

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (8): 2342.doi: 10.7503/cjcu20210177
李辉阳1, 朱思颖1, 李莎1, 张桥保2( ), 赵金保1, 张力1(
), 赵金保1, 张力1( )
)
收稿日期:2021-03-16
出版日期:2021-08-10
发布日期:2021-08-05
通讯作者:
张桥保
E-mail:zhangqiaobao@xmu.edu.cn;zhangli81@xmu.edu.cn
作者简介:张 力, 男, 博士, 教授级高工, 主要从事新型负极材料和电池非活性材料研发和性能优化研究. E-mail: 基金资助:
LI Huiyang1, ZHU Siying1, LI Sha1, ZHANG Qiaobao2( ), ZHAO Jinbao1, ZHANG Li1(
), ZHAO Jinbao1, ZHANG Li1( )
)
Received:2021-03-16
Online:2021-08-10
Published:2021-08-05
Contact:
ZHANG Qiaobao
E-mail:zhangqiaobao@xmu.edu.cn;zhangli81@xmu.edu.cn
Supported by:摘要:
硅氧化物(SiOx, 0<x≤2)具有高的比容量和低的嵌锂电位, 且体积膨胀率显著低于纯硅负极, 因而被认为是替代传统石墨负极材料的理想选择之一. 然而SiOx负极在首次嵌锂过程中表面形成的固体电解质界面膜(SEI)以及大量的不可逆产物, 造成其首次库伦效率偏低, 严重阻碍了SiOx负极的实际应用. 本文从SiOx的结构模型出发, 系统阐述了SiOx负极的嵌锂机理以及首次库伦效率低的原因; 归纳了SiOx负极首次库伦效率的提升策略及其研究进展; 并对提升SiOx负极首次库伦效率的未来发展方向进行了展望.
中图分类号:
TrendMD:
李辉阳, 朱思颖, 李莎, 张桥保, 赵金保, 张力. 锂离子电池硅氧化物负极首次库伦效率的影响因素与提升策略. 高等学校化学学报, 2021, 42(8): 2342.
LI Huiyang, ZHU Siying, LI Sha, ZHANG Qiaobao, ZHAO Jinbao, ZHANG Li. Influencing Factors and Promotion Strategies of the First-cycle Coulombic Efficiency of Silicon Suboxide Anodes in Lithium-ion Batteries. Chem. J. Chinese Universities, 2021, 42(8): 2342.
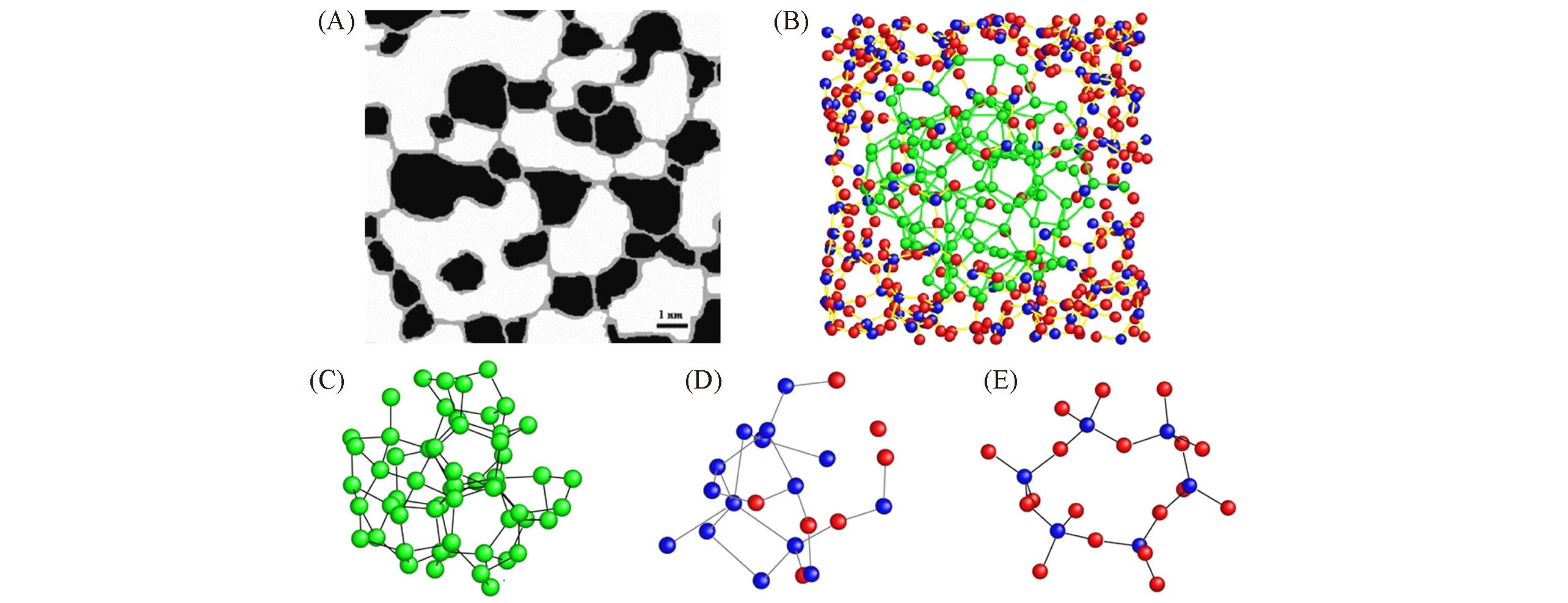
Fig.1 Schematic of the interface clusters mixture model of amorphous SiO(A)[30], schematic illustration of the reconstructed heterostructure model of amorphous SiO(B), atomic models of amorphous Si(C), the model of interfacial silicon suboxide(D) and the model of amorphous SiO(E)[32](A) Copyright 2003, Elsevier; (B—E) Copyright 2016, Springer Nature.
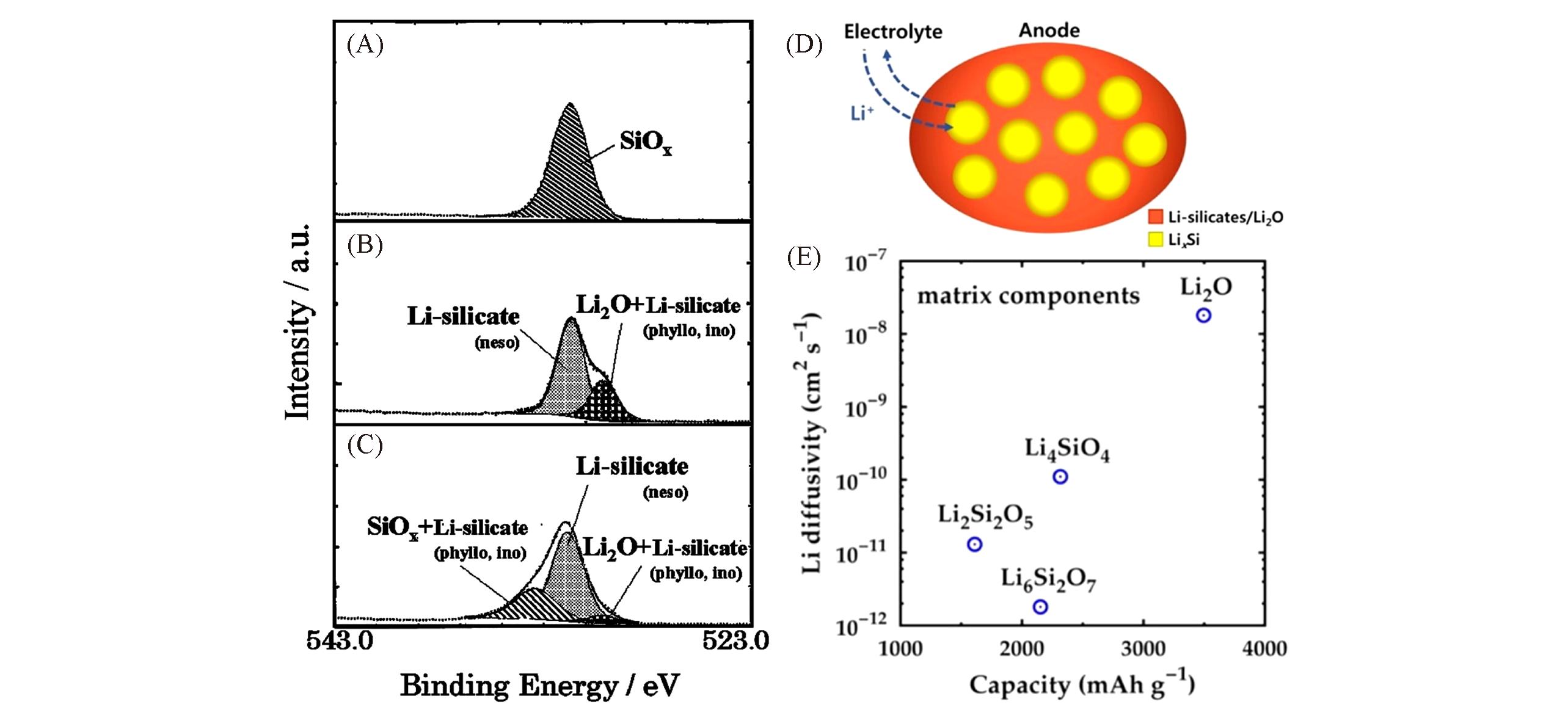
Fig.2 O1s spectra for SiO anodes in as?deposited state(A), charged state(B), and discharged state(C)[33], schematic diagram of SiO anode during lithiation?delithiation cycles(D), the diffusivity of Li2O and lithium silicate(E)[38](A—C) Copyright 2005, Electrochemical Society, Inc; (D—E) Copyright 2015, American Chemical Society.
