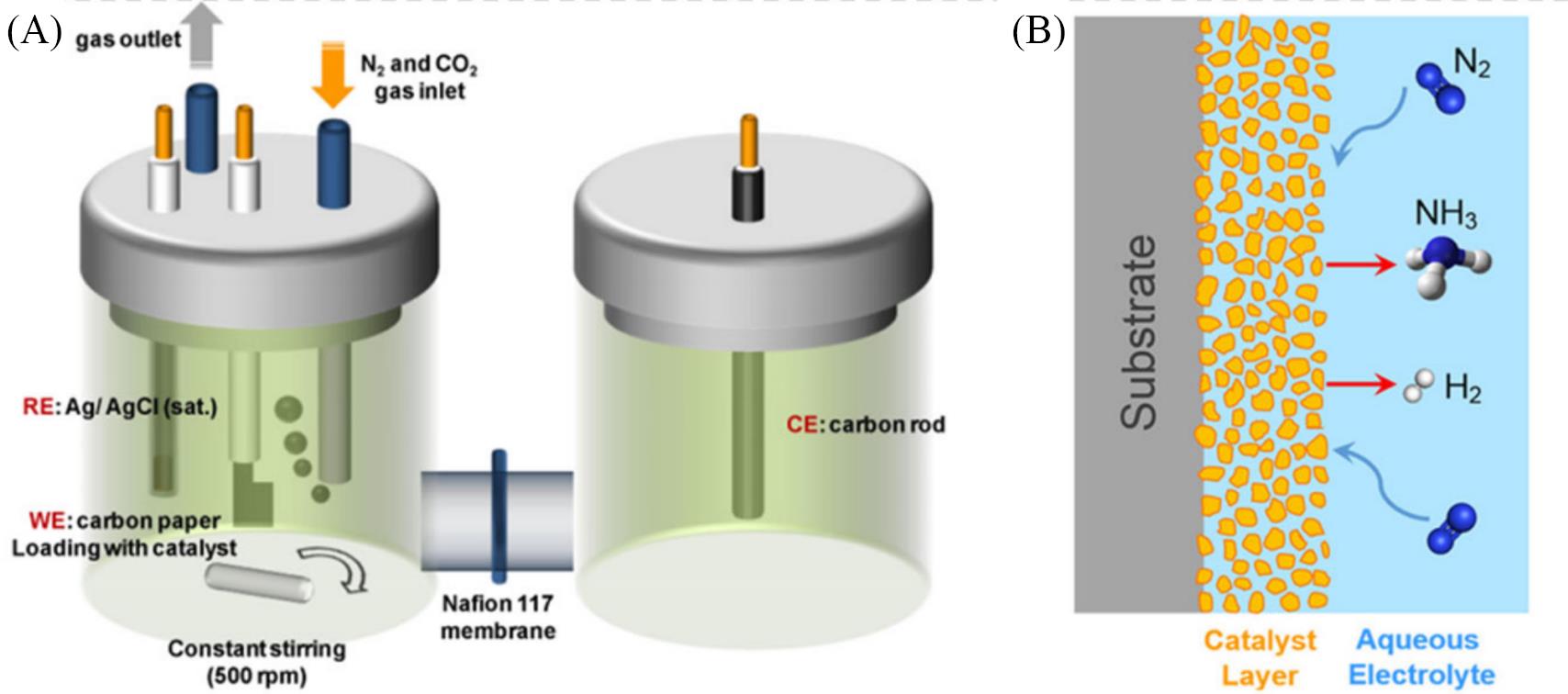
Fig.4 Optimization of catalytic system for urea synthesis(A) The typical H cell for urea electrocatalytic synthesis; (B) schematic illustration of solid-liquid interface in H cell[65].Copyright 2020, American Chemical Society.
