

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (1): 11.doi: 10.7503/cjcu20200476
所属专题: 分子筛功能材料 2021年,42卷,第1期
王健羽1,张强1,闫文付1,于吉红1,2
收稿日期:2020-07-21
出版日期:2021-01-10
发布日期:2021-01-12
基金资助:
WANG Jianyu1, ZHANG Qiang1, YAN Wenfu1, YU Jihong1,2( )
)
Received:2020-07-21
Online:2021-01-10
Published:2021-01-12
Contact:
YU Jihong
E-mail:jihong@jlu.edu.cn
Supported by:摘要:
沸石分子筛由于具有独特的形选催化作用及可调的酸性, 已成为化学工业中最重要的固体催化材料. 沸石分子筛的合成主要基于碱性条件下的水热晶化, OH?被认为起到催化硅铝物种的解聚及聚合作用. 近年来, 研究者发现了羟基自由基加速分子筛的水热晶化机制. 通过利用紫外光照射或芬顿反应等物理或化学方法向分子筛合成体系引入羟基自由基, 可以实现沸石分子筛的加速晶化及高硅沸石分子筛的合成. 理论计算结果表明, 羟基自由基可以促进Si—O—Si 键的断裂和重新生成, 从而显著加快分子筛成核并促进硅原子进入骨架. 本综述介绍了羟基自由基在沸石分子筛晶化方面的最新研究进展, 探讨了羟基自由基的主要作用和优势, 并对沸石分子筛合成的羟基自由基路线发展前景进行了展望.
中图分类号:
TrendMD:
王健羽, 张强, 闫文付, 于吉红. 羟基自由基在沸石分子筛合成中的作用. 高等学校化学学报, 2021, 42(1): 11.
WANG Jianyu, ZHANG Qiang, YAN Wenfu, YU Jihong. Roles of Hydroxyl Radicals in Zeolite Synthesis. Chem. J. Chinese Universities, 2021, 42(1): 11.
