

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (5): 1315.doi: 10.7503/cjcu20200675
石颖1,2, 胡广剑1,3, 吴敏杰1,3, 李峰1,2,3( )
)
收稿日期:2020-09-11
出版日期:2021-05-10
发布日期:2020-12-25
通讯作者:
李峰
E-mail:fli@imr.ac.cn
基金资助:
SHI Ying1,2, HU Guangjian1,3, WU Minjie1,3, LI Feng1,2,3( )
)
Received:2020-09-11
Online:2021-05-10
Published:2020-12-25
Contact:
LI Feng
E-mail:fli@imr.ac.cn
Supported by:摘要:
综合评述了低温等离子体技术的基本原理、 常用方法及其在锂离子电池材料领域中的研究进展, 重点评述了等离子体技术在锂离子电池正极、 负极、 隔膜及固态电解质等重要组分中的材料制备与表面改性方面的主要研究结果和应用优势, 并对其所面临的挑战和未来的应用方向进行了展望.
中图分类号:
TrendMD:
石颖, 胡广剑, 吴敏杰, 李峰. 低温等离子体在锂离子电池材料中的应用. 高等学校化学学报, 2021, 42(5): 1315.
SHI Ying, HU Guangjian, WU Minjie, LI Feng. Applications of Low Temperature Plasma for the Materials in Li-ion Batteries. Chem. J. Chinese Universities, 2021, 42(5): 1315.
| Plasma technology | Applications for Li?ion battery | Advantage |
|---|---|---|
| Glow discharge plasma | Surface processing, such as etching and doping | Simple, inexpensive, wide pressure range |
| Dielectric barrier discharge plasma(DBD) | Synthesis and polymerization of materials | Simple excitation, wide pressure range, large plasma discharge zone |
| Radio frequency(RF) plasma | Synthesis of electrode materials and solid?state electrolytes, surface etching | Less charge accumulation |
| Spark plasma sintering(SPS) | Synthesis of electrode materials and inorganic solid?state electrolytes | Short holding times, reduced temperature, pressure to achieve dense materials |
| Plasma?enhanced chemical vapor deposition(PECVD) | Synthesis of thin films and coatings | Lower temperature for deposition and high film quality |
| Magnetron sputtering | Synthesis of thin films and inorganic solid?state electrolytes | High film purity and density, good contact with the substrate and low damage to the substrate, controllable film thickness |
| Plasma spray | Synthesis of thin films and coatings | High film quality and density, no damage to substrate |
表1 中归纳了目前锂离子电池中常用的等离子体技术及其应用优势.
Table 1 Plasma technologies commonly used for Li-ion batteries
| Plasma technology | Applications for Li?ion battery | Advantage |
|---|---|---|
| Glow discharge plasma | Surface processing, such as etching and doping | Simple, inexpensive, wide pressure range |
| Dielectric barrier discharge plasma(DBD) | Synthesis and polymerization of materials | Simple excitation, wide pressure range, large plasma discharge zone |
| Radio frequency(RF) plasma | Synthesis of electrode materials and solid?state electrolytes, surface etching | Less charge accumulation |
| Spark plasma sintering(SPS) | Synthesis of electrode materials and inorganic solid?state electrolytes | Short holding times, reduced temperature, pressure to achieve dense materials |
| Plasma?enhanced chemical vapor deposition(PECVD) | Synthesis of thin films and coatings | Lower temperature for deposition and high film quality |
| Magnetron sputtering | Synthesis of thin films and inorganic solid?state electrolytes | High film purity and density, good contact with the substrate and low damage to the substrate, controllable film thickness |
| Plasma spray | Synthesis of thin films and coatings | High film quality and density, no damage to substrate |
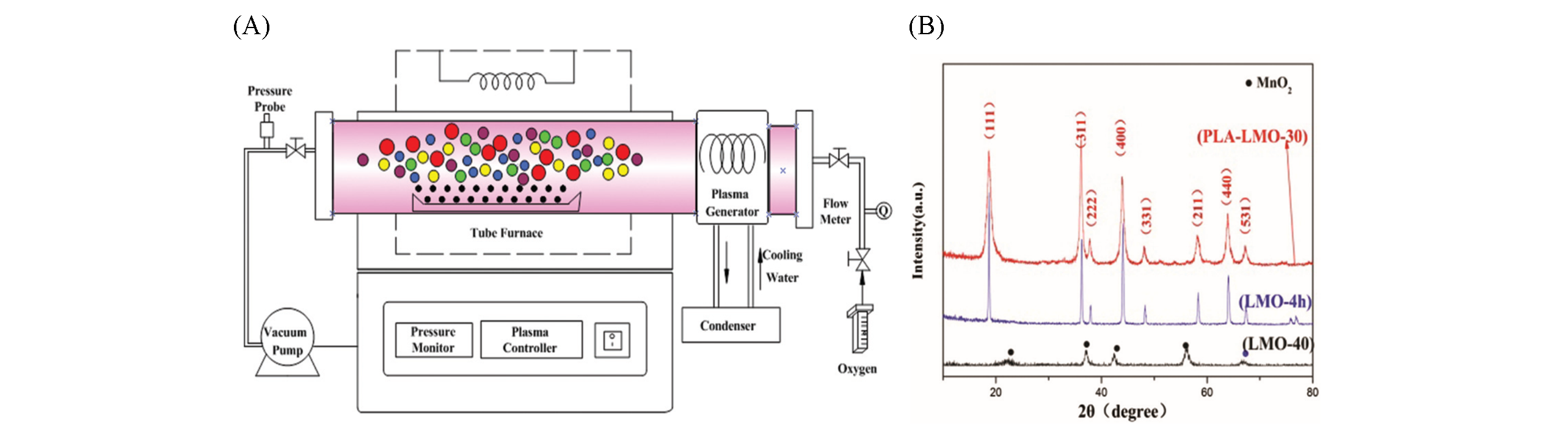
Fig.2 Plasma?enhanced low temperature preparation of LiMn2O4[24](A) Schematic diagram of the plasma-enhanced tube furnace for the preparation of LiMn2O4; (B) XRD patterns for the as-prepared spinel LiMn2O4 by the plasma-enhanced low temperature solid-state method(PLA-LMO-30) and the conventional thermal annealing method(LMO-40 and LMO-4 h). Copyright 2016, Royal Society of Chemistry.