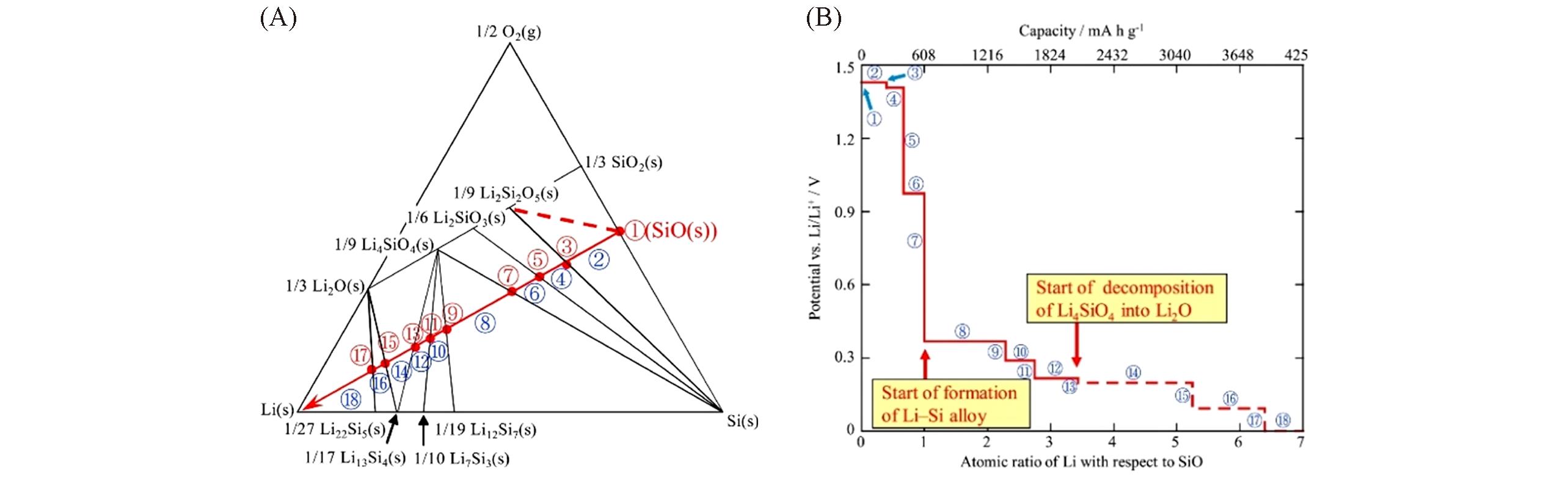
Fig.3 Ternary phase diagram showing transition of formed phases during the Li insertion for a SiO anode(A), and transition of equilibrium electrode potential during the Li insertion(B)[39]Copyright 2016, Elsevier.
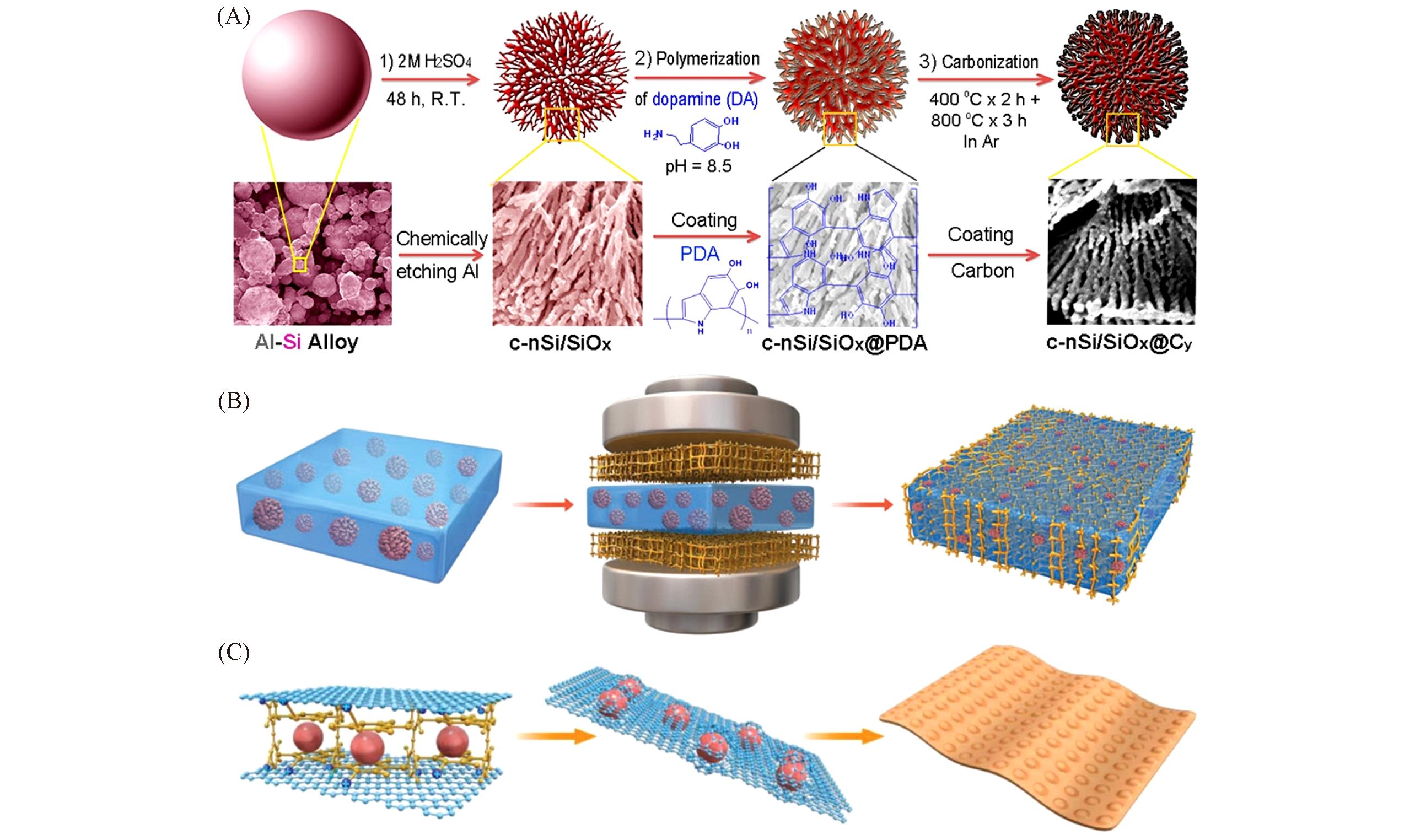
Fig.4 Schematic showing the fabrication process of the coralloid?like nanostructured Si/SiOx@carbon composites, c?nSi/SiOx@Cy(A)[58], schematic of the fabrication process of the SiOx/asphalt membrane and binder?free anode materials(B)[59], schematic of the encapsulation of SiOxnanoparticles into a conductive graphene bubble film(C)[60](A) Copyright 2017, American Chemical Society;(B) Copyright 2017, the Royal Society of Chemistry;(C) Copyright 2018, Wiley-VCH.
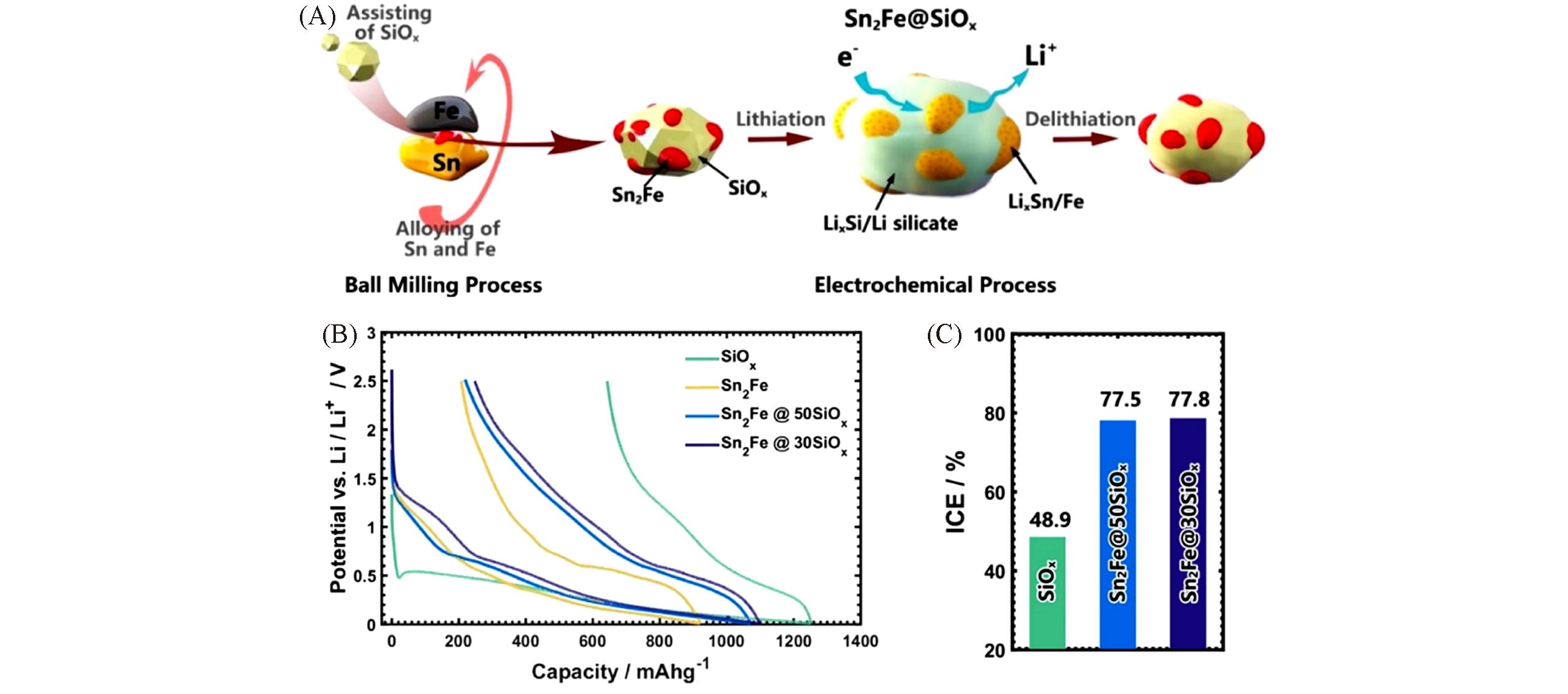
Fig.5 Schematic of fabrication process of the Sn2Fe@SiOx composites(A), the initial discharge/charge profiles of the SiOx, Sn2Fe, and Sn2Fe@SiOx hybrid electrodes(B), and initial Coulombic efficiency(ICE) values of various SiOx?based anode(C)[65]Copyright 2018, Elsevier.