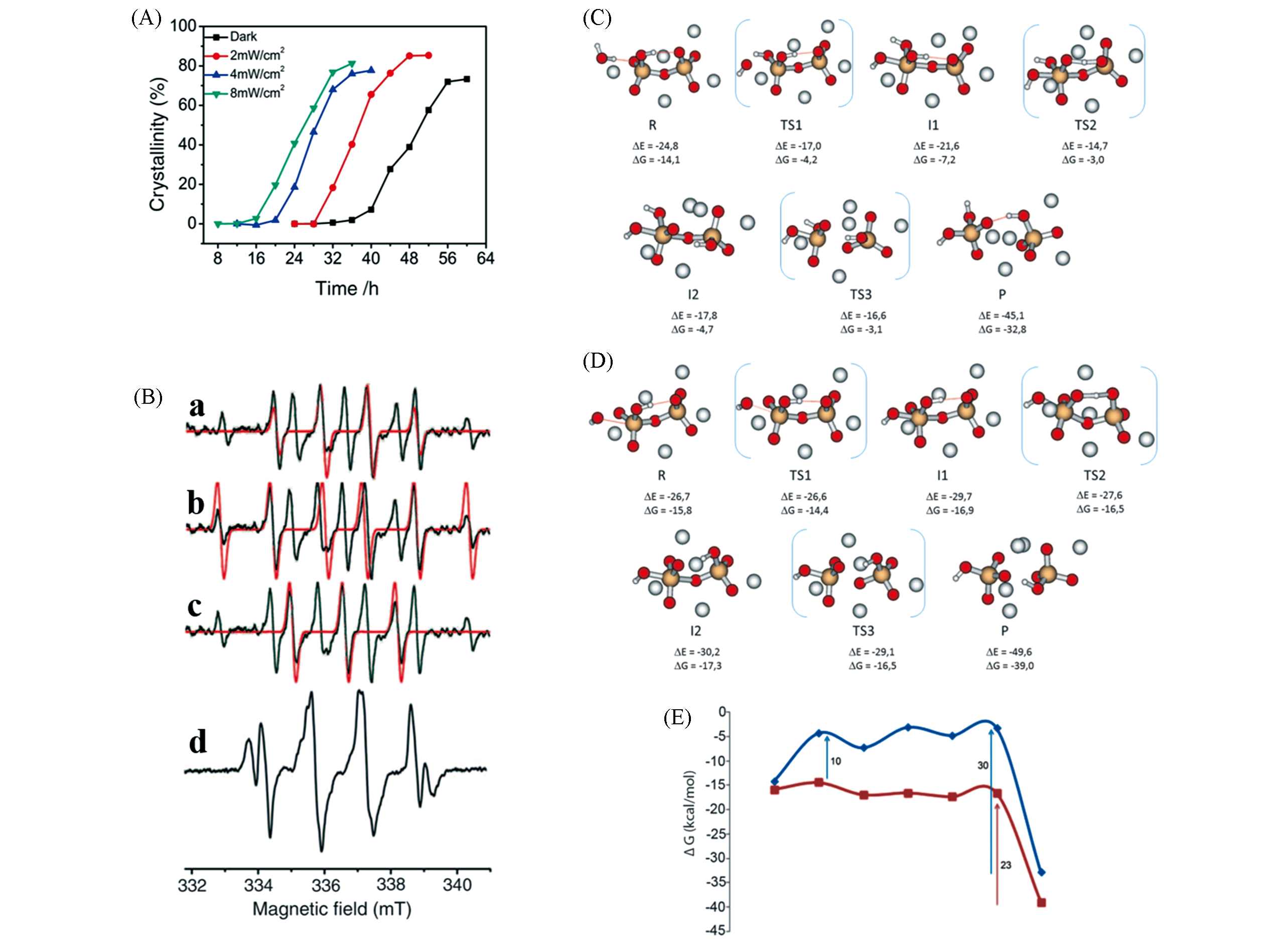
Fig.2 Crystallization curves of zeolite NaA under UV conditions with different irradiances(A); comparison of simulated and experimental EPR spectra of DMPO?·OH adduct(a), DMPO?·Si adduct(b), and oxidized DMPO radicals(c), EPR spectrum of initial synthesis gel containing BMPO after 10 h under dark condition(d)(B); reactions of OH-(C) and ·OH(D) with [SiO2(OH)―O―SiO3]Na5 system and Gibbs free energy profiles for the reaction of OH-(blue) and ·OH(red) with [SiO2(OH)―O―SiO3]Na5 system(E)[14]Copyright 2016, American Association for the Advancement of Science.
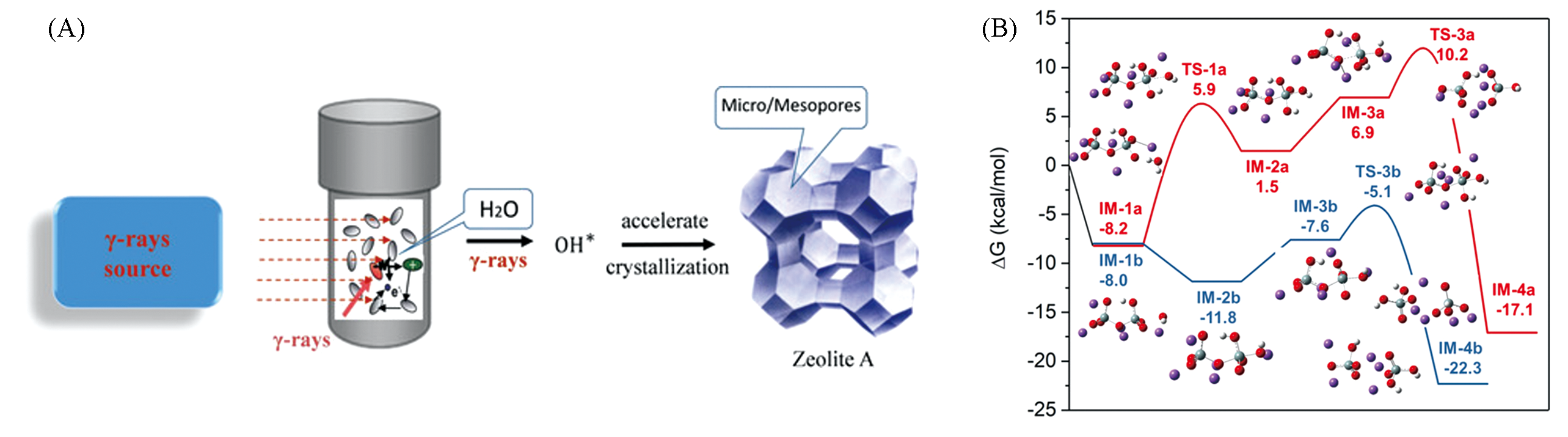
Fig.3 Accelerated synthesis of zeolites under Gamma rays(γ?rays) irradiation(A) and Gibbs free energy profiles for the depolymerization of silicate by ·OH radicals(blue) and OH-(red)(B)[15]Copyright 2020, Wiley?VCH.