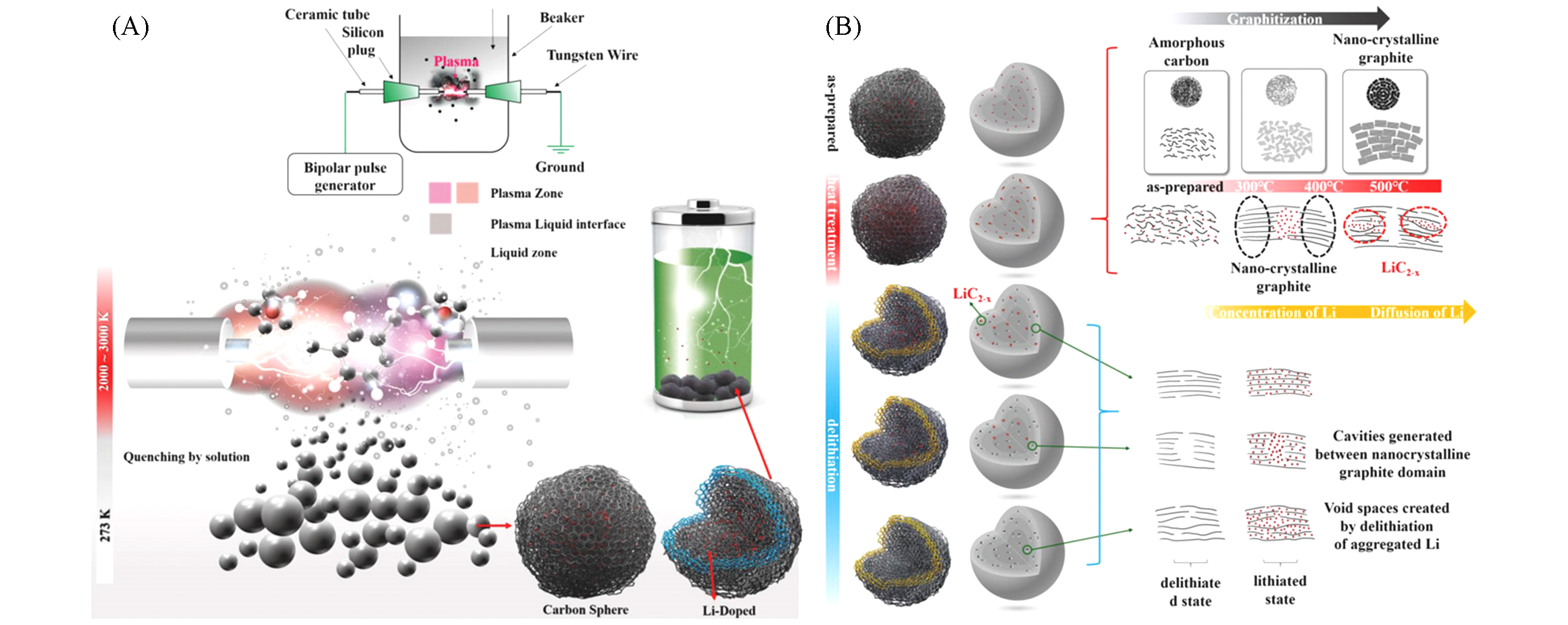
Fig.3 Schematics for the Li?doped carbon material by solution plasma process[36](A) Schematics showing concept of anode material and solution plasma process; (B) schematic showing formation of Li domains and delithiation process. Copyright 2018, Wiley.
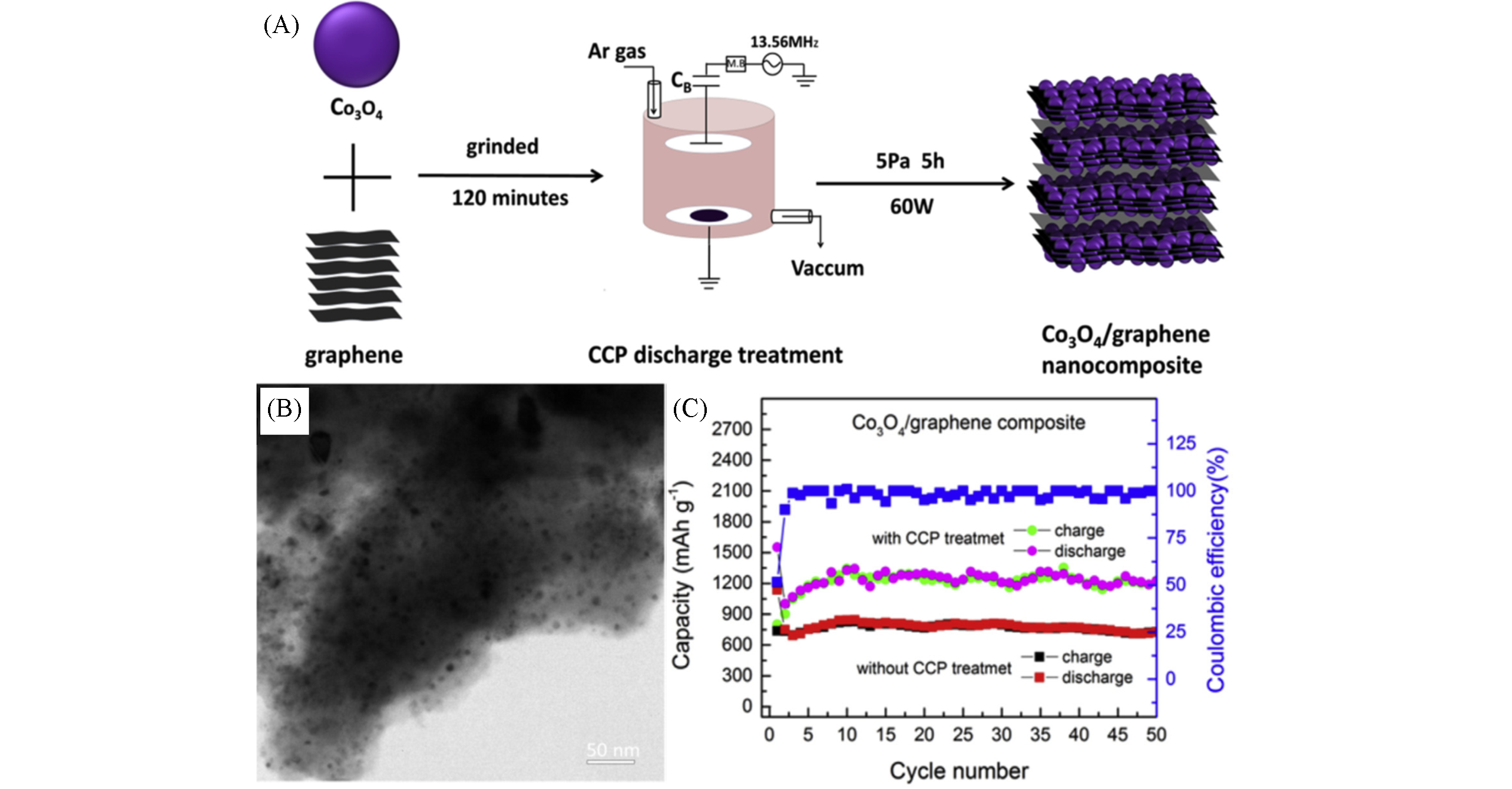
Fig.4 Co3O4 /graphene nanocomposites prepared by low pressure capacitively?coupled?plasma treatment[44](A) Schematics for the synthesis process of Co3O4/graphene nanocomposites by CCP treatment; (B) TEM images of graphene/Co3O4 nanocomposites; (C) the cycling performance and coulombic efficiency of Co3O4/graphene nanocomposites with/without CCP treatment at the current density of 125 mA/g. Copyright 2017, Elsevier.