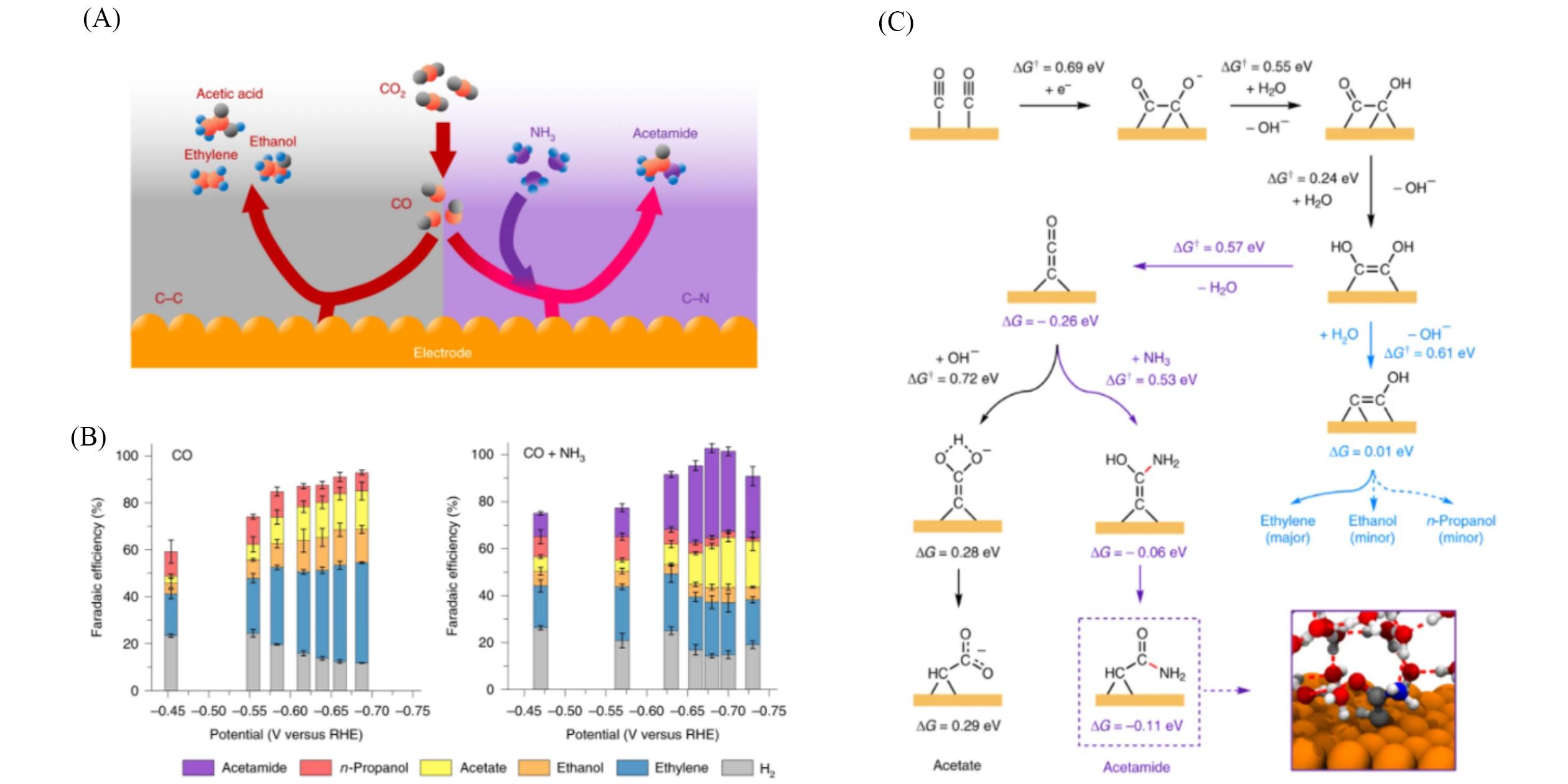
Fig.5 Schematic diagram of the formation of carbon⁃nitrogen bonds induced by introducing ammonia gas in the electrocatalytic reduction reaction of carbon monoxide(A), Faradaic efficiency of each product obtained by co⁃electrolysis of carbon monoxide and ammonia gas(B), co⁃electrolysis of carbon monoxide and ammonia gas, the mechanism diagram of each product and the energy barrier obtained by theoretical calculation of each step reaction(C)[66]
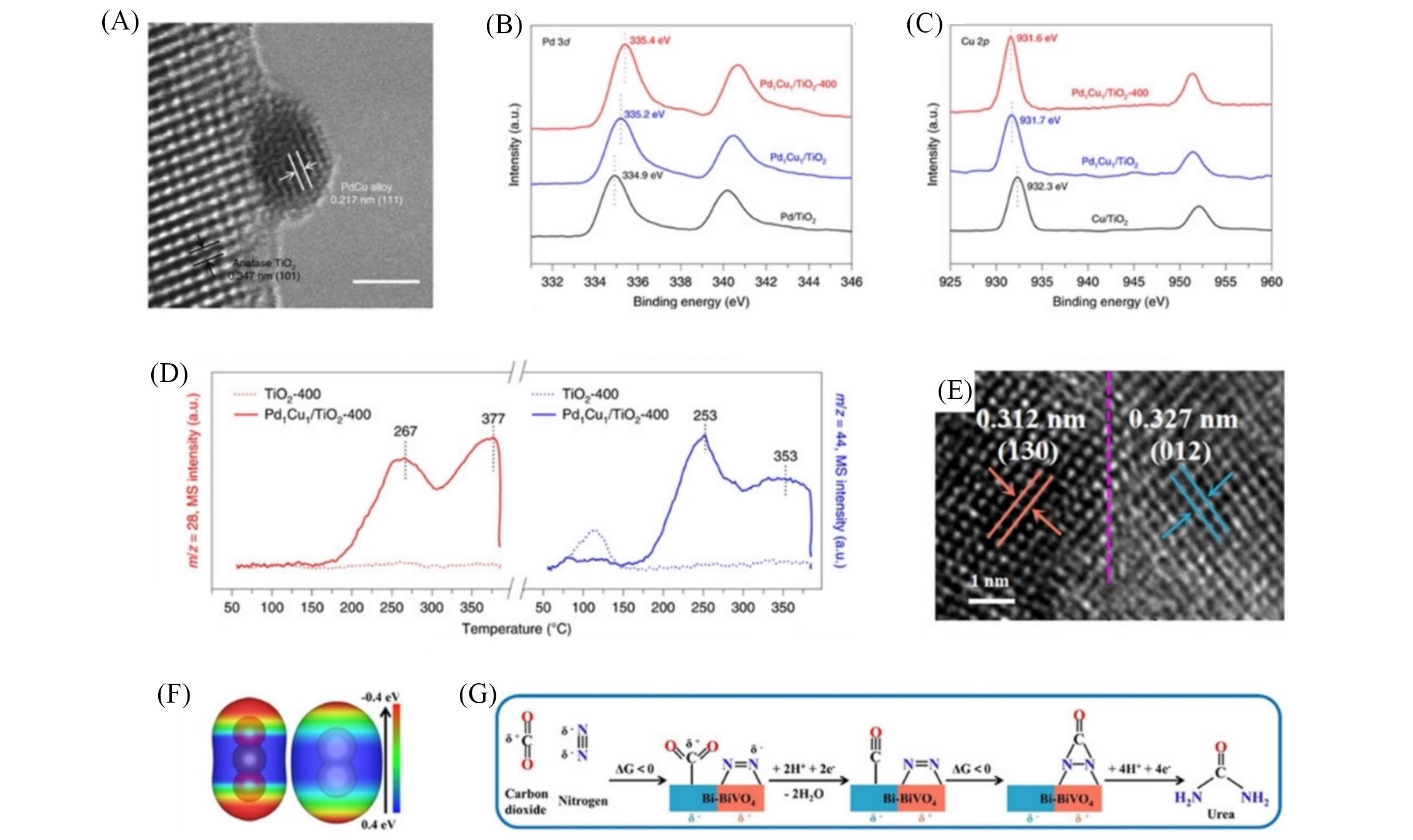
Fig.6 Design of electrocatalysts for coupling of nitrogen and carbon dioxide for urea synthesis(A) High-resolution TEM image of PdCu/TiO2-400 catalyst; (B) Pd3d XPS spectra of Pd/TiO2, PdCu/TiO2, and PdCu/TiO2-400; (C) Cu2p XPS spectra of Cu/TiO2, PdCu/TiO2 and PdCu/TiO2-400; (D) competitive chemisorption of nitrogen and carbon dioxide on TiO2-400 and PdCu/TiO2-400[45]; (E) high-resolution TEM image of Bi/BiVO4 hybrid; (F) electron density isosurface of carbon dioxide and nitrogen molecules; (G) the proposed reaction pathway of urea formation on Bi/BiVO4 hybrid, the urea yield rate[71].