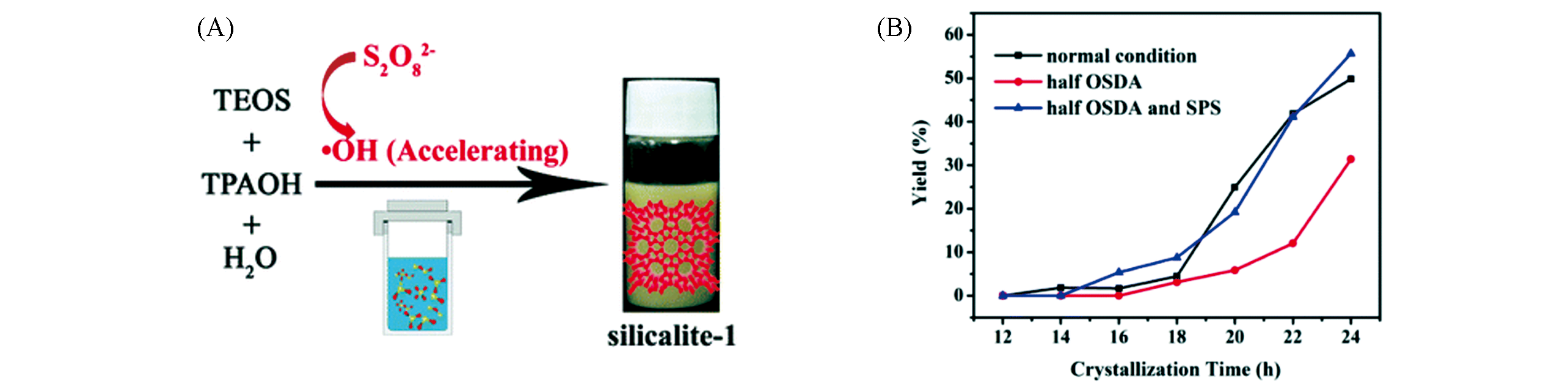
Fig.4 Schematic illustration of ·OH radical?assisted route for the synthesis of silicalite?1 in the presence of sodium persulfate(A) and yield of products with different amounts of TPAOH and sodium persulfate(B)[16]Copyright 2018, the Royal Society of Chemistry.
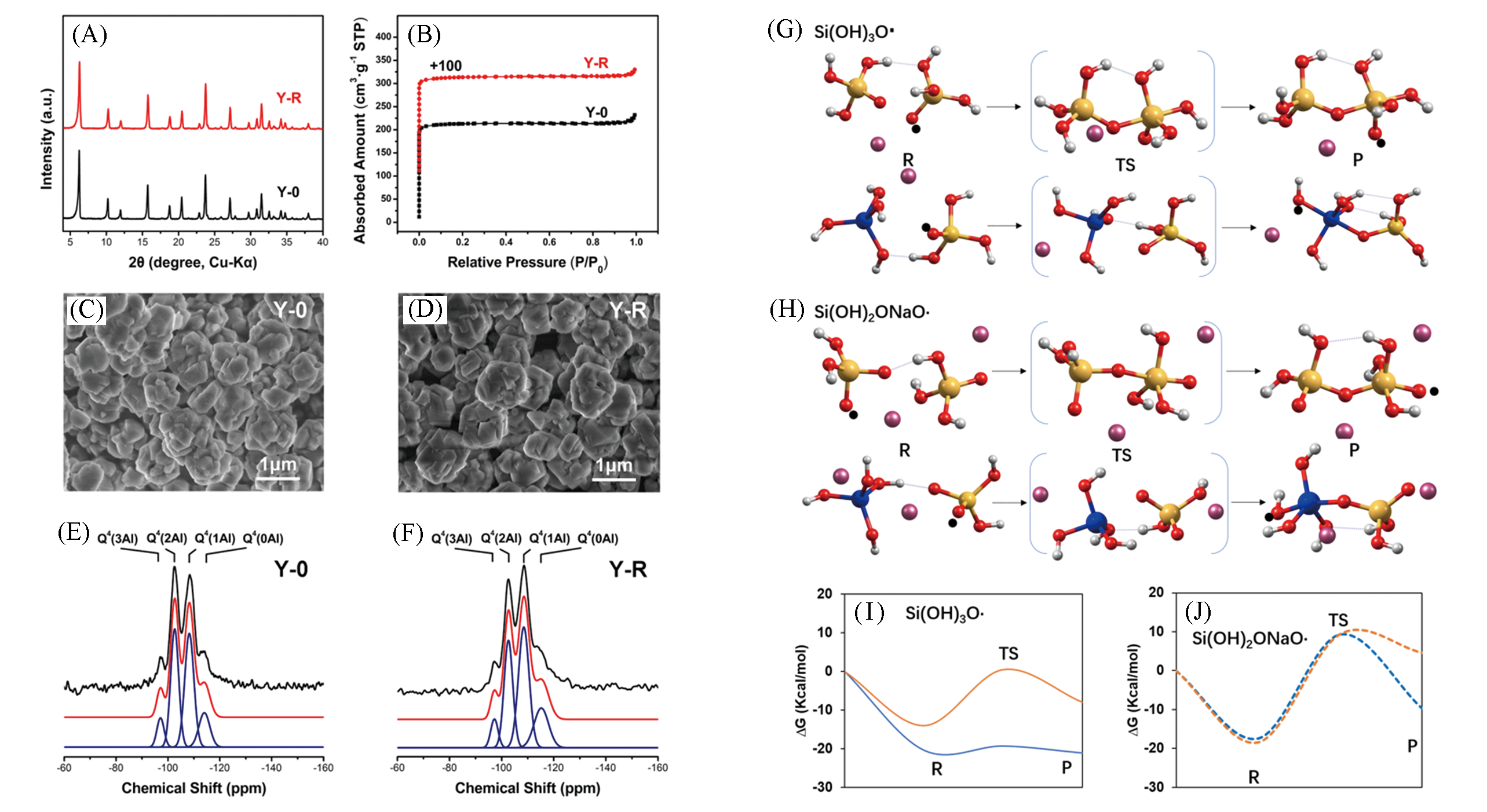
Fig.6 Characterizations of zeolite Y synthesized via conventional route(Y?0) and ·OH radical?assisted route(Y?R)(A—F); optimized geometries of the structures(G, H) and Gibbs free energy profiles(I, J) for condensation of the