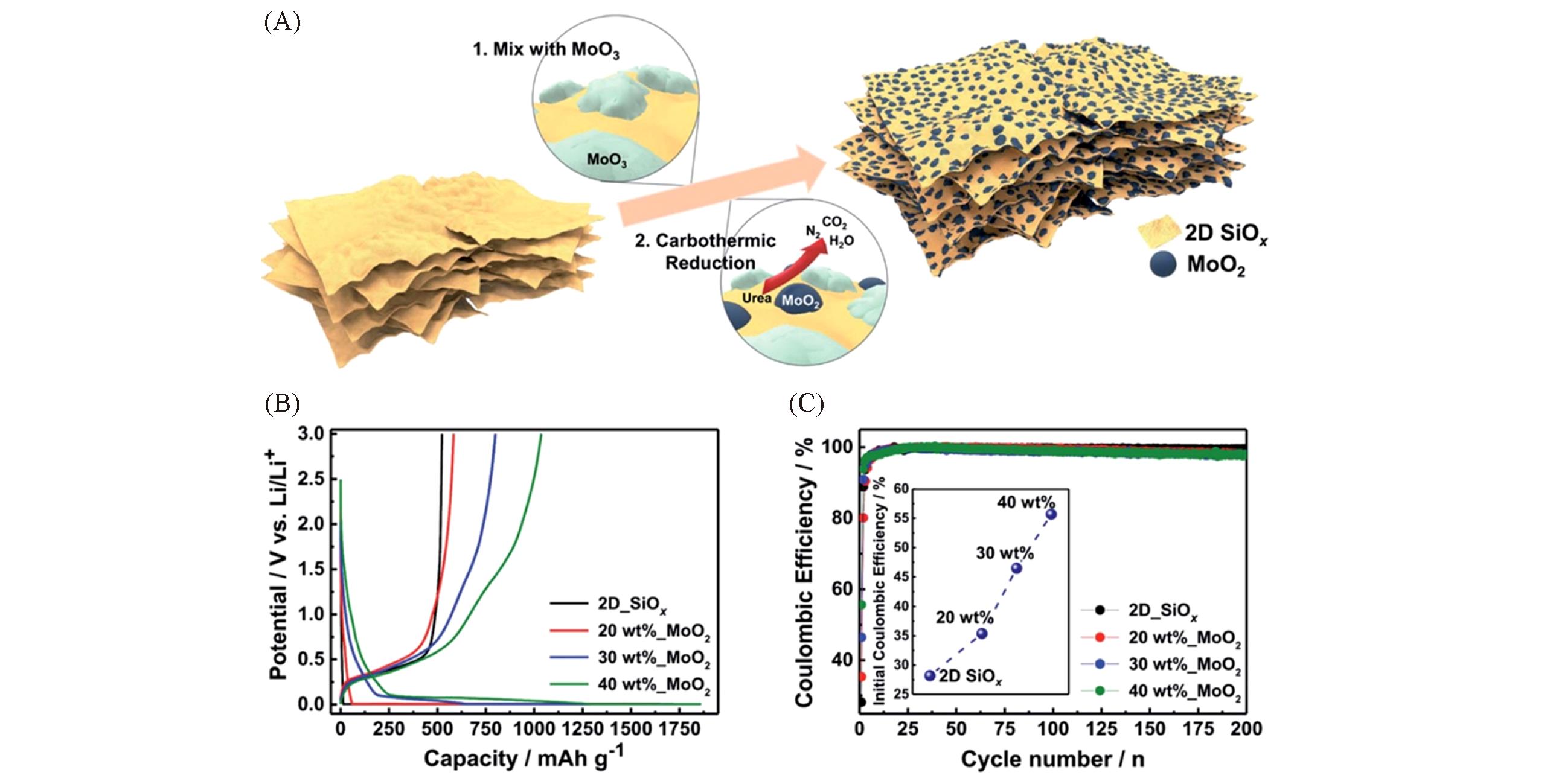
Fig.6 Schematic of fabrication process of the 2D?SiOx/0D?MoO2 nanocomposites(A), galvanostatic charge/discharge profiles(B) and Coulombic efficiency(C) of the 2D?SiOx/0D?MoO2 nanocomposites with the different amount of MoO2[69]Copyright 2020, the Royal Society of Chemistry.
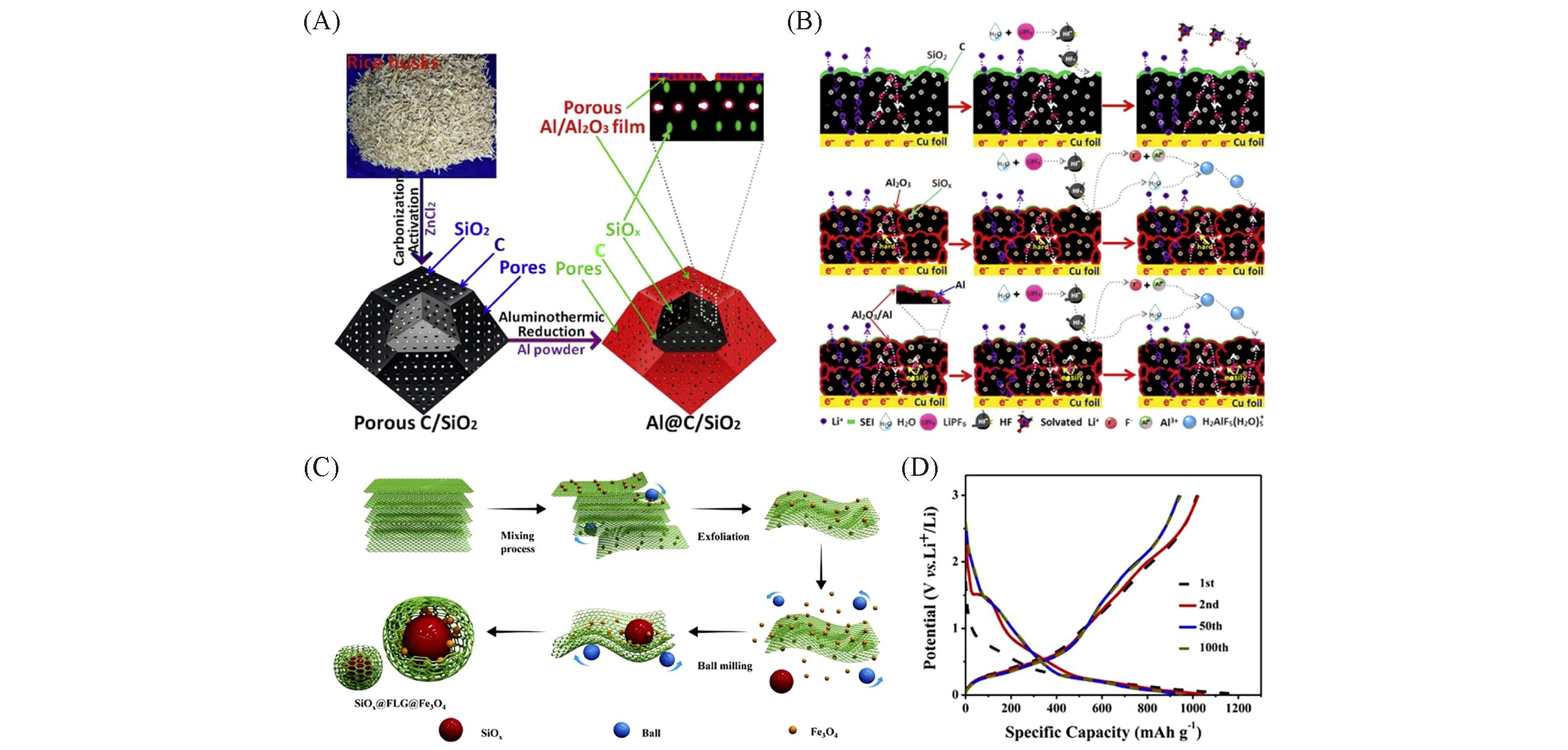
Fig.7 Schematic of the process to prepare Al@C/SiO2 composites(A), schematic illustration of the surface repair method for bare, Al2O3?coated, and porous Al/Al2O3?coated porous C/SiO2 composites during the charge/discharge process(B)[70], schematic diagram showing the process to synthesize FLG(few?layered graphene)?wrapped and Fe3O4?pillared SiOx composites(C), and the charge/discharge curves of SiOx@Fe3O4@FLG composite electrode with different cycles(D)[71](A, B) Copyright 2019, Elsevier; (C, D) Copyright 2019, Elsevier.