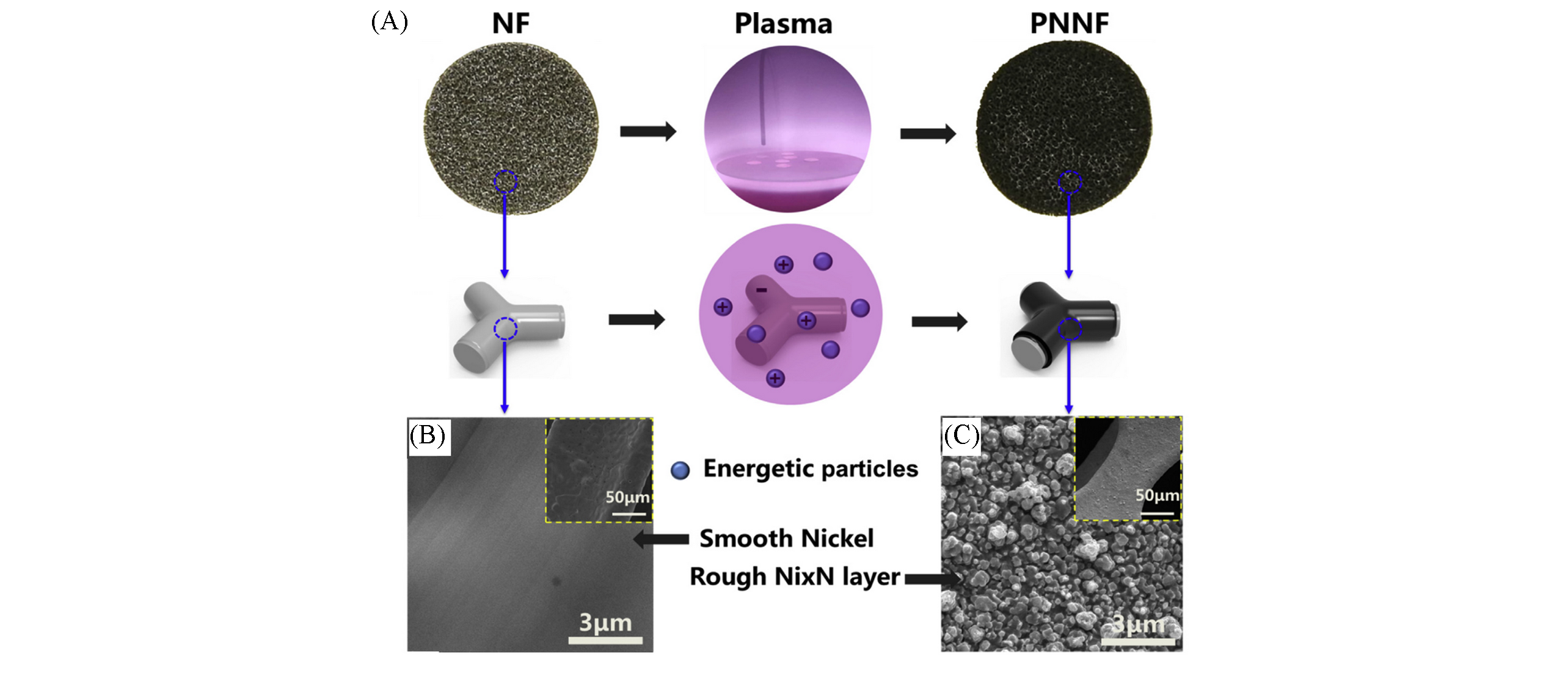
Fig.5 Plasma nitriding process of nitride decorated nickel foams(PNNF)[53](A) The schematics of the plasma nitriding process of PNNF; (B) SEM images of nickel foam(NF); (C) SEM images of PNNF. Copyright 2019, Elsevier.
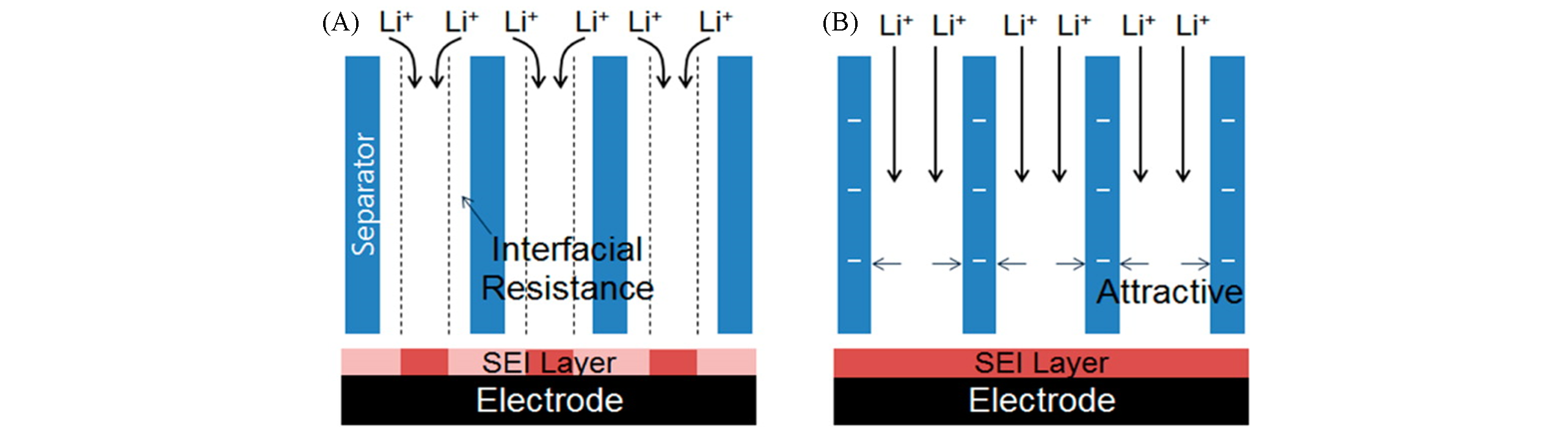
Fig.6 Illustrative comparison of lithium ion flux through pores of the separators and the resulting SEI layers[69](A) Lithium ion flux in cells with bare separators; (B) lithium ion flux in cells with negatively charged separators.Copyright 2019, American Chemical Society.