radicals with Al(OH)4Na(orange) and Si(OH)3ONa(blue)[20](A) XRD patterns; (B) N2 adsorption?desorption isotherms; (C, D) SEM images; (E, F) 29Si MAS NMR spectra. These results indicated that zeolite Y synthesized via conventional and ·OH radical?assisted routes are similar in the crystallinities, textural properties and morphologies.Copyright 2020, Wiley?VCH.
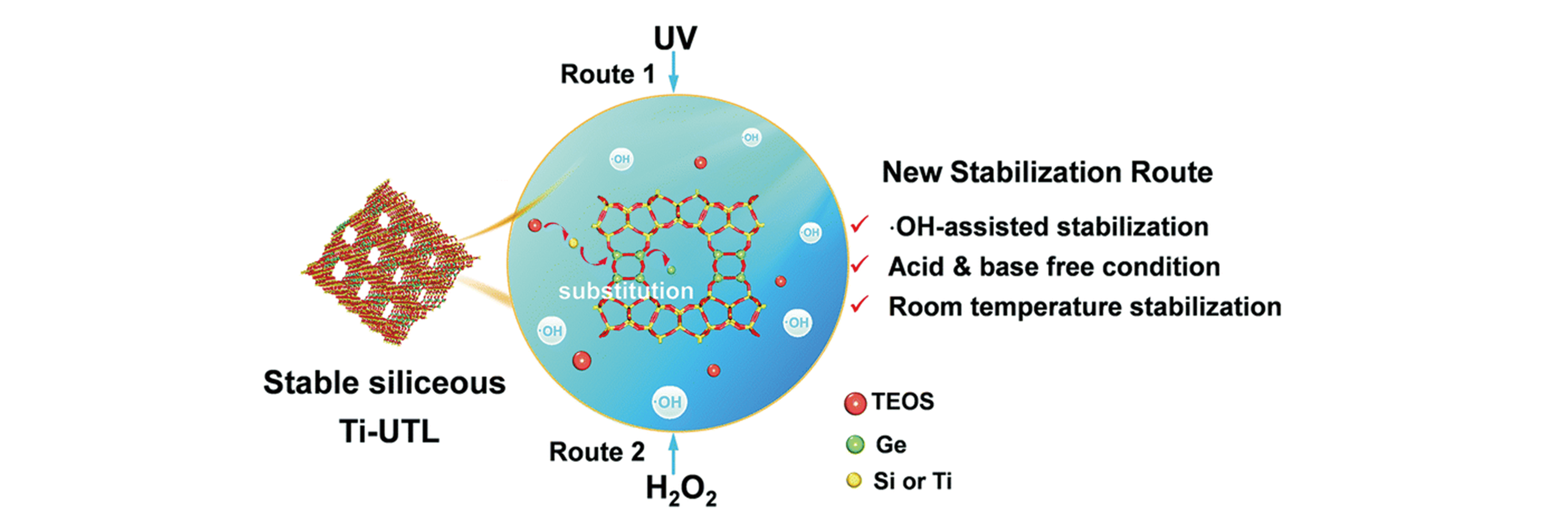
Fig.7 Schematic illustration of the isomorphous substitution of Ge with Si under neutral conditions and room temperature[21]Copyright 2019, the Royal Society of Chemistry.