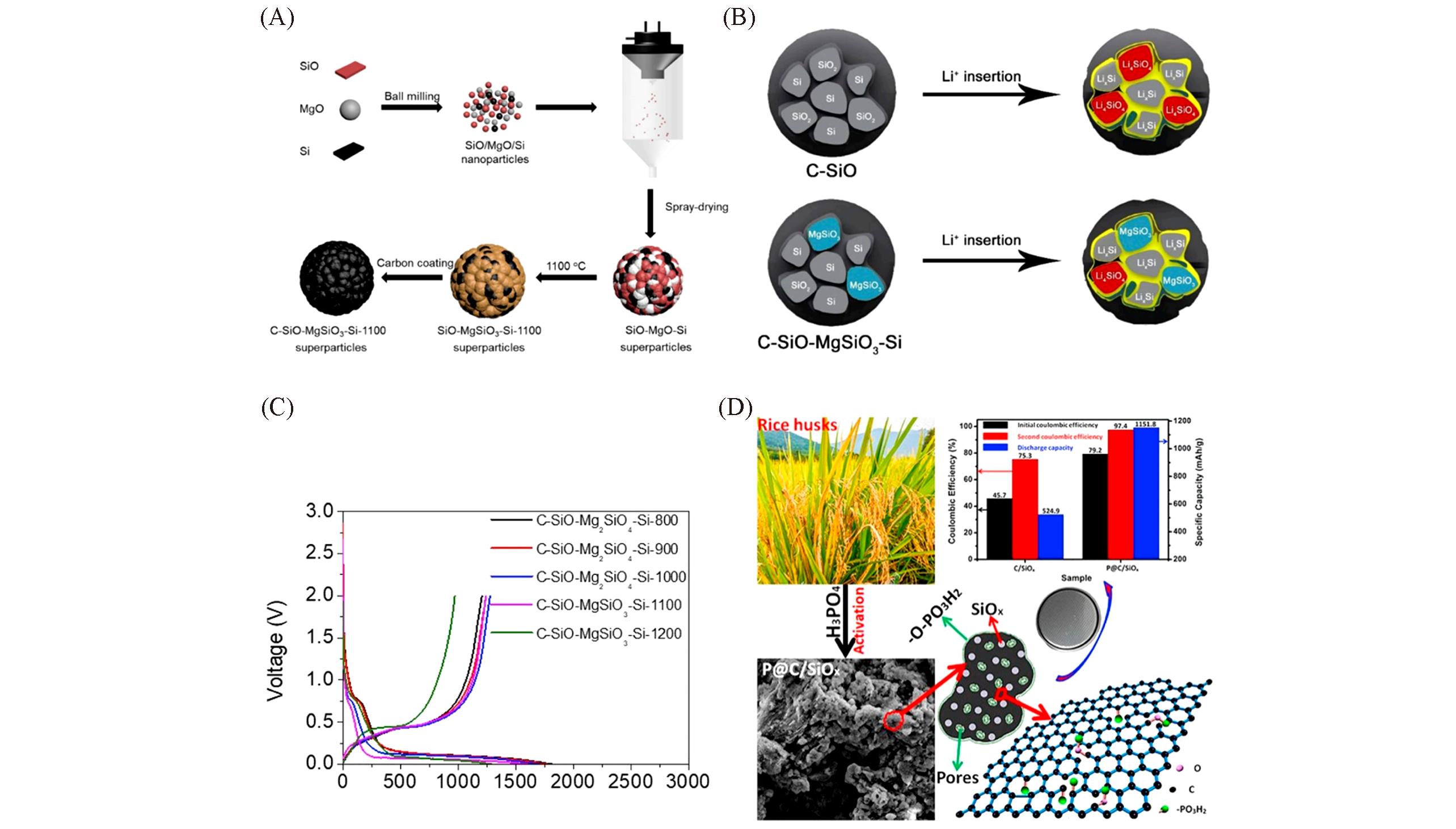
Fig.8 Schematic of the synthesis process of C?SiO?MgSiO3?Si superparticles(A), schematic showing the lithiation of C?SiO and C?SiO?MgSiO3?Si superparticles(B), the charge/discharge profiles of C?SiO?Mg2SiO4?Si and C?SiO?MgSiO3?Si superparticles for the first cycle(C)[72], schematic illustration for the preparation process of P@C/SiOx composites and the electrochemical properties(D)[73](A—C) Copyright 2019, Elsevier. (D) Copyright 2020, Elsevier.
| Year | Material | ICE(%) | Reversible capacity/(mA·h·g-1)) | Current density/(A·g-1) | Electrolyte system | Binder | Ref. |
|---|---|---|---|---|---|---|---|
| 2007 | SiOx?C | 76 | 800 | 0.1 | 1 mol/L LiPF6 in EC/ DEC | PVDF | [ |
| 2017 | cnSi/SiOx@Cy | 85.53 | 2200 | 1 | 1 mol/L LiPF6 in EC/DEC/DMC+15%FEC | Sodium alginate | [ |
| 2017 | SiOx/C/ Ni | 76.3 | 1200 | 0.2 | 1 mol/L LiPF6 in EC/DEC/DMC+5%FEC | No | [ |
| 2018 | SiOx@G | 69 | 1275 | 0.1 | 1 mol/L LiPF6 in EC/DEC/DMC+5%FEC | CMC/SBR | [ |
| 2019 | SiO@CNTs/C | 60.6 | 821.7 | 1 | 1 mol/L LiPF6 in EC/DEC+5%FEC | CMC | [ |
| 2021 | SiOx@CNTs/C | 88 | 902 | 1 | 1 mol/L LiPF6 in EC/DEC/EMC+FEC | CMC | [ |
| 2010 | SiAl0.2O | 67.4 | 1510 | 0.06 | 1 mol/L LiPF6 in EC/DEC/EMC | PAI | [ |
| 2018 | Sn2Fe@SiOx | 78 | 849 | 0.2 | 1 mol/L LiPF6 in EC/DEC+5%FEC | CMC | [ |
| 2012 | TiO2?coated SiO | 72 | 1000 | 0.2 | 1 mol/L LiPF6 in EC/DEC/EMC+10%FEC | PAI | [ |
| 2013 | SiO/Fe2O3 | 68 | 1893 | 0.16 | 1 mol/L LiPF6 in EC/DEC/DMC | CMC | [ |
| 2020 | 2DSiOx/0D?MoO2 | 55.7 | 1051.6 | 0.2 | 1 mol/L LiPF6 in EC/EMC+2%FEC | PAA | [ |
| 2019 | Al@C/SiO2 | 80.47 | 1385 | 0.1 | 1 mol/L LiPF6 in EC/DMC | PVDF | [ |
| 2019 | SiOx@Fe3O4@FLG | 84.90 | 833.4 | 0.5 | 1 mol/L LiPF6 in EC/DEC/DMC+10%FEC | PVDF | [ |
| 2019 | C?SiO?MgSiO3?Si | 78.3 | 1608 | 0.15 | 1 mol/L LiPF6 in EC/DEC | PVDF | [ |
| 2020 | P@C/SiO | 79.2 | 1151.8 | 0.1 | 1 mol/L LiPF6 in EC/DEC/EMC | PVDF | [ |
Table 1 Methods for structural design to improve ICE of SiOx anode
| Year | Material | ICE(%) | Reversible capacity/(mA·h·g-1)) | Current density/(A·g-1) | Electrolyte system | Binder | Ref. |
|---|---|---|---|---|---|---|---|
| 2007 | SiOx?C | 76 | 800 | 0.1 | 1 mol/L LiPF6 in EC/ DEC | PVDF | [ |
| 2017 | cnSi/SiOx@Cy | 85.53 | 2200 | 1 | 1 mol/L LiPF6 in EC/DEC/DMC+15%FEC | Sodium alginate | [ |
| 2017 | SiOx/C/ Ni | 76.3 | 1200 | 0.2 | 1 mol/L LiPF6 in EC/DEC/DMC+5%FEC | No | [ |
| 2018 | SiOx@G | 69 | 1275 | 0.1 | 1 mol/L LiPF6 in EC/DEC/DMC+5%FEC | CMC/SBR | [ |
| 2019 | SiO@CNTs/C | 60.6 | 821.7 | 1 | 1 mol/L LiPF6 in EC/DEC+5%FEC | CMC | [ |
| 2021 | SiOx@CNTs/C | 88 | 902 | 1 | 1 mol/L LiPF6 in EC/DEC/EMC+FEC | CMC | [ |
| 2010 | SiAl0.2O | 67.4 | 1510 | 0.06 | 1 mol/L LiPF6 in EC/DEC/EMC | PAI | [ |
| 2018 | Sn2Fe@SiOx | 78 | 849 | 0.2 | 1 mol/L LiPF6 in EC/DEC+5%FEC | CMC | [ |
| 2012 | TiO2?coated SiO | 72 | 1000 | 0.2 | 1 mol/L LiPF6 in EC/DEC/EMC+10%FEC | PAI | [ |
| 2013 | SiO/Fe2O3 | 68 | 1893 | 0.16 | 1 mol/L LiPF6 in EC/DEC/DMC | CMC | [ |
| 2020 | 2DSiOx/0D?MoO2 | 55.7 | 1051.6 | 0.2 | 1 mol/L LiPF6 in EC/EMC+2%FEC | PAA | [ |
| 2019 | Al@C/SiO2 | 80.47 | 1385 | 0.1 | 1 mol/L LiPF6 in EC/DMC | PVDF | [ |
| 2019 | SiOx@Fe3O4@FLG | 84.90 | 833.4 | 0.5 | 1 mol/L LiPF6 in EC/DEC/DMC+10%FEC | PVDF | [ |
| 2019 | C?SiO?MgSiO3?Si | 78.3 | 1608 | 0.15 | 1 mol/L LiPF6 in EC/DEC | PVDF | [ |
| 2020 | P@C/SiO | 79.2 | 1151.8 | 0.1 | 1 mol/L LiPF6 in EC/DEC/EMC | PVDF | [ |
| Method | Material | Introduction | ICE of full cell(%) | Matched cathode | Ref. |
|---|---|---|---|---|---|
| Prelithiation additive | SiO | SLMP was directly sprayed on the surface of the electrode | 89.77 | LiNi1/3Mn1/3Co1/3O2 | [ |