| 1 | Zhu D. D., Liu J. L., Qiao S. Z., Adv. Mater., 2016, 28(18), 3423—3452 |
| 2 | Sun Z., Ma T., Tao H., Fan Q., Han B. X., Chem, 2017, 3(4), 560—587 |
| 3 | Chen X., Guo Y., Du X., Adv. Energy Mater., 2019, 10(3), 1903172 |
| 4 | Seh Z. W., Kibsgaard J., Dickens C. F., Science, 2017, 355(6321), eaad4998 |
| 5 | Gao S., Lin Y., Jiao X., Li D. Q., Yang J. L., Xie Y., Nature, 2016, 529(7584), 68—71 |
| 6 | Kim C., Jeon H. S., Eom T., Min B., Hwang J., J. Am. Chem. Soc., 2015, 137(43), 13844—13850 |
| 7 | Wu J., Yadav R. M., Liu M., Zou A. L., Zhou X. D., Yakobson B. I., Lou J., Pulickel M. A., ACS Nano, 2015, 9(5), 5364—5371 |
| 8 | Cao Z., Kim D., Hong D. A., Wen X. D., Nichols E. M, Keunhong J., Jeffrey A. R., Yang P. D., Christopher J. C., J. Am. Chem. Soc., 2016, 138(26), 8120—8125 |
| 9 | Rosen J., Hutchings G. S., Lu Q., ACS Catal., 2015, 5(7), 4293—4299 |
| 10 | Chen C., He N. H., Wang S. Y., Small Sci., 2021, 1, 2100070 |
| 11 | Comer B. M., Fuentes P., Dimkpa C. O., Joule, 2019, 3(7), 1578—1605 |
| 12 | Service R. F., Science, 2014, 345(6197), 610 |
| 13 | Martín A. J., Shinagawa T., Pérez⁃Ramírez J., Chem, 2019, 5(2), 263—283 |
| 14 | Yan D., Li H., Chen C., Xie C., Zou Y. Q., Wang S. Y., Small Methods, 2018, 3(6), 1800331 |
| 15 | Guo C., Ran J., Vasileff A., Energy Environ. Sci., 2018, 11(1), 45—56 |
| 16 | Licht S., Cui B., Wang B., Science, 2014, 345(6197), 637—640 |
| 17 | Soloveichik G., Nat. Catal., 2019, 2(5), 377—380 |
| 18 | Jiao F., Xu B. J., Adv. Mater., 2019, 31, 1805173 |
| 19 | Tao L., Wang Y., Zou Y., Che W., Chen C., Zheng J. Y., Lyu Y. H., Wang S. Y., Adv. Energy Mater., 2019, 10(11), 1901227 |
| 20 | Wang J., Yu L., Hu L., Nat. Commun., 2018, 9(1), 1795 |
| 21 | Mo R. C., Zhang X. R., Chen Z. Y., Huang S. L., Li Y. J., Liang L. Z., Tian Z. Q., Shen P. K., Int. J. Hydrog. Energy, 2021, 46, 15991—16002 |
| 22 | Scher H. D., Geisz J. F., Deutsch T. G., Turner J. A., Energy Environ. Sci., 2014, 7, 2951—2956 |
| 23 | Jin J., Walczak K., Singh M. R., Karp C., Energy Environ. Sci., 2014, 7, 3371—3380 |
| 24 | Bae D., Seger B., Vesborg P. C., Hanse O., Chorkendorff I., Chem. Soc. Rev., 2017, 46, 1933—1954 |