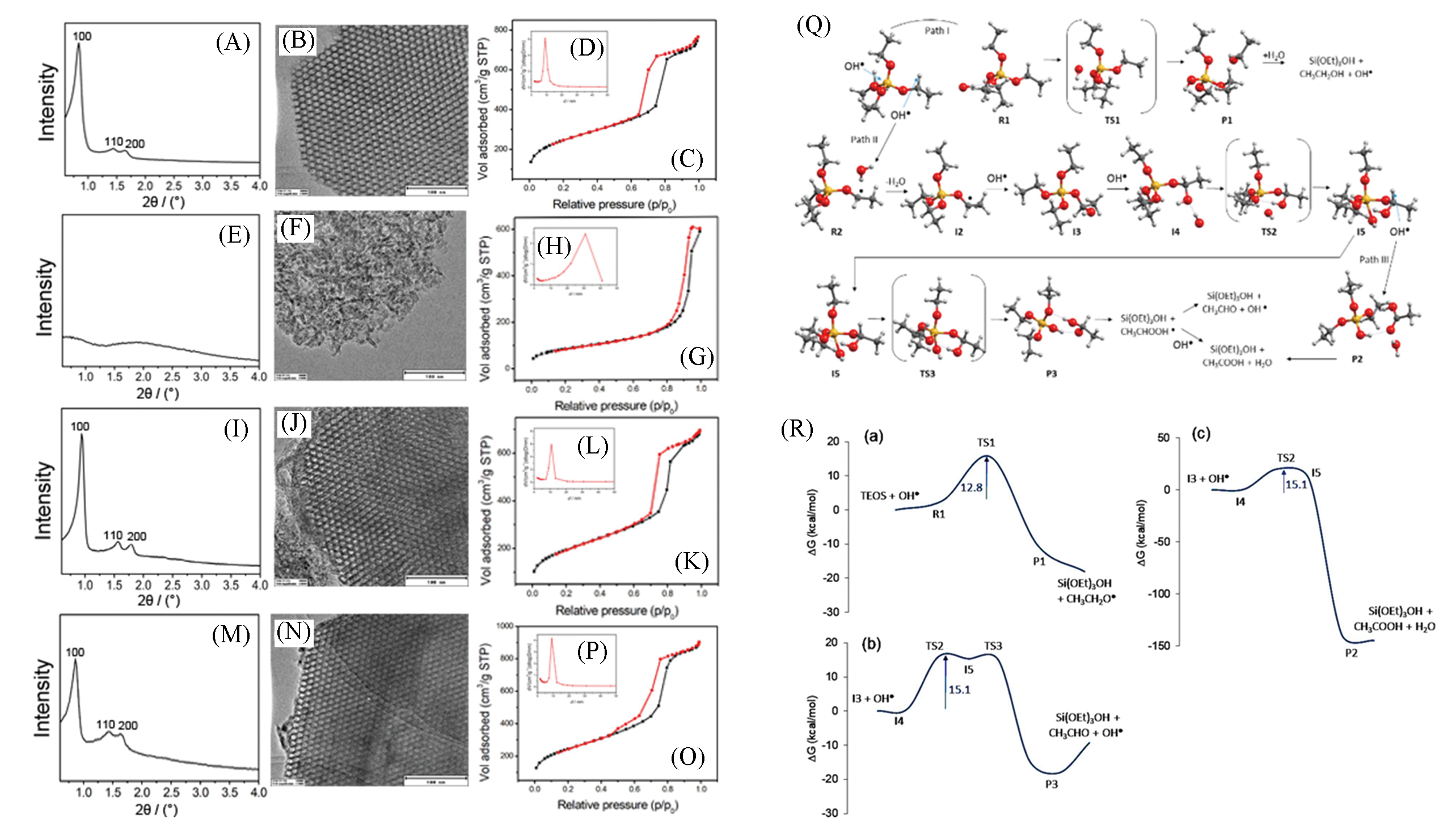
Fig.8 Characterizations of SBA?15 samples synthesized under different conditions(A—P); hydrolysis reactions of TEOS catalyzed by ·OH radicals(Q) and Gibbs free energy profiles for the attack of ·OH to TEOS(R)[22](A)―(D) Under UV irradiation; (E)―(H) without addition of radicals or acid; (I)―(L) with addition of Na2S2O8; (M)―(P) with addition of Fenton’s reagents.Copyright 2018, American Chemical Society.