| SiO | SLMP was dispersed in xylene solvent and then coated on the surface of the electrode | 88.12 | LiNi1/3Mn1/3Co1/3O2 | [ | |
| SiOx | SiOx and molten lithium are thermally alloyed to form LixSi/Li2O composite for prelithiation | 94 | LiFePO4 | [ | |
Self?discharge prelithiation | SiOx | Inserting a resistance buffer layer(RBL) between SiOx anode and Li foil toregulate the rate and degree of prelithiation | 87 | NCM622 | [ |
Electrochemical prelithiation | c?SiOx | Assembling a temporary battery and introduce a rheostat in the external circuit to control the prelithiation rate | 85.34 | LiNi0.8Co0.15Al0.05O2 | [ |
Chemical prelithiation | SiOx | Employing molecularly engineered BP derivatives to adjust the reduction potential of Li?arene(LAC) below 0.2 V drives active Li accommodation in SiOx anodes | 86 | NCM523 | [ |
SiOx/C | Controllable LiBp complex solution prelithiation and high?temperature calcination process were combined to pregenerate LixSiOy in the interior of SiOx/C | 86 | NCM811 | [ |
Table 2 Various prelithiation methods
| Method | Material | Introduction | ICE of full cell(%) | Matched cathode | Ref. |
|---|---|---|---|---|---|
| Prelithiation additive | SiO | SLMP was directly sprayed on the surface of the electrode | 89.77 | LiNi1/3Mn1/3Co1/3O2 | [ |
| SiO | SLMP was dispersed in xylene solvent and then coated on the surface of the electrode | 88.12 | LiNi1/3Mn1/3Co1/3O2 | [ | |
| SiOx | SiOx and molten lithium are thermally alloyed to form LixSi/Li2O composite for prelithiation | 94 | LiFePO4 | [ | |
Self?discharge prelithiation | SiOx | Inserting a resistance buffer layer(RBL) between SiOx anode and Li foil toregulate the rate and degree of prelithiation | 87 | NCM622 | [ |
Electrochemical prelithiation | c?SiOx | Assembling a temporary battery and introduce a rheostat in the external circuit to control the prelithiation rate | 85.34 | LiNi0.8Co0.15Al0.05O2 | [ |
Chemical prelithiation | SiOx | Employing molecularly engineered BP derivatives to adjust the reduction potential of Li?arene(LAC) below 0.2 V drives active Li accommodation in SiOx anodes | 86 | NCM523 | [ |
SiOx/C | Controllable LiBp complex solution prelithiation and high?temperature calcination process were combined to pregenerate LixSiOy in the interior of SiOx/C | 86 | NCM811 | [ |
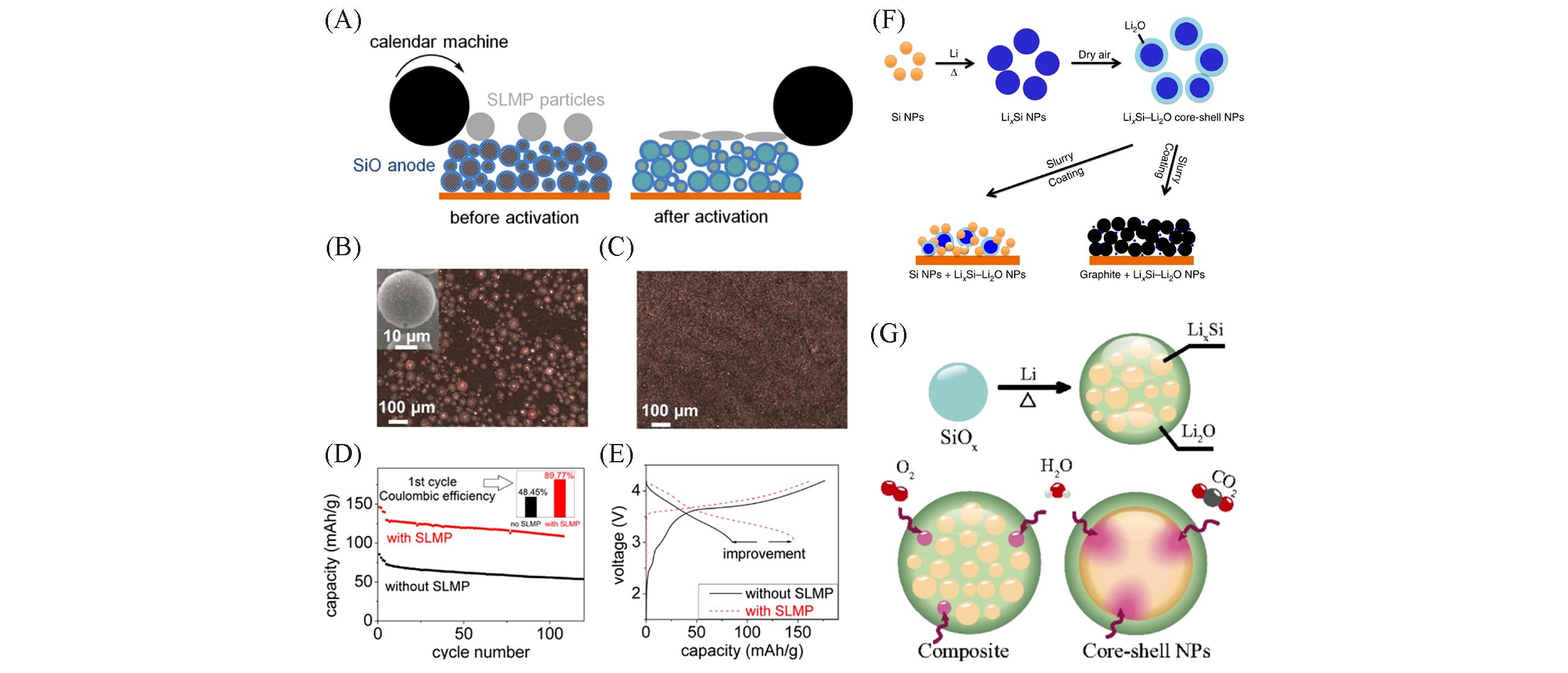
Fig.9 Schematics illustration showing SLMP particles loaded on the SiO anode(A), SEM image of SLMP particles loaded on the SiO electrode before activation(B), SEM image of SiO electrode surface after adding the electrolyte to the SiO electrode with activated SLMP for 12 h(C), cycle performance(D) and first cycle voltage curves(E) of SiO/NMC full cell with or without the SLMP[77], schematic of Si NPs react with melted Li to form LixSi NPs(F)[81], comparison of different behaviors of LixSi/Li2O core shell NPs and LixSi/Li2O composite at the ambient condition(G)[82](A—E) Copyright 2014, American Chemical Society; (F) Copyright 2014, Springer Nature; (G) Copyright 2016, National Academy of Science.
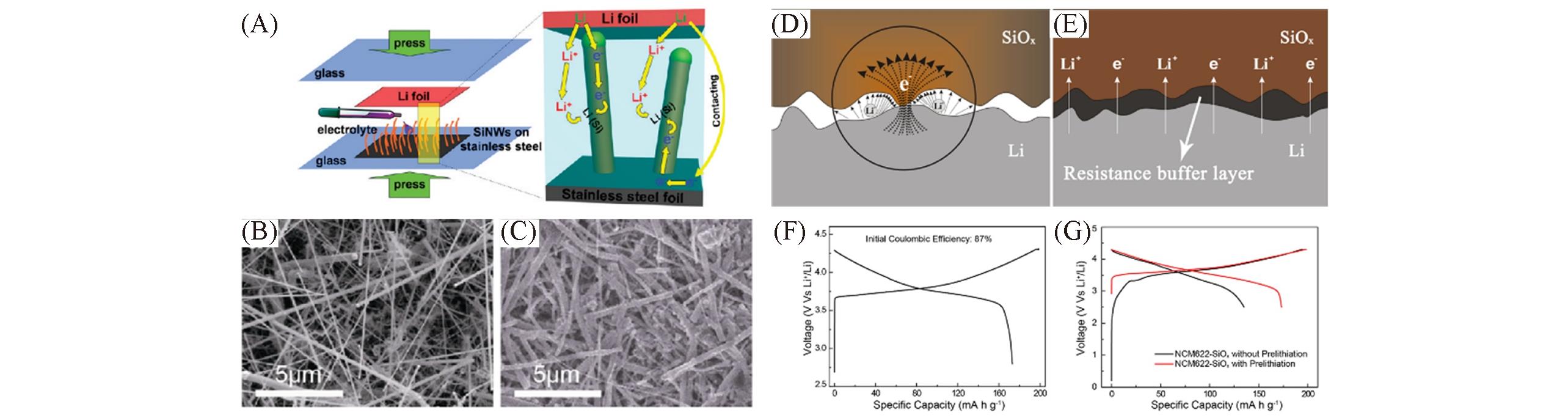
Fig.10 Schematic illustration of the prelithiation of SiNWs on stainless steel(SS) foil and the Li+ and internal electron pathways during the prelithiation(A), SEM images of SiNWs before(B) and after 10 min(C) prelithiation[83], schematic of Li+ and electron transfer in the direct contact prelithiation process(D) and resistance buffer layer regulated prelithiation process(E), the initial discharge/charge profiles of the NCM622?Li half?cell(F), and the initial discharge/charge profiles of NCM622?SiOx full cell with and without prelithiation(G)[84](A—C) Copyright 2011, American Chemical Society; (D—G) Copyright 2019, American Chemical Society.