| 25 | Shen S., Lindley S. A., Chen X., Zhang J. Z., Energy Environ. Sci., 2016, 9, 2744—2775 |
| 26 | White J. L., Baruch M. F., Pander J. E., Hu Y., Fortmeyer I. C., Park J. E., Chem. Rev., 2015, 115, 12888—12935 |
| 27 | Zhang X. R., Zhu X. R., Bo S. W., Chen C., Qiu M. Y., Wei X. X., Chen W., Zheng J. Y., Jiang S. P., Wang S. Y., 2022, Nat. Commun., 13, 5337 |
| 28 | Lv C., Lee C., Zhong L. X., Liu H. J., Liu J. W., Yang L., Yan C. S., Yan Q. Y., Yu G. H., Sci. Adv., 2019, 5(1), eaat5778 |
| 29 | Wei M., Huang L., Huang S. L., Chen Z. Y., Lyu D. D., Zhang X. R., Wang S. B., Tian Z. Q., Shen P. K., J. Catal., 2020, 381, 385—394 |
| 30 | Zhao Y. X., Zhao Y. F., Shi R., Wang B., Geoffrey I. N., Wu L. Z., Tung C. H., Zhang T. R., Adv. Mater., 2019 , 31, 1806482 |
| 31 | Hirakawa H., Hashimoto M., Shiraishi Y., Hirai T., J. Am. Chem. Soc., 2017, 139, 10929 |
| 32 | Liu G., Zhao Y., Sun C., Li F., Lu G. Q., Cheng M. H., Angew. Chem. Int. Ed., 2008, 47, 4516 |
| 33 | Wang S., Hai X., Ding X., Chang K., Xiang Y., Meng X., Yang Z., Chen H., Ye J., Adv. Mater., 2017, 29, 1701774 |
| 34 | Kumari S., Pishgar S., Schwarting M. E., Chem. Commun., 2018, 54(95), 13347—13350 |
| 35 | Iwamoto M., Akiyama M., Aihara K., ACS Catal., 2017, 7(10), 6924—6929 |
| 36 | Xin X., Xu T., Yin J., Wang L., Wang C., Appl. Catal. B, 2015, 354, 176—177 |
| 37 | Mcenaney J. M., Singh A. R., Schwalbe J. A., Energy Environ. Sci., 2017, 10(7), 1621—1630 |
| 38 | Hawtof R., Ghosh S., Guarr E., Sci. Adv., 2019, 5(1), eaat5778 |
| 39 | Chen J. G., Crooks R. M., Seefeldt L. C., Science, 2018, 360(6391), eaar6611 |
| 40 | Erisman J. W., Sutton M. A., Nat. Geosci., 2008, 1(10), 636—639 |
| 41 | Li S. Q., Wang Y. N., Du Y., Zhu X. D., Gao J., Zhang Y. C., Wu G., Small, 2023, 2206776 |
| 42 | Ma J. L., Bao D., Shi M. M., Chem, 2017, 2(4), 525—532 |
| 43 | Lazouski N., Schiffer Z. J., Williams K., Joule, 2019, 3(4), 1127—1139 |
| 44 | Geng Z., Liu Y., Kong X., Miao S., Si R., Zeng J., Adv. Mater., 2018, 1803498 |
| 45 | Chen C., Zhu X., Nat. Chem., 2020, 12, 717—724 |
| 46 | Yan D., Li Y., Huo J., Chen R., Dai L. M., Wang S. Y., Adv. Mater., 2017, 29(48), 1606459 |