| 1 | Čejka J., Corma A., Zones S., Zeolites and Catalysis Synthesis, Reactions and Applications, Wiley⁃VCH Verlag GmbH & Co. KGaA, Weinheim, 2010 |
| 2 | Kulprathipanja S., Zeolites in Industrial Separation and Catalysis, Wiley⁃VCH Verlag GmbH & Co. KGaA, Weinheim, 2010 |
| 3 | Xiao F., Meng X., Zeolites in Sustainable Chemistry, Springer⁃Verlag, Berlin Heidelberg, 2016 |
| 4 | Yang R. T., Adsorbents: Fundamentals and Applications, Wiley⁃Interscience, New York, 2003 |
| 5 | Yang R. T., Gas Separation by Adsorption Processes, Butterworth, Boston, 1987 |
| 6 | Townsend R. P., Harjula R., Ion Exchange in Molecular Sieves by Conventional Techniques, Springer⁃Verlag, Berlin Heidelberg, 2002 |
| 7 | Li Y., Yu J., Chem. Rev., 2014, 114, 7268—7316 |
| 8 | Dusselier M., Davis M. E., Chem. Rev., 2018, 118, 5265—5329 |
| 9 | Martínez C., Corma A., Coord. Chem. Rev., 2011, 255, 1558—1580 |
| 10 | Xu R. R., Pang W. Q., Yu J. H., Huo Q. S., Chen J. S., Molecular Sieve and Porous Material Chemistry, Science Press, Beijing, 2004(徐如人, 庞文琴, 于吉红, 霍启升, 陈接胜. 分子筛与多孔材料化学, 北京: 科学出版社, 2004) |
| 11 | Barrer R. M., J. Chem. Soc., 1948, 127, 2158—2163 |
| 12 | http://www.iza⁃structure.org/databases/ |
| 13 | Cundy C. S., Cox P. A., Chem. Rev., 2003, 103, 663—701 |
| 14 | Feng G., Cheng P., Yan W., Boronat M., Li X., Su J. H., Wang J., Li Y., Corma A., Xu R., Yu J., Science, 2016, 351, 1188—1191 |
| 15 | Chen X., Qiu M., Li S., Yang C., Shi L., Zhou S., Yu G., Ge L., Yu X., Liu Z., Sun N., Zhang K., Wang H., Wang M., Zhong L., Sun Y., Angew. Chem. Int. Ed., 2020, 59, 11325—11329 |
| 16 | Cheng P., Feng G., Sun C., Xu W., Su J. H., Yan W., Yu J., Inorg. Chem. Front., 2018, 5, 2106—2110 |
| 17 | Cheng P., Song M., Zhang H., Xuan Y., Wu C., J. Mater. Sci., 2019, 54, 4573—4578 |
| 18 | Han Z., Zhang F., Zhao X., Micropor. Mesopor. Mater., 2019, 290, 109679 |
| 19 | Huang J., Hu J., Du W., Liu H., Qian F., Wang M., J. Mater. Chem. A, 2017, 5, 18801—18807 |
| 20 | Wang J., Liu P., Boronat M., Ferri P., Xu Z., Liu P., Shen B., Wang Z., Yu J., Angew. Chem. Int. Ed., 2020, 59, 17225—17228 |
| 21 | Shi D., Xu L., Chen P., Ma T., Lin C., Wang X., Xu D., Sun J., Chem. Commun., 2019, 55, 1390—1393 |
| 22 | Feng G., Wang J., Boronat M., Li Y., Su J. H., Huang J., Ma Y., Yu J., J. Am. Chem. Soc., 2018, 140, 4770—4773 |
| 23 | Buxton G. V., Greenstock C. L., Helman W. P., Ross A. B., J. Phys. Chem. Ref. Data, 1988, 17, 513—531 |
| 24 | Von Sonntag C., Schuchmann H. P., Methods Enzymol., 1994, 233, 3—20 |
| 25 | Xu G., Chance M. R., Chem. Rev., 2007, 107, 3514—3543 |
| 26 | Gligorovski S., Strekowski R., Barbati S., Vione D., Chem. Rev., 2015, 115, 13051—13092 |