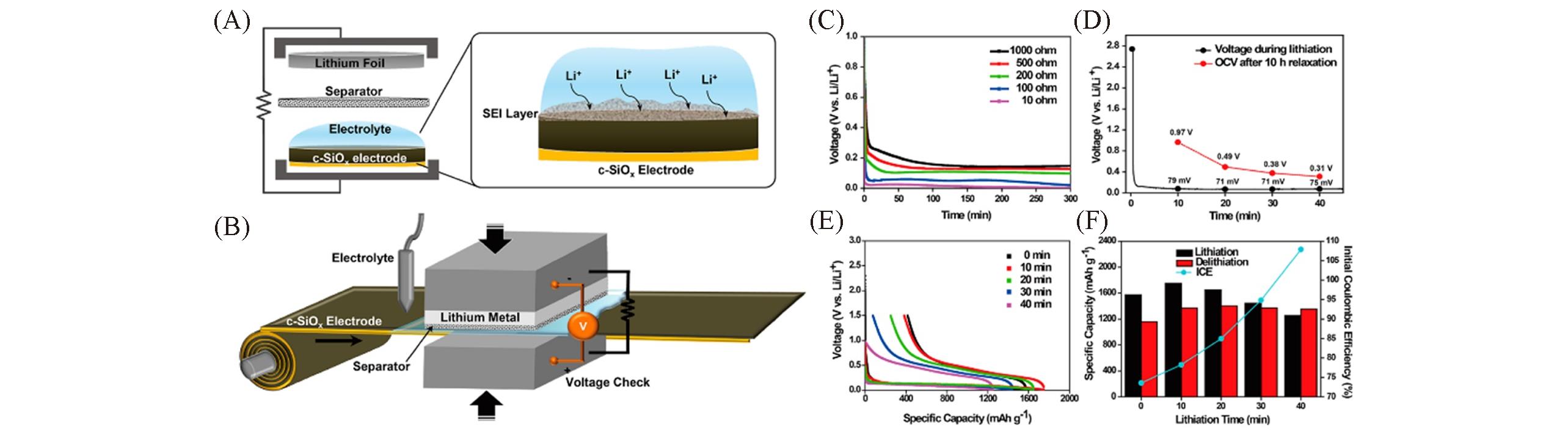
Fig.11 Schematic diagrams showing the prelithiation process of c?SiOx electrode(A) and its scalable roll?to?roll process scheme(B), lithiation voltage profiles of the external circuit containing different resistances(C), voltage profile during the external shorting with 100 Ω incorporated in the external circuit and open circuit voltages(OCVs) after 10 h of relaxation at different prelithiation points(D), the initial discharge/charge profiles of c?SiOx with different prelithiation durations(E), the specific capacity and ICE after different prelithiation times(F)[85]Copyright 2016, American Chemical Society.
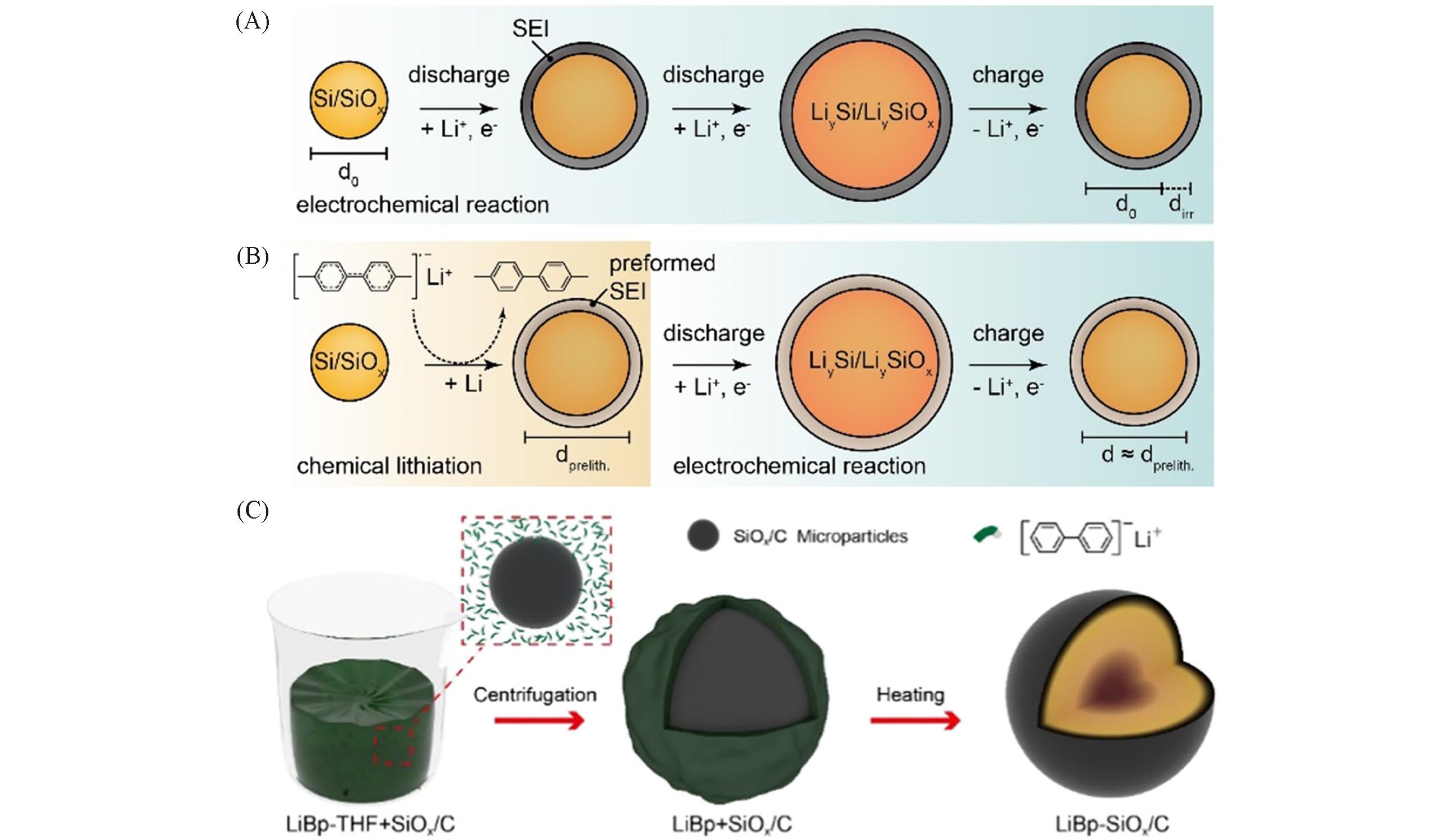
Fig.12 Lithiation/delithiation of pristine Si/SiOx during the initial electrochemical cycle accompanied with SEI formation and irreversible lithium trapping(A), lithiation/delithiation of prelithiated Si/SiOx using Li?arene complex beforehand with volume expansion and active lithium loss prior to cell assembly(B)[88], schematic of the prelithiation of LiBp?SiOx/C(C)[89](A, B) Copyright 2020, Wiley-VCH. (C) Copyright 2020, American Chemical Society.