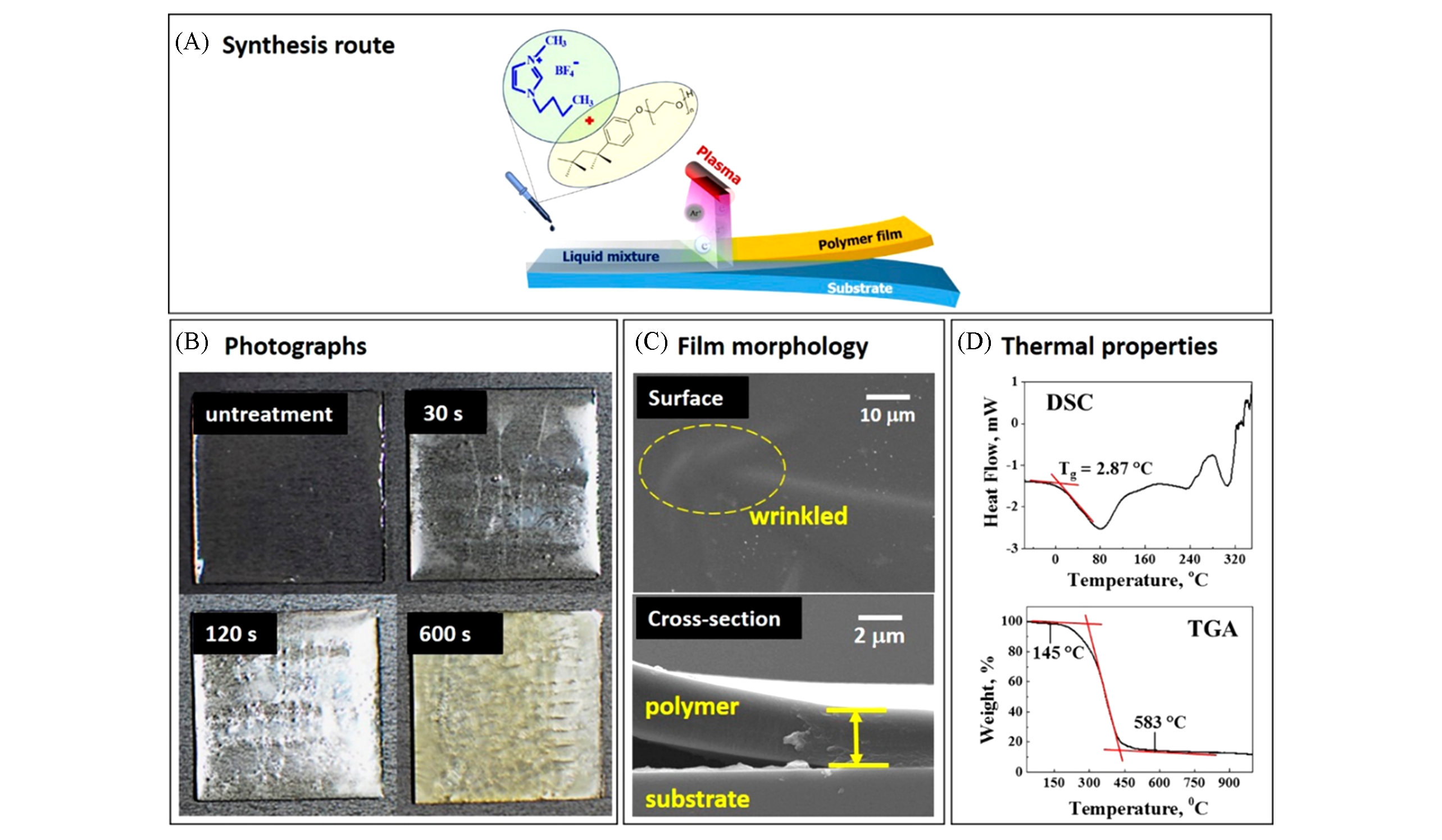
Fig.7 Interfacial liquid plasma polymerization of the polymer electrolyte from ionic liquids(IL)[81](A) Scheme illustrating the synthesis route of the polymer electrolyte from ionic liquid and surfactant via interfacial liquid plasma polymerization; (B) photographs of polymer electrolyte films on glass substrates with different plasma exposure times; (C) surface and cross-sectional morphology of polymer electrolyte film; (D) thermal properties of polymer electrolyte. Copyright 2016, American Chemical Society.
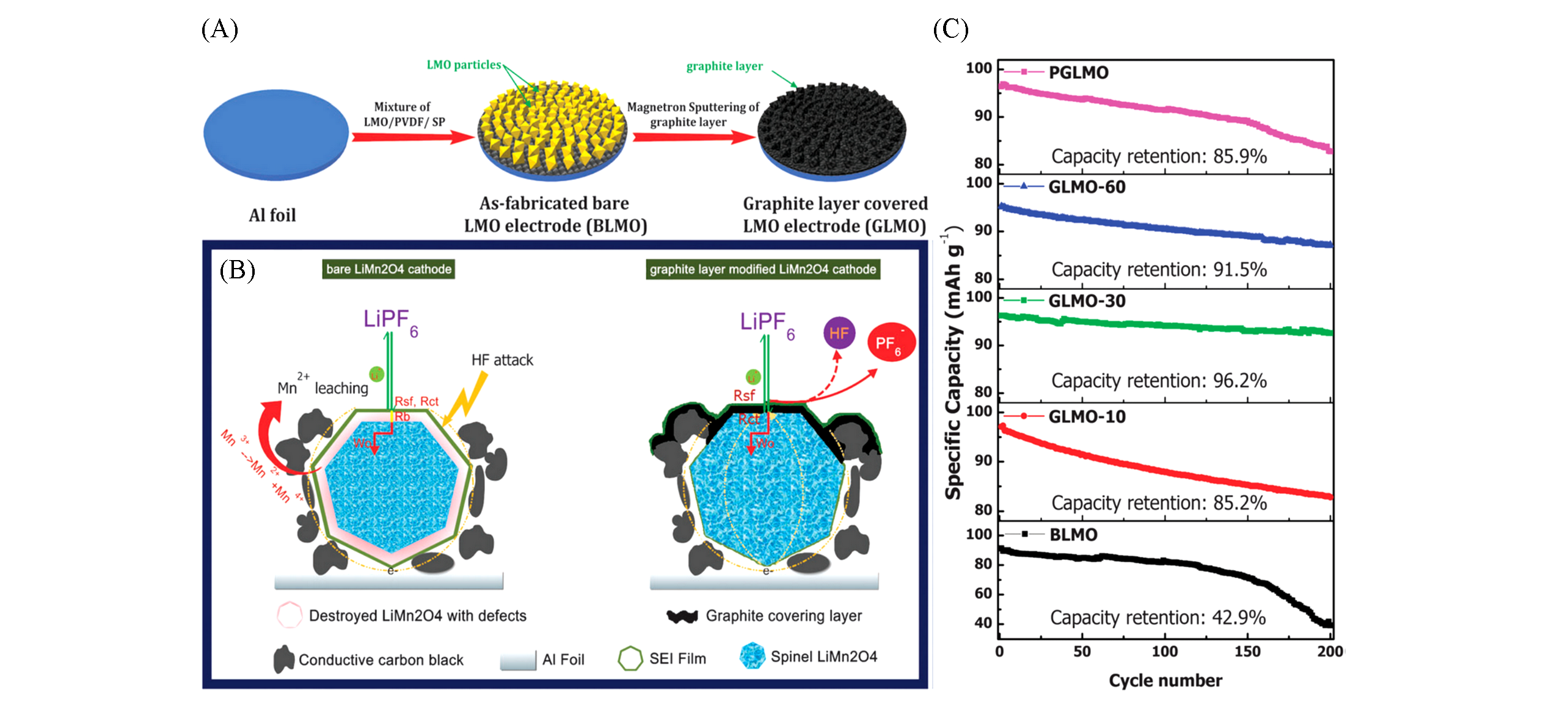
Fig.8 Nano?thin graphite layer covered LiMn2O4 electrode via room?temperature DC magnetron sputtering[83](A) Schematic diagram for the preparation of the graphite layer covered LiMn2O4 electrode; (B) schematic conduction/diffusion of electron and lithium ions during the electrochemical kinetics process in the BLMO and GLMO-30 electrode, respectively; (C) cycle performance of the electrodes at 1C rate under 55 ℃. Copyright 2014, Royal Society of Chemistry.