| 27 | Garoma T., Gurol M. D., Environ. Sci. Technol., 2004, 38, 5246—5252 |
| 28 | Reisz E., Schmidt W., Schuchmann H., Von Sonntag C., Environ. Sci. Technol., 2003, 37, 1941—1948 |
| 29 | Song S., Xu X., Xu L., He Z., Ying H., Chen J., Yan B., Ind. Eng. Chem. Res., 2008, 47, 1386—1391 |
| 30 | Tezcanli⁃Güyer G., Ince N. H., Ultrasonics, 2004, 42, 603—609 |
| 31 | Catalkaya E. C., Kargi F., J. Hazard. Mater., 2007, 139, 244—253 |
| 32 | Kusic H., Koprivanac N., Bozic A. L., Chem. Eng. J., 2006, 123, 127—137 |
| 33 | Yasuda H., Polym. Prepr.(Am. Chem. Soc., Div. Polym. Chem.), 1975, 16, 57—59 |
| 34 | Chu P. K., Chen J. Y., Wang L. P., Huang N., Mater. Sci. Eng., R, 2002, 36, 143—206 |
| 35 | Dainton F. S., J. Am. Chem. Soc., 1956, 78, 1278—1279 |
| 36 | Weeks J. L., Matheson M. S., J. Am. Chem. Soc., 1956, 78, 1273—1278 |
| 37 | Ferradini C., Jay⁃Gerin J. P., Res. Chem. Intermed., 2000, 29, 549—565 |
| 38 | Kruus P., Beutel L., Aranda R., Penchuk J., Otson R., Chemosphere, 1998, 36, 1811—1824 |
| 39 | Weissler A., J. Am. Chem. Soc., 1959, 81, 1077—1081 |
| 40 | Makino K., Mossoba M. M., Riesz P., J. Phys. Chem., 1983, 87, 1369—1377 |
| 41 | Hart E. J., Henglein A., J. Phys. Chem., 1985, 89, 4342—4347 |
| 42 | Barb W. G., Baxendale J. H., George P., Hargrave K. R., Nature, 1949, 163, 692—694 |
| 43 | Barb W. G., Baxendale J. H., George P., Hargrave K. R., Trans. Faraday Soc., 1950, 47, 462—500 |
| 44 | Barb W. G., Baxendale J. H., George P., Hargrave K. R., Trans. Faraday Soc., 1950, 47, 591—616 |
| 45 | Haber F., Weiss J., Proc. R. Soc. London, Ser. A, 1934, 147, 332—351 |
| 46 | Bautista P., Mohedano A. F., Casas J. A., Zazo J. A., Rodriguez J. J., J. Chem. Technol. Biotechnol., 2008, 83, 1323—1338 |
| 47 | Brillas E., Sirés I., Oturan M. A., Chem. Rev., 2009, 109, 6570—6631 |
| 48 | Pignatello J. J., Oliveros E., MacKay A., Crit. Rev. Environ. Sci. Technol., 2006, 36, 1—84 |
| 49 | Ntampegliotis K., Riga A., Karayannis V., Bontozoglou V., Papapolymerou G., J. Hazard. Mater., 2006, 136, 75—84 |
| 50 | Bremner D. H., Molina R., Martínez F., Melero J. A., Segura Y., Appl. Catal., B, 2009, 90, 380—388 |
| 51 | Chakinala A. G., Gogate P. R., Burgess A. E., Bremner D. H., Chem. Eng. J., 2009, 152, 498—502 |
| 52 | Pradhan A. A., Gogate P. R., J. Hazard. Mater., 2010, 173, 517—522 |
| 53 | El-Desoky H. S., Ghoneim M. M., El-Sheikh R., Zidan N. M., J. Hazard. Mater., 2010, 175, 858—865 |
| 54 | Snook M. E., Hamilton G. A., J. Am. Chem. Soc., 1974, 96, 860—869 |
| 55 | Beitz T., Bechmann W., Mitzner R., J. Phys. Chem. A, 1998, 102, 6760—6765 |