| 1 | Cano Z. P., Banham D., Ye S., Hintennach A., Lu J., Fowler M., Chen Z., Nat. Energy,2018, 3(4), 279—289 |
| 2 | Lu J., Chen Z. H., Ma Z. F., Pan F., Curtiss L. A., Amine K., Nat. Nanotechnol.,2016, 11(12), 1031—1038 |
| 3 | Janek J., Zeier W. G., Nat. Energy,2016, 1(9), 16141—16144 |
| 4 | Li P., Hwang J. Y., Sun Y. K., ACS Nano,2019, 13(2), 2624—2633 |
| 5 | Cheng X. B., Zhang R., Zhao C. Z., Zhang Q., Chem. Rev.,2017, 117(15), 10403—10473 |
| 6 | Maier J., Nat. Mater.,2005, 4(11), 805—815 |
| 7 | Dimov N., Kugino S., Yoshio M., Electrochim. Acta,2003, 48(11), 1579—1587 |
| 8 | Chae S., Choi S. H., Kim N., Sung J., Cho J., Angew. Chem. Int. Ed.,2020, 59(1), 110—135 |
| 9 | Ashuri M., He Q., Shaw L. L., Nanoscale,2016, 8(1), 74—103 |
| 10 | Casimir A., Zhang H., Ogoke O., Amine J. C., Lu J., Wu G., Nano Energy,2016, 27, 359—376 |
| 11 | Cao C., Abate I. I., Sivonxay E., Shyam B., Jia C., Moritz B., Devereaux T. P., Persson K. A., Steinrück H. G., Toney M. F., Joule,2019, 3(3), 762—781 |
| 12 | Liu Z., Zhao Y., He R., Luo W., Meng J., Yu Q., Zhao D., Zhou L., Mai L., Energy Storage Mater.,2019, 19, 299—305 |
| 13 | Zhang L., Zhang L., Zhang J., Hao W., Zheng H., J. Mater. Chem. A, 2015, 3(30), 15432—15443 |
| 14 | Zhang Q., Chen H., Luo L., Zhao B., Luo H., Han X., Wang J., Wang C., Yang Y., Zhu T., Liu M., Energy Environ Sci.,2018, 11(3), 669—681 |
| 15 | Yoshio M., Tsumura T., Dimov N., J. Power Sources,2005, 146(1/2), 10—14 |
| 16 | Wu H., Cui Y., Nano Today,2012, 7(5), 414—429 |
| 17 | Szczech J. R., Jin S., Energy Environ Sci.,2011, 4(1), 56—72 |
| 18 | Lee J. K., Oh C., Kim N., Hwang J. Y., Sun Y. K., J. Mater. Chem. A,2016, 4(15), 5366—5384 |
| 19 | Li J. Y., Xu Q., Li G., Yin Y. X., Wan L. J., Guo Y. G., Mater. Chem. Front.,2017, 1(9), 1691—1708 |
| 20 | Kwon T. W., Choi J. W., Coskun A., Chem. Soc. Rev.,2018, 47(6), 2145—2164 |
| 21 | Chen T., Wu J., Zhang Q., Su X., J. Power Sources,2017, 363, 126—144 |
| 22 | Liu Z., Yu Q., Zhao Y., He R., Xu M., Feng S., Li S., Zhou L., Mai L., Chem. Soc. Rev.,2019, 48(1), 285—309 |
| 23 | Schnurre S. M., Gröbner J., Schmid⁃Fetzer R., J. Non⁃Cryst. Solids,2004, 336(1), 1—25 |
| 24 | AlKaabi K., Prasad D. L., Kroll P., Ashcroft N. W., Hoffmann R., J. Am. Chem. Soc.,2014, 136(9), 3410—3423 |
| 25 | Philipp H. R., J. Phys. Chem. Solids,1971, 32(8),1935—1945 |
| 26 | Philipp H. R., J. Non⁃Cryst. Solids,1972, 8—10, 627—632 |
| 27 | Brady G. W., J. Phys. Chem.,1959, 63(7), 1119—1120 |
| 28 | Temkin R. J., J. Non⁃Cryst. Solids,1975, 17(2), 215—230 |
| 29 | Fuglein E., Schubert U., Chem. Mater.,1999, 11(4), 865—866 |
| 30 | Hohl A., Wieder T., van Aken P. A., Weirich T. E., Denninger G., Vidal M., Oswald S., Deneke C., Mayer J., Fuess H., J. Non⁃Cryst. Solids,2003, 320(1—3), 255—280 |
| 31 | Schulmeister K., Mader W., J. Non⁃Cryst. Solids,2003, 320(1—3), 143—150 |
| 32 | Hirata A., Kohara S., Asada T., Arao M., Yogi C., Imai H., Tan Y., Fujita T., Chen M., Nat. Commun.,2016, 7, 11591 |
| 33 | Miyachi M., Yamamoto H., Kawai H., Ohta T., Shirakata M., J. Electrochem. Soc.,2005, 152(10), A2089—A2091 |
| 34 | Yamada M., Inaba A., Ueda A., Matsumoto K., Iwasaki T., Ohzuku T., J. Electrochem. Soc.,2012, 159(10), A1630—A1635 |
| 35 | Yamamura H., Nobuhara K., Nakanishi S., Iba H., Okada S., J. Ceram. Soc. Jpn.,2011, 119(1395), 855—860 |
| 36 | Yu B. C., Hwa Y., Park C. M., Sohn H. J., J. Mater. Chem. A, 2013, 1(15), 4820—4825 |
| 37 | Yu B. C., Hwa Y., Kim J. H., Sohn H. J., Electrochim. Acta,2014, 117, 426—430 |
| 38 | Jung S. C., Kim H. J., Kim J. H., Han Y. K., J. Phy. Chem. C,2015, 120(2), 886—892 |
| 39 | Yasuda K., Kashitani Y., Kizaki S., Takeshita K., Fujita T., Shimosaki S., J. Power Sources,2016, 329, 462—472 |
| 40 | Kim M. K., Jang B. Y., Lee J. S., Kim J. S., Nahm S., J. Power Sources,2013, 244, 115—121 |
| 41 | Xu K., Chem. Rev.,2014, 114(23), 11503—11618 |
| 42 | Philippe B., Dedryvère R., Allouche J., Lindgren F., Gorgoi M., Rensmo H., Gonbeau D., Edström K., Chem. Mater.,2012, 24(6), 1107—1115 |
| 43 | Radvanyi E., Porcher W., de Vito E., Montani A., Franger S., Jouanneau Si Larbi S., Phys. Chem. Chem. Phys.,2014, 16(32), 17142—17153 |
| 44 | Han J., Chen G., Yan T., Liu H., Shi L., An Z., Zhang J., Zhang D., Chem. Eng. J.,2018, 347, 273—279 |
| 45 | Zhang F., Yang J., Emergent Mater.,2020, 3(3), 369—380 |
| 46 | Jiao M., Wang Y., Ye C., Wang C., Zhang W., Liang C., J. Alloys Compd.,2020, 842, 155774 |
| 47 | Li X., Sun X., Hu X., Fan F., Cai S., Zheng C., Stucky G. D., Nano Energy,2020, 77, 105143 |
| 48 | Zhang L., Hao W., Wang H., Zhang L., Feng X., Zhang Y., Chen W., Pang H., Zheng H., J. Mater. Chem. A, 2013, 1(26), 7601—7611 |
| 49 | Zhang J., Zhang L., Xue P., Zhang L., Zhang X., Hao W., Tian J., Shen M., Zheng H., J. Mater. Chem. A, 2015, 3(15), 7810—7821 |
| 50 | Zeng Y., Huang Y., Liu N., Wang X., Zhang Y., Guo Y., Wu H. H., Chen H., Tang X., Zhang Q., J. Energy. Chem.,2021, 54, 727—735 |
| 51 | Li Q., Chen D., Li K., Wang J., Zhao J., Electrochim. Acta,2016, 202, 140—146 |
| 52 | Yang Y., Li J., Chen D., Fu T., Sun D., Zhao J., ChemElectroChem, 2016, 3(5), 757—763 |
| 53 | Zhang Y., Li K., Ji P., Chen D., Zeng J., Sun Y., Zhang P., Zhao J., J. Mater. Sci.,2017, 52(7), 3630—3641 |
| 54 | Cao Z., Xia B., Xie X., Zhao J., Electrochim. Acta,2019, 313, 311—320 |
| 55 | Xu Q., Sun J. K., Yin Y. X., Guo Y. G., Adv. Funct. Mater.,2018, 28(8), 1705235 |
| 56 | Kim J. H., Sohn H. J., Kim H., Jeong G., Choi W., J. Power Sources,2007, 170(2), 456—459 |
| 57 | Wu Z. L., Ji S. B., Liu L. K., Xie T., Tan L., Tang H., Sun R. G., Rare Met.,2021, 40(5), 1110—1117 |
| 58 | Zhuang X., Song P., Chen G., Shi L., Wu Y., Tao X., Liu H., Zhang D., ACS Appl. Mater. Interfaces,2017, 9(34), 28464—28472 |
| 59 | Xu Q., Sun J. K., Li G., Li J. Y., Yin Y. X., Guo Y. G., Chem. Commun.,2017, 53(89), 12080—12083 |
| 60 | Xu Q., Sun J. K., Yu Z. L., Yin Y. X., Xin S., Yu S. H., Guo Y. G., Adv. Mater.,2018, 30(25), 1707430 |
| 61 | Li J., Wang L., Liu F., Liu W., Luo C., Liao Y., Li X., Qu M., Wan Q., Peng G., ChemistrySelect,2019, 4(10), 2918—2925 |
| 62 | Tian H., Tian H., Yang W., Zhang F., Yang W., Zhang Q., Wang Y., Liu J., Silva S. R. P., Liu H., Wang G., Adv. Funct. Mater.,2021, 2101796 |