| 47 | Cao N., Zheng G., Nano Res., 2018, 11(6), 2992—3008 |
| 48 | Wang M., Liu S., Qian T., Florian T. L., Schmidt A. K., Markus S. S., Jahnke T., Manfred L., Reinhard D., Nat. Commun., 2019, 10(1), 341 |
| 49 | He C., Wu Z. Y., Zhao L., ACS Cataly., 2019, 9(8), 7311—7317 |
| 50 | Liu M., Zhao Han Jiang S., Xia B. Y., Chen Y., J. Mater. Chem. A, 2018, 5(6), 3211 |
| 51 | Chen S., Perathoner C., Ampelli C., Mebrahtu D., Su G., Angew. Chem. Int. Ed., 2017, 56, 2699—2703 |
| 52 | Han J., Liu Z. G., Ma Y., Cui G., Xie F., Wang F., Wu Y., Gao S., Xu Y., Sun X., Nano Energy, 2018, 52, 264—270 |
| 53 | Yu X., Han P., Wei Z., Huang L., Gu Z., Zheng G., Joule, 2018, 2, 1610 |
| 54 | Liu Q. A., Jiang L. J., J. Mater. Chem. A, 2018, 6, 4102 |
| 55 | Wang Y., Zhang Y., Liu Z., Angew. Chem. Int. Ed., 2017, 56(21), 5867—5871 |
| 56 | Lin Y. X., Zhang S. N., Xue Z. H., Nat. Commun., 2019, 10(1), 4380 |
| 57 | Tong W., Huang B., Wang P., Angew. Chem. Int. Ed., 2020, 59(7), 2649—2653 |
| 58 | Wang Y., Yan D., Zou Y. Q., Chen R., Chen C., Zheng J. Y., Wang S. Y., Adv. Sci., 2018, 5(8), 1800064 |
| 59 | Qiao B., Wang A., Yang X., Allard L., Jiang Z., Cui Y., Liu J., Li J., Zhang T., Nature Chem., 2011 , 3, 634—641 |
| 60 | Liu H. M., Timoshenko J., Bai L. C., Li Q. Y., Rüscher M., Sun C. H., Cuenya B. R., Luo J. S., ACS Catal., 2023, 13(2), 1513—1521 |
| 61 | Tao H. C., Choi C., Ding L. X., Chem, 2019, 5(10), 204—214 |
| 62 | Ross M. B., De Luna P., Li Y., Dinh C. T., Kim D., Yang P., Sargent E. H., Nat. Catal., 2019 , 2, 648—658 |
| 63 | Sun Y. W., Zhou J. Q., Qian T., Yan C. L., ACS Appl. Mater. Interfaces, 2019, 11, 35, 32008—32014 |
| 64 | Shi M. M., Bao D., Wulan B. R., Li Y. H., Zhang Y. F., Yan J. M., Jiang Q., Adv. Mater., 2017, 29(17), 1606550 |
| 65 | Hu L., Xing Z., Feng X., ACS Energy Lett., 2020, 5(2), 430—436 |
| 66 | Jouny M., Lv J. J., Cheng T., Jiao F., Nat. Chem., 2019, 11, 846—851 |
| 67 | Tahta A., Hermawan V., Novit A., Zhi W. S., Shu Y., Mater. Today Energy, 2021, 22, 100864 |
| 68 | Jiao F., Pan X. L., Gong K., Bao X. H., Angew. Chem. Int. Ed., 2018, 57, 4692—4696 |