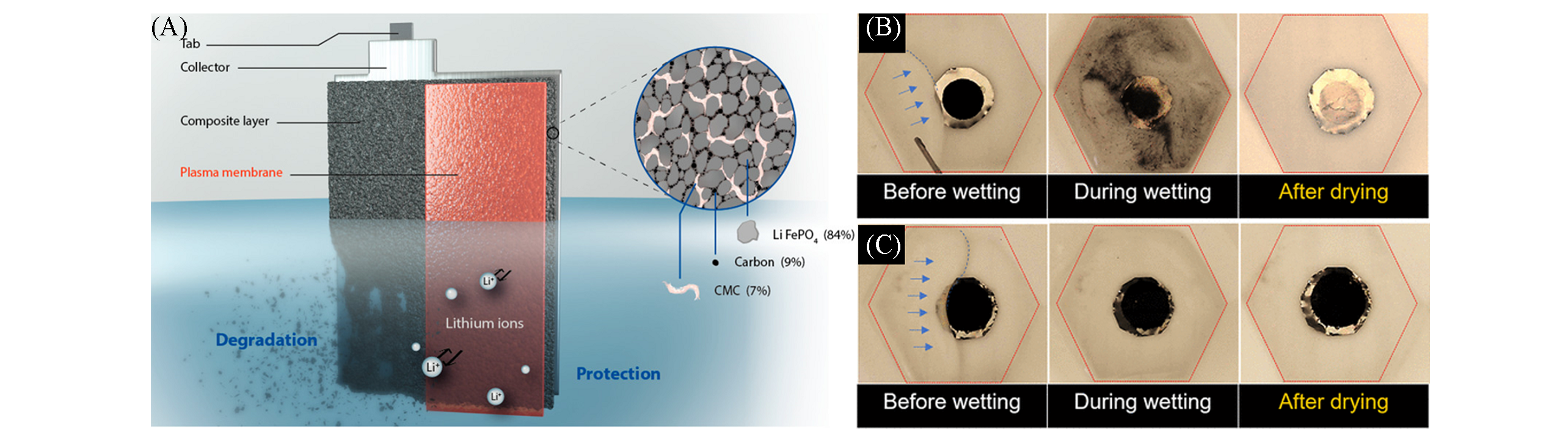
Fig.9 Water?soluble CMC?based LiFePO4 electrode for aqueous Li?ion battery systems protected by a plasma induced thin membrane[88](A) Schematic for the electrode protected by a thin membrane obtained by the plasma treatment; (B) stability of the untreated electrode before, during, and after immersion in water; (C) comparative result obtained with plasma treated electrodes. Copyright 2020, American Chemical Society.
| 1 | Armand M., Tarascon J. M., Nature, 2008, 451(7179), 652—657 |
| 2 | van Noorden R., Nature, 2014, 507(7490), 26—28 |
| 3 | Evarts E. C., Nature, 2015, 526(7575), S93—S95 |
| 4 | Nava⁃Avendano J., Veilleux J., J. Phys. D⁃Appl. Phys., 2017, 50(16), 163001 |
| 5 | He B., Yang Y., Yuen M. F., Chen X. F., Lee C. S., Zhang W. J., Nano Today, 2013, 8(3), 265—289 |
| 6 | Cheruthazhekatt S., Cernak M., Slavicek P., Havel J., J. Appl. Biomed., 2010, 8(2), 55—66 |
| 7 | Lu T., Qiao Y. Q., Liu X. Y., Interface Focus, 2012, 2(3), 325—336 |
| 8 | Desmet T., Morent R., de Geyter N., Leys C., Schacht E., Dubruel P., Biomacromolecules, 2009, 10(9), 2351—2378 |
| 9 | Lloyd G., Friedman G., Jafri S., Schultz G., Fridman A., Harding K., Plasma Process. Polym., 2010, 7(3/4), 194—211 |
| 10 | Zheng Z. W., Deng K. M., Ren L., Wang Y. J., Chem. J. Chinese Universities, 2012, 33(6), 1350—1354(郑志雯, 邓凯敏, 任力, 王迎军. 高等学校化学学报, 2012, 33(6), 1350—1354) |
| 11 | Sankaran R. M., Kortshagen U., J. Phys. D⁃Appl. Phys., 2015, 48(31), 310301 |
| 12 | Kortshagen U. R., Sankaran R. M., Pereira R. N., Girshick S. L., Wu J. J., Aydil E. S., Chem. Rev., 2016, 116(18), 11061—11127 |
| 13 | Seo J. H., Hong B. G., Nucl. Eng. Technol., 2012, 44(1), 9—20 |
| 14 | Chen X., Hu Z., Wang X. Z., Wu Q., Chen Y., Yang S. G., Du Y. W., Chem. J. Chinese Universities, 2001, 22(5), 731—733(陈新, 胡征, 王喜章, 吴强, 陈懿, 杨绍光, 都有为. 高等学校化学学报, 2001, 22(5), 731—733) |
| 15 | Joseph J., Murdock A. T., Seo D. H., Han Z. J., O’Mullane A. P., Ostrikov K., Adv. Mater. Technol., 2018, 3(9), 1800070 |
| 16 | Liu A. G., Low Temperature Plasma Surface Strengthening Technologies, Harbin Institute of Technology Press, Harbin, 2015, 6—39(刘爱国. 低温等离子体表面强化技术, 哈尔滨: 哈尔滨工业大学出版社, 2015, 6—39) |
| 17 | Tyczkowski J.,Ed.: Shao Y., Electrochemical Cells⁃New Advances in Fundamental Researches and Applications, InTech, Croatia, 2012, 105—138 |
| 18 | Dou S., Tao L., Wang R. L., El Hankari S., Chen R., Wang S. Y., Adv. Mater., 2018, 30(21), 1705850 |
| 19 | Kali R., Mukhopadhyay A., J. Power Sources, 2014, 247, 920—931 |
| 20 | Galy J., Dolle M., Hungria T., Rozier P., Monchoux J. P., Solid State Sci., 2008, 10(8), 976—981 |
| 21 | Meyyappan M., Delzeit L., Cassell A., Hash D., Plasma Sources Sci. Technol., 2003, 12(2), 205—216 |
| 22 | Tanaka M., Kageyama T., Sone H., Yoshida S., Okamoto D., Watanabe T., Nanomaterials, 2016, 6(4), 6040060 |
| 23 | Ajayi B. P., Thapa A. K., Cvelbar U., Jasinski J. B., Sunkara M. K., Chem. Eng. Sci., 2017, 174, 302—310 |
| 24 | Jiang Q. Q., Zhang H., Wang S. Y., Green Chem., 2016, 18(3), 662—666 |
| 25 | Jiang Q. Q., Xu L., Huo J., Zhang H., Wang S. Y., RSC Adv., 2015, 5(92), 75145—75148 |
| 26 | Dumont-Botto E., Bourbon C., Patoux S., Rozier P., Dolle M., J. Power Sources, 2011, 196(4), 2274—2278 |
| 27 | Ouyang L. Z., Cao Z. J., Wang H., Hu R. Z., Zhu M., J. Alloy. Compd., 2017, 691, 422—435 |
| 28 | Needham S. A., Calka A., Wang G. X., Mosbah A., Liu H. K., Electrochem. Commun., 2006, 8(3), 434—438 |