| 56 | Anipsitakis G. P., Dionysiou D. D., Environ. Sci. Technol., 2004, 38, 3705—3712 |
| 57 | Pennington D. E., Haim A., J. Am. Chem. Soc., 1968, 90, 3700—3704 |
| 58 | Hayon E., Treinin A., Wilf J., J. Am. Chem. Soc., 1972, 94, 47—57 |
| 59 | Bektaşoğlu B., Özyürek M., Güçlü K., Apak R., Talanta, 2008, 77, 90—97 |
| 60 | Tai C., Gu X., Zou H., Guo Q., Talanta, 2002, 58, 661—667 |
| 61 | Ma Z., Zhao B., Yuan Z., Anal. Chim. Acta, 1999, 389, 213—218 |
| 62 | Jen J. F., Leu M. F., Yang T. C., J. Chromatogr. A, 1998, 796, 283—288 |
| 63 | Tai C., Peng J. F., Liu J. F., Jiang G. B., Zou H., Anal. Chim. Acta, 2004, 527, 73—80 |
| 64 | Hu Y., Zhang Z., Yang C., Ultrason. Sonochem., 2008, 15, 665—672 |
| 65 | Cameron M. D., Poyer J. F., Aust S. D., J. Cataract Refractive Surg., 2001, 27, 463—470 |
| 66 | Zhao H., Joseph J., Zhang H., Karoui H., Kalyanaraman B., Free Radicals Biol. Med., 2001, 31, 599—606 |
| 67 | Xu R., Pang W., Yu J., Huo Q., Chen J., Chemistry of Zeolites and Related Porous Materials, Wiley⁃VCH, Singapore, 2007 |
| 68 | Zhao D., Feng J., Huo Q., Melosh N., Fredrickson G. H., Chmelka B. F., Stucky G. D., Science, 1998, 279, 548—552 |
| 69 | Zhao D., Huo Q., Feng J., Chmelka B. F., Stucky G. D., J. Am. Chem. Soc., 1998, 120, 6024—6036 |
| [1] | 程媛媛, 郗碧莹. ·OH自由基引发CH3SSC |
| [2] | 梁宇, 刘欢, 宫丽阁, 王春晓, 王春梅, 于凯, 周百斌. 联咪唑修饰{SiW12O40}杂化物的合成及超级电容性能[J]. 高等学校化学学报, 2022, 43(1): 20210556. |
| [3] | 李健, 于明明, 孙源, 冯文华, 冯兆池, 吴剑峰. 水溶液pH对甲烷低温氧化制备甲醇的影响[J]. 高等学校化学学报, 2021, 42(3): 776. |
| [4] | 张会双, 高延晓, 王秋娴, 李向南, 刘文凤, 杨书廷. CTAB辅助水热合成高镍三元材料LiNi0.6Co0.2Mn0.2O2及其高低温性能研究[J]. 高等学校化学学报, 2021, 42(3): 819. |
| [5] | 王冶, 张晓思, 孙丽婧, 李冰, 刘琳, 杨淼, 田鹏, 刘仲毅, 刘中民. 有机硅烷辅助合成特殊形貌SAPO分子筛[J]. 高等学校化学学报, 2021, 42(3): 683. |
| [6] | 闻嘉丽, 张钧豪, 姜久兴. 超大孔分子筛, 十年再回顾[J]. 高等学校化学学报, 2021, 42(1): 101. |
| [7] | 吴勤明, 王叶青, 孟祥举, 肖丰收. 硅铝沸石分子筛晶化过程再思考[J]. 高等学校化学学报, 2021, 42(1): 21. |
| [8] | 刘珊珊, 柴玉超, 关乃佳, 李兰冬. 分子筛材料在小分子吸附分离中的应用[J]. 高等学校化学学报, 2021, 42(1): 268. |
| [9] | 王博伦, 宗思宇, 李激扬. 光致发光沸石分子筛复合材料的研究进展[J]. 高等学校化学学报, 2021, 42(1): 299. |
| [10] | 刘亚冰,李明阳,田戈,阿拉腾沙嘎,裴桐鹤,聂婧思. 基于2-氨基吡啶的两个簇基超分子化合物的合成、 结构及催化性能[J]. 高等学校化学学报, 2020, 41(5): 995. |
| [11] | 卓孟宁,李飞,蒋浩,陈倩文,李鹏,王立章. SnO2/GDE阴极的制备及电催化还原CO2产甲酸性能[J]. 高等学校化学学报, 2020, 41(3): 530. |
| [12] | 董乐, 黄星亮, 任俊杰, 代小平, 刘宗俨, 田洪锋, 王志东, 吴晓彤. 硅溶胶粒度与分布对高硅铝比镁碱沸石合成的影响机理[J]. 高等学校化学学报, 2020, 41(11): 2449. |
| [13] | 章凌,段宏昌,谭争国,吴勤明,孟祥举,肖丰收. 用于柴油车尾气消除反应(NH3-SCR)的八元环沸石分子筛研究进展[J]. 高等学校化学学报, 2020, 41(1): 19. |
| [14] | 周海, 陈豪, 郭娅, 康敏. 介孔Co3O4多面体的制备及电化学性能[J]. 高等学校化学学报, 2019, 40(7): 1374. |
| [15] | 高宁萧, 徐玉龙, 刘勇. 豆奶粉提取碳点及含碳点荧光纳米纤维的制备[J]. 高等学校化学学报, 2019, 40(3): 555. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||