| 69 | Geng J., Ji S. H., Jin M., Zhang C., Xu M., Wang G. Z., Liang C. H., Zhang H. M., Angew. Chem. Int. Ed., 2023, 62, e202210958 |
| 70 | Yuan M. L., Chen J. W., Xu Y., Zhang G. J., Energy Environ. Sci., 2021, 14, 6605—6615 |
| 71 | Yuan M. L., Chen J. W., Bai Y. L., Zhang G. J., Angew. Chem. Int. Ed., 2021, 60(19), 10910—10918 |
| 72 | Yuan M. L., Chen J. W., Zhao T. K., Li S. W., Zhang G. J., Chem. Sci., 2021, 12, 6048—6058 |
| 73 | Hetian C., Albertus D., Xiao J. W., ACS Appl. Mater. Interfaces, 2019, 11, 36571—36579 |
| 74 | Handoko A. D., Khoo K. H., Tan T. L., Jin H. M., She Z. W., J. Mater. Chem. A, 2018, 6, 21885—21890 |
| [1] | 池丽萍, 牛壮壮, 廖洁, 唐凯斌, 高敏锐. 过渡金属氧化物插层化学及其电催化应用的新进展[J]. 高等学校化学学报, 2023, 44(5): 248. |
| [2] | 徐佳宁, 白文静, 楼雨寒, 于海鹏, 窦烁. 电催化氧化木质素解聚: 温和高效的生物质增值策略[J]. 高等学校化学学报, 2023, 44(5): 304. |
| [3] | 李轩, 亓帅, 周伟良, 李小杰, 景玲胭, 冯超, 蒋兴星, 杨恒攀, 胡琪, 何传新. 纤维基氧化还原电催化剂的研究进展[J]. 高等学校化学学报, 2023, 44(5): 316. |
| [4] | 刘至辰, 张宏伟, 张博稳, 陈鹏, 袁珮. 吸附法制备金属/碳催化剂用于5-羟基甲基糠醛高效电催化氧化的研究[J]. 高等学校化学学报, 2023, 44(1): 20220631. |
| [5] | 匡华艺, 陈晨. 贵金属纳米框架设计合成及电催化性能的研究进展[J]. 高等学校化学学报, 2023, 44(1): 20220586. |
| [6] | 杨庆凤, 吕良, 赖小勇. 中空MOFs材料制备及电催化应用的研究进展[J]. 高等学校化学学报, 2023, 44(1): 20220666. |
| [7] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [8] | 姚青, 俞志勇, 黄小青. 单原子催化剂的合成及其能源电催化应用的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220323. |
| [9] | 范建玲, 唐灏, 秦凤娟, 许文静, 谷鸿飞, 裴加景, 陈文星. 氮掺杂超薄碳纳米片复合铂钌单原子合金催化剂的电化学析氢性能[J]. 高等学校化学学报, 2022, 43(9): 20220366. |
| [10] | 林高鑫, 王家成. 单原子掺杂二硫化钼析氢催化的进展和展望[J]. 高等学校化学学报, 2022, 43(9): 20220321. |
| [11] | 汪思聪, 庞贝贝, 刘潇康, 丁韬, 姚涛. XAFS技术在单原子电催化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220487. |
| [12] | 韩付超, 李福进, 陈良, 贺磊义, 姜玉南, 徐守冬, 张鼎, 其鲁. CoSe2/C复合电催化材料修饰隔膜对高载量锂硫电池性能的影响[J]. 高等学校化学学报, 2022, 43(8): 20220163. |
| [13] | 王茹涵, 贾顺涵, 吴丽敏, 孙晓甫, 韩布兴. CO2参与电化学构筑C—N键制备重要化学品[J]. 高等学校化学学报, 2022, 43(7): 20220395. |
| [14] | 彭奎霖, 李桂林, 江重阳, 曾少娟, 张香平. 电解液调控CO2电催化还原性能微观机制的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220238. |
| [15] | 王丽君, 李欣, 洪崧, 詹新雨, 王迪, 郝磊端, 孙振宇. 调节氧化镉-炭黑界面高效电催化CO2还原生成CO[J]. 高等学校化学学报, 2022, 43(7): 20220317. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||