| 29 | Sun W., Hu R. Z., Liu H., Zeng M. Q., Yang L. C., Wang H. H., Zhu M., J. Power Sources, 2014, 268, 610—618 |
| 30 | Shigeta M., Murphy A. B., J. Phys. D⁃Appl. Phys., 2011, 44(17), 174025 |
| 31 | Yoshida T., Pure Appl. Chem., 1994, 66(6), 1223—1230 |
| 32 | Ketterer B., Vasilchina H., Seemann K., Ulrich S., Besser H., Pfleging W., Kaiser T., Adelhelm C., International Journal of Materials Research, 2008, 99(10), 1171—1176 |
| 33 | Chiu K. F., Chen C. C., Chiang M. H., Ho W. H., J. Electrochem. Soc., 2010, 157(2), A130—A135 |
| 34 | Pentyala N., Guduru R. K., Mohanty P. S., Electrochim. Acta, 2011, 56(27), 9851—9859 |
| 35 | He M., Zhou H. P., Ding G. Q., Zhang Z. D., Ye X., Cai D., Wu M. Q., Carbon, 2019, 146, 194—199 |
| 36 | Kang J., Kim H. V., Chae S. A., Kim K. H., Small, 2018, 14(20), 1704394 |
| 37 | Poizot P., Laruelle S., Grugeon S., Dupont L., Tarascon J. M., Nature, 2000, 407(6803), 496—499 |
| 38 | Kasavajjula U., Wang C. S., Appleby A. J., J. Power Sources, 2007, 163(2), 1003—1039 |
| 39 | Marcinek A., Hardwick L. J., Richardson T. J., Song X., Kostecki R., J. Power Sources, 2007, 173(2), 965—971 |
| 40 | Bo Z., Mao S., Han Z. J., Cen K. F., Chen J. H., Ostrikov K., Chem. Soc. Rev., 2015, 44(8), 2108—2121 |
| 41 | Yang J. H., de Guzman R. C., Salley S. O., Ng K. Y. S., Chen B. H., Cheng M. M. C., J. Power Sources, 2014, 269, 520—525 |
| 42 | Wei Y., Yu H., Li H. T., Ming H., Pan K. M., Huang H., Liu Y., Kang Z. H., Mater. Res. Bull., 2013, 48(10), 4072—4077 |
| 43 | Zhou Q., Zhao Z. B., Wang Z. Y., Dong Y. F., Wang X. Z., Gogotsi Y., Qiu J. S., Nanoscale, 2014, 6(4), 2286—2291 |
| 44 | Long H. F., Zhang M. Y., Wang Q., Xing L. L., Wang S., Xue X. Y., J. Alloy. Compd., 2017, 701, 200—207 |
| 45 | Tsai H. S., Hsu C. H., Chi C. C., Wang Y. C., Liu F. W., Tang S. Y., Tsai C. J., Ouyang H., Chueh Y. L., Liang J. H., Inorg. Chem. Front., 2019, 6(1), 172—175 |
| 46 | Liu Y. Y., Zhang L., Zhao Y. C., Shen T. D., Yan X. L., Yu C., Wang H. Q., Zeng H., J. Alloy. Compd., 2019, 787, 996—1003 |
| 47 | Chang X. H., Zheng X. Y., Guo Y. R., Chen J., Zheng J., Li X. G., Nano Res., 2018, 11(5), 2724—2732 |
| 48 | Lin D. C., Liu Y. Y., Cui Y., Nat. Nanotechnol., 2017, 12(3), 194—206 |
| 49 | Cheng X. B., Zhang R., Zhao C. Z., Zhang Q., Chem. Rev., 2017, 117(15), 10403—10473 |
| 50 | Zhang R., Li N. W., Cheng X. B., Yin Y. X., Zhang Q., Guo Y. G., Adv. Sci., 2017, 4(3), 1600445 |
| 51 | Hu Z., Li Z., Xia Z., Jiang T., Wang G., Sun J., Sun P., Yan C., Zhang L., Energy Storage Materials, 2019, 22, 29—39 |
| 52 | Luan J. Y., Zhang Q., Yuan H. Y., Sun D., Peng Z. G., Tang Y. G., Ji X. B., Wang H. Y., Adv. Sci., 2019, 6(20), 1901433 |
| 53 | Zhu J. F., Chen J., Luo Y., Sun S. Q., Qin L. G., Xu H., Zhang P. G., Zhang W., Tian W. B., Sun Z. M., Energy Storage Materials, 2019, 23, 539—546 |
| 54 | Kamaya N., Homma K., Yamakawa Y., Hirayama M., Kanno R., Yonemura M., Kamiyama T., Kato Y., Hama S., Kawamoto K., Mitsui A., Nat. Mater., 2011, 10(9), 682—686 |
| 55 | Wu M. F., Wen Z. Y., Liu Y., Wang X. Y., Huang L. Z., J. Power Sources, 2011, 196(19), 8091—8097 |
| 56 | Li Y. B., Sun Y. M., Pei A., Chen K. F., Vailionis A., Li Y. Z., Zheng G. Y., Sun J., Cui Y., Acs Central Sci., 2018, 4(1), 97— 104 |
| 57 | Chen K., Pathak R., Gurunga A., Adhamash E. A., Bahrami B., He Q. Q., Qiao H., Smirnova A. L., Wu J. J., Qiao Q. Q., Zhou Y., Energy Storage Materials, 2019,(18), 389—396 |
| 58 | Li Y., Yin Y., Guo K., Xue X. Z., Zou Z. Q., Li X. M., He T., Yang H., J. Power Sources, 2013, 241, 288—294 |