| 63 | Jeong G., Kim Y. U., Krachkovskiy S. A., Lee C. K., Chem. Mater.,2010, 22(19), 5570—5579 |
| 64 | Miyachi M., Yamamoto H., Kawai H., J. Electrochem. Soc.,2007, 154(4), A376—A380 |
| 65 | Zhang H., Hu R., Liu Y., Cheng X., Liu J., Lu Z., Zeng M., Yang L., Liu J., Zhu M., Energy Storage Mater.,2018, 13, 257—266 |
| 66 | Jeong G., Kim J. H., Kim Y. U., Kim Y. J., J. Mater. Chem.,2012, 22(16), 7999—8004 |
| 67 | Zhou M., Gordin M. L., Chen S., Xu T., Song J., Lv D., Wang D., Electrochem. Commun.,2013, 28, 79—82 |
| 68 | Yamamura H., Nakanishi S., Iba H., J. Power Sources,2013, 232, 264—269 |
| 69 | Kim S., Yoo H., Kim H., RSC Adv.,2020, 10(36), 21375—21381 |
| 70 | Cui J., Yang J., Man J., Li S., Yin J., Ma L., He W., Sun J., Hu J., Electrochim. Acta,2019, 300, 470—481 |
| 71 | Liao C., Wu S., Chem. Eng. J.,2019, 355, 805—814 |
| 72 | Zhang Y., Guo G., Chen C., Jiao Y., Li T., Chen X., Yang Y., Yang D., Dong A., J. Power Sources,2019, 426, 116—123 |
| 73 | Zhang H., Liu K., Liu Y., Lang Z., He W., Ma L., Man J., Jia G., Cui J., Sun J., J. Power Sources,2020, 447, 227400 |
| 74 | Vaughey J. T., Liu G., Zhang J. G., MRS Bull.,2014, 39(5), 429—435 |
| 75 | Jarvis C. R., Lain M. J., Yakovleva M. V., Gao Y., J. Power Sources,2006, 162(2), 800—802 |
| 76 | Xiang B., Wang L., Liu G., Minor A. M., J. Electrochem. Soc.,2013, 160(3), A415—A419 |
| 77 | Zhao H., Wang Z., Lu P., Jiang M., Shi F., Song X., Zheng Z., Zhou X., Fu Y., Abdelbast G., Xiao X., Liu Z., Battaglia V. S., Zaghib K., Liu G., Nano Lett.,2014, 14(11), 6704—6710 |
| 78 | Cassel F., Chua D., Lane M., Yakovleva M., Gao Y., Au G., ECS Trans.,2008, 11, 157—166 |
| 79 | Jarvis C. R., Lain M. J., Gao Y., Yakovleva M., J. Power Sources,2005, 146(1/2), 331—334 |
| 80 | Ai G., Wang Z., Zhao H., Mao W., Fu Y., Yi R., Gao Y., Battaglia V., Wang D., Lopatin S., Liu G., J. Power Sources,2016, 309, 33—41 |
| 81 | Zhao J., Lu Z., Liu N., Lee H. W., McDowell M. T., Cui Y., Nat. Commun.,2014, 5, 5088 |
| 82 | Zhao J., Lee H. W., Sun J., Yan K., Liu Y., Liu W., Lu Z., Lin D., Zhou G., Cui Y., Proc. Natl. Acad. Sci. USA,2016, 113(27), 7408—7413 |
| 83 | Liu N., Hu L., McDowell M. T., Jackson A., Cui Y., ACS Nano,2011, 5(8), 6487—6493 |
| 84 | Meng Q., Li G., Yue J., Xu Q., Yin Y. X., Guo Y. G., ACS Appl. Mater. Interfaces,2019, 11(35), 32062—32068 |
| 85 | Kim H. J., Choi S., Lee S. J., Seo M. W., Lee J. G., Deniz E., Lee Y. J., Kim E. K., Choi J. W., Nano Lett.,2016, 16(1), 282—288 |
| 86 | Scott M. G., Whitehead A. H., Owen J. R., J. Electrochem. Soc.,1998, 145(5), 1506—1510 |
| 87 | Tabuchi T., Yasuda H., Yamachi M., J. Power Sources,2005, 146(1/2), 507—509 |
| 88 | Jang J., Kang I., Choi J., Jeong H., Yi K. W., Hong J., Lee M., Angew. Chem. Int. Ed.,2020, 59(34), 14473—14480 |
| 89 | Yang M. Y., Li G., Zhang J., Tian Y. F., Yin Y. X., Zhang C. J., Jiang K. C., Xu Q., Li H. L., Guo Y. G., ACS Appl. Mater. Interfaces,2020, 12(24), 27202—27209 |
| 90 | Zhang L., Zhang L., Chai L., Xue P., Hao W., Zheng H., J. Mater. Chem. A, 2014, 2(44), 19036—19045 |
| 91 | Zhu X., Zhang F., Zhang L., Zhang L., Song Y., Jiang T., Sayed S., Lu C., Wang X., Sun J., Liu Z., Adv. Funct. Mater., 2018, 28(11), 1705015 |
| 92 | Yom J. H., Hwang S. W., Cho S. M., Yoon W. Y., J. Power Sources,2016, 311, 159—166 |
| 93 | Guo C., Wang D., Liu T., Zhu J., Lang X., J. Mater. Chem. A,2014, 2(10), 3521—3527 |
| 94 | Park E., Park M. S., Lee J., Kim K. J., Jeong G., Kim J. H., Kim Y. J., Kim H., ChemSusChem, 2015, 8(4), 688—694 |
| 95 | Zhang J., Zhang C., Liu Z., Zheng J., Zuo Y., Xue C., Li C., Cheng B., J. Power Sources,2017, 339, 86—92 |
| 96 | Yuge R., Toda A., Fukatsu K., Tamura N., Manako T., Nakahara K., Nakano K., J. Electrochem. Soc.,2013, 160(10), A1789—A1793 |
| 97 | Ren Y., Li M., J. Power Sources,2016, 306, 459—466 |
| 98 | Li Z., He Q., He L., Hu P., Li W., Yan H., Peng X., Huang C., Mai L., J. Mater. Chem. A, 2017, 5(8), 4183—4189 |
| 99 | Cui J., Cui Y., Li S., Sun H., Wen Z., Sun J., ACS Appl. Mater. Interfaces,2016, 8(44), 30239—30247 |
| 100 | Komaba S., Shimomura K., Yabuuchi N., Ozeki T., Yui H., Konno K., J. Phys. Chem. C,2011, 115(27), 13487—13495 |
| 101 | Guerfi A., Charest P., Dontigny M., Trottier J., Lagacé M., Hovington P., Vijh A., Zaghib K., J. Power Sources,2011, 196(13), 5667—5673 |
| 102 | Zhao T., Meng Y., Yin H., Guo K., Ji R., Zhang G., Zhang Y., Chem. Phys. Lett.,2020, 742, 137145 |
| 103 | Hu Z., Zhao L., Jiang T., Liu J., Rashid A., Sun P., Wang G., Yan C., Zhang L., Adv. Funct. Mater., 2019, 29(45), 1906548 |
| 104 | Choi N. S., Yew K. H., Lee K. Y., Sung M., Kim H., Kim S. S., J. Power Sources,2006, 161(2), 1254—1259 |
| 105 | Chen L., Wang K., Xie X., Xie J., J. Power Sources,2007, 174(2), 538—543 |
| 106 | Nguyen C. C., Choi H., Song S. W., J. Electrochem. Soc.,2013, 160(6), A906—A914 |
| [1] | 贾洋刚, 邵霞, 程婕, 王朋朋, 冒爱琴. 赝电容控制型钙钛矿高熵氧化物La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3负极材料的制备及储锂性能[J]. 高等学校化学学报, 2022, 43(8): 20220157. |
| [2] | 鲍俊全, 郑仕兵, 苑旭明, 史金强, 孙田将, 梁静. 有机盐PTO(KPD)2作为高性能锂离子电池正极材料的研究[J]. 高等学校化学学报, 2021, 42(9): 2911. |
| [3] | 卓增庆, 潘锋. 基于软X射线光谱的锂电池材料的电子结构与演变的研究进展[J]. 高等学校化学学报, 2021, 42(8): 2332. |
| [4] | 吴卓彦, 李至, 赵旭东, 王倩, 陈顺鹏, 常兴华, 刘志亮. 一步法高效制备纳米Si/C复合材料及其在高性能锂离子电池中的应用[J]. 高等学校化学学报, 2021, 42(8): 2500. |
| [5] | 易聪华, 苏华坚, 钱勇, 李琼, 杨东杰. 木质素纳米炭的制备及作为锂离子电池负极的性能研究[J]. 高等学校化学学报, 2021, 42(6): 1807. |
| [6] | 李世恒, 王超, 鲁振达. 锂离子电池硅基负极材料的预锂化研究进展[J]. 高等学校化学学报, 2021, 42(5): 1530. |
| [7] | 石颖, 胡广剑, 吴敏杰, 李峰. 低温等离子体在锂离子电池材料中的应用[J]. 高等学校化学学报, 2021, 42(5): 1315. |
| [8] | 毛尔洋, 王莉, 孙永明. 锂离子电池高容量合金基含锂负极材料的研究进展[J]. 高等学校化学学报, 2021, 42(5): 1552. |
| [9] | 刘铁峰, 张奔, 盛欧微, 佴建威, 王垚, 刘育京, 陶新永. 硅负极黏结剂的研究进展[J]. 高等学校化学学报, 2021, 42(5): 1446. |
| [10] | 王弈艨, 刘凯, 王保国. 高镍三元正极材料的表面包覆策略[J]. 高等学校化学学报, 2021, 42(5): 1514. |
| [11] | 王任衡, 肖哲, 李艳, 孙一翎, 范姝婷, 郑俊超, 钱正芳, 贺振江. 固相烧结法制备锂离子电池正极材料Li2FeP2O7及其电化学性能研究[J]. 高等学校化学学报, 2021, 42(4): 1299. |
| [12] | 巴智晨, 梁大鑫, 谢延军. MXenes复合材料的发展: 界面调控及结构设计[J]. 高等学校化学学报, 2021, 42(4): 1225. |
| [13] | 周战, 马录芳, 谭超良. 层状钒青铜纳米片的制备及其锂离子电池阳极材料性能[J]. 高等学校化学学报, 2021, 42(2): 662. |
| [14] | 孙全虎, 卢天天, 何建江, 黄长水. 含异原子石墨炔基电极材料的研究进展[J]. 高等学校化学学报, 2021, 42(2): 366. |
| [15] | 詹舒辉, 赵亚松, 杨乃亮, 王丹. 石墨炔孔结构: 设计、 合成和应用[J]. 高等学校化学学报, 2021, 42(2): 333. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||