| 59 | Phan L. T., Yoon S. M., Moon M. W., Polymers⁃Basel, 2017, 9(9), 9090417 |
| 60 | Jiang Q. Q., Li Z., Wang S. Y., Zhang H., RSC Adv., 2015, 5(113), 92995—93001 |
| 61 | Fang J., Kelarakis A., Lin Y. W., Kang C. Y., Yang M. H., Cheng C. L., Wang Y., Giannelis E. P., Tsai L. D., Phys. Chem. Chem. Phys., 2011, 13(32), 14457—14461 |
| 62 | Correia D. M., Ribeiro C., Sencadas V., Botelho G., Carabineiro S. A. C., Ribelles J. L. G., Lanceros⁃Mendez S., Prog. Org. Coat., 2015, 85, 151—158 |
| 63 | Jin S. Y., Manuel J., Zhao X., Park W. H., Ahn J. H., J. Ind. Eng. Chem., 2017, 45, 15—21 |
| 64 | Huang C., Lin C. C., Tsai C. Y., Juang R. S., Plasma Process. Polym., 2013, 10(5), 407—415 |
| 65 | Son J., Kim M. S., Lee H. W., Yu J. S., Kwon K. H., J. Nanosci. Nanotechnol., 2014, 14(12), 9368—9372 |
| 66 | Chen H., Lin Q., Xu Q., Yang Y., Shao Z. P., Wang Y., J. Membr. Sci., 2014, 458, 217—224 |
| 67 | Kim J. Y., Lim D. Y., Energies, 2010, 3(4), 866—885 |
| 68 | Li X. F., He J. L., Wu D. Z., Zhang M. Z., Meng J. W., Ni P. H., Electrochim. Acta, 2015, 167, 396—403 |
| 69 | Han M., Kim D. W., Kim Y. C., ACS Appl. Mater. Interfaces, 2016, 8(39), 26073—26081 |
| 70 | Shi Q. A., Vitchuli N., Nowak J., Lin Z., Guo B. K., McCord M., Bourham M., Zhang X. W., J. Polym. Sci. Pt. B⁃Polym. Phys., 2011, 49(2), 115—122 |
| 71 | Colombo V., Fabiani D., Focarete M. L., Gherardi M., Gualandi C., Laurita R., Zaccaria M., Plasma Process. Polym., 2014, 11(3), 247—255 |
| 72 | Laurita R., Zaccaria M., Gherardi M., Fabiani D., Merlettini A., Pollicino A., Focarete M. L., Colombo V., Plasma Process. Polym., 2016, 13(1), 124—133 |
| 73 | Jeon H., Jin S. Y., Park W. H., Lee H., Kim H. T., Ryou M. H., Lee Y. M., Electrochim. Acta, 2016, 212, 649—656 |
| 74 | Qin S. C., Wang M., Wang C. L., Jin Y. C., Yuan N. N., Wu Z. C., Zhang J., Adv. Mater. Interfaces, 2018, 5(19), 1800579 |
| 75 | Zhu H. Z., Liu J., J. Power Sources, 2018, 391, 10—25 |
| 76 | Aboulaich A., Bouchet R., Delaizir G., Seznec V., Tortet L., Morcrette M., Rozier P., Tarascon J. M., Viallet V., Dolle M., Adv. Energy Mater., 2011, 1(2), 179—183 |
| 77 | Wei X. L., Rechtin J., Olevsky E. A., Metals, 2017, 7(9), 7090372 |
| 78 | Westover A. S., Kercher A. K., Kornbluth M., Naguib M., Palmer M. J., Cullen D. A., Dudney N. J., ACS Appl. Mater. Interfaces, 2020, 12(10), 11570—11578 |
| 79 | Ogumi Z., Uchimoto Y., Takehara Z., J. Power Sources, 1989, 26(3/4), 457—460 |
| 80 | Ogumi Z., Uchimoto Y., Takehara Z., Kanamori Y., J. Electrochem. Soc., 1988, 135(10), 2649—2650 |
| 81 | Tran Q. C., Bui V. T., Dao V. D., Lee J. K., Choi H. S., ACS Appl. Mater. Interfaces, 2016, 8(25), 16125—16135 |
| 82 | Marcinek M. L., Wilcox J. W., Doeff M. M., Kostecki R. M., J. Electrochem. Soc., 2009, 156(1), A48—A51 |
| 83 | Wang J. X., Zhang Q. B., Li X. H., Wang Z. X., Guo H. J., Xu D. G., Zhang K. L., Phys. Chem. Chem. Phys., 2014, 16(30), 16021—16029 |
| 84 | Wang K. X., Wang C. Z., Yang H., Wang X. B., Cao F., Wu Q. C., Peng H. L., Nano Res., 2020, doi: s12274⁃020⁃2780⁃2 |
| 85 | Luo W., Lin C. F., Zhao O., Noked M., Zhang Y., Rubloff G. W., Hu L. B., Adv. Energy Mater., 2017, 7(2), 1601526 |
| 86 | Qiu B., Wang J., Xia Y. G., Wei Z., Han S. J., Liu Z. P., ACS Appl. Mater. Interfaces, 2014, 6(12), 9185—9193 |
| 87 | Chang W., Choi J. W., Im J. C., Lee J. K., J. Power Sources, 2010, 195(1), 320—326 |
| 88 | Profili J., Rousselot S., Tomassi E., Briqueleur E., Ayme⁃Perrot D., Stafford L., Dolle M., ACS Sustain. Chem. Eng., 2020, 8(12), 4728—4733 |
| 89 | Rao P., Cui P., Yang L., Wang M. S., Wang S., Cai H., Wang Y., Zhao X. S., Wilkinson D. P., Zhang J. J., J. Power Sources, 2020, 453, 227858 |
| 90 | Liu F. Z., Leung Y. H., Djurisic A. B., Ng A. M. C., Chan W. K., Ng K. L., Wong K. S., Liao C. Z., Shih K., Surya C., J. Phys. Chem. C, 2014, 118(39), 22760—22767 |
| 91 | Xu L., Jiang Q. Q., Xiao Z. H., Li X. Y., Huo J., Wang S. Y., Dai L. M., Angew. Chem. Int. Ed., 2016, 55(17), 5277—5281 |
| 92 | Wang Q., He H. N., Luan J. Y., Tang Y. G., Huang D., Peng Z. G., Wang H. Y., Electrochim. Acta, 2019, 309, 242—252 |
| 93 | Dou S., Tao L., Huo J., Wang S. Y., Dai L. M., Energ Environ. Sci., 2016, 9(4), 1320—1326 |
| 94 | Lan C. K., Chuang S. I., Bao Q., Liao Y. T., Duh J. G., J. Power Sources, 2015, 275, 660—667 |
| 95 | Zhu J. F., Chen J., Xu H., Sun S. Q., Xu Y., Zhou M., Gao X., Sun Z. M., ACS Appl. Mater. Interfaces, 2019, 11(19), 17384—17392 |
| 96 | Conder J., Urbonaite S., Streich D., Novak P., Gubler L., RSC Adv., 2015, 5(97), 79654—79660 |
| 97 | Ahn J. H., Shin H. J., Abbas S., Lee K. Y., Ha H. Y., J. Mater. Chem. A, 2019, 7(8), 3772—3782 |
| 98 | Duan L. F., Zhao L. J., Cong H., Zhang X. Y., Lu W., Xue C. L., Small, 2019, 15(7), 1804347 |
| 99 | Hsieh Y. Y., Zhang L., DeArmond D., Kanakaraj S. N., Adusei P. K., Alvarez N. T., Fang Y. B., Daum J., Shanov V., Carbon, 2018, 139, 1093—1103 |
| 100 | Huang S. T., Fan W. G., Guo X. X., Meng F. H., Liu X. Y., ACS Appl. Mater. Interfaces, 2014, 6(23), 21567—21575 |
| 101 | Han C. P., Veeramani V., Hsu C. C., Jena A., Chang H., Yeh N. C., Hu S. F., Liu R. S., Nanotechnology, 2018, 29(50), 505401 |
| 102 | Chen J. Z., Liao W. Y., Hsieh W. Y., Hsu C. C., Chen Y. S., J. Power Sources, 2015, 274, 894—898 |
| 103 | He H. N., Huang D., Pang W. K., Sun D., Wang Q., Tang Y. G., Ji X. B., Guo Z. P., Wang H. Y., Adv. Mater., 2018, 30(26), 1801013 |
| 104 | Ma L. T., Chen S. M., Pei Z. X., Li H. F., Wang Z. F., Liu Z. X., Tang Z. J., Zapien J. A., Zhi C. Y., ACS Nano, 2018, 12(8), 8597—8605 |
| [1] | 贾洋刚, 邵霞, 程婕, 王朋朋, 冒爱琴. 赝电容控制型钙钛矿高熵氧化物La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3负极材料的制备及储锂性能[J]. 高等学校化学学报, 2022, 43(8): 20220157. |
| [2] | 赵盛, 霍志鹏, 钟国强, 张宏, 胡立群. 改性钆/硼/聚乙烯纳米复合材料的制备及对中子和伽马射线的屏蔽性能[J]. 高等学校化学学报, 2022, 43(6): 20220039. |
| [3] | 刘坤, 尹远, 耿文强, 夏昊天, 李华. 不同组分过渡金属氧化物催化剂对介质阻挡放电固氮的影响机制[J]. 高等学校化学学报, 2022, 43(11): 20220278. |
| [4] | 鲍俊全, 郑仕兵, 苑旭明, 史金强, 孙田将, 梁静. 有机盐PTO(KPD)2作为高性能锂离子电池正极材料的研究[J]. 高等学校化学学报, 2021, 42(9): 2911. |
| [5] | 李辉阳, 朱思颖, 李莎, 张桥保, 赵金保, 张力. 锂离子电池硅氧化物负极首次库伦效率的影响因素与提升策略[J]. 高等学校化学学报, 2021, 42(8): 2342. |
| [6] | 卓增庆, 潘锋. 基于软X射线光谱的锂电池材料的电子结构与演变的研究进展[J]. 高等学校化学学报, 2021, 42(8): 2332. |
| [7] | 吴卓彦, 李至, 赵旭东, 王倩, 陈顺鹏, 常兴华, 刘志亮. 一步法高效制备纳米Si/C复合材料及其在高性能锂离子电池中的应用[J]. 高等学校化学学报, 2021, 42(8): 2500. |
| [8] | 易聪华, 苏华坚, 钱勇, 李琼, 杨东杰. 木质素纳米炭的制备及作为锂离子电池负极的性能研究[J]. 高等学校化学学报, 2021, 42(6): 1807. |
| [9] | 毛尔洋, 王莉, 孙永明. 锂离子电池高容量合金基含锂负极材料的研究进展[J]. 高等学校化学学报, 2021, 42(5): 1552. |
| [10] | 刘铁峰, 张奔, 盛欧微, 佴建威, 王垚, 刘育京, 陶新永. 硅负极黏结剂的研究进展[J]. 高等学校化学学报, 2021, 42(5): 1446. |
| [11] | 王弈艨, 刘凯, 王保国. 高镍三元正极材料的表面包覆策略[J]. 高等学校化学学报, 2021, 42(5): 1514. |
| [12] | 王任衡, 肖哲, 李艳, 孙一翎, 范姝婷, 郑俊超, 钱正芳, 贺振江. 固相烧结法制备锂离子电池正极材料Li2FeP2O7及其电化学性能研究[J]. 高等学校化学学报, 2021, 42(4): 1299. |
| [13] | 孙全虎, 卢天天, 何建江, 黄长水. 含异原子石墨炔基电极材料的研究进展[J]. 高等学校化学学报, 2021, 42(2): 366. |
| [14] | 周战, 马录芳, 谭超良. 层状钒青铜纳米片的制备及其锂离子电池阳极材料性能[J]. 高等学校化学学报, 2021, 42(2): 662. |
| [15] | 高小雅, 左自成, 李玉良. 石墨炔电化学电池界面构筑[J]. 高等学校化学学报, 2021, 42(2): 321. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||