

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (4): 591.doi: 10.7503/cjcu20190651
• 庆祝《高等学校化学学报》复刊40周年专栏 • 上一篇 下一篇
韩方杰1,2,代梦娇1,2,梁芷珊3,宋忠乾1,韩冬雪1,2,3,*( ),牛利1,2,3
),牛利1,2,3
收稿日期:2019-12-11
出版日期:2020-04-10
发布日期:2020-02-07
通讯作者:
韩冬雪
E-mail:dxhan@gzhu.edu.cn
基金资助:
HAN Fangjie1,2,DAI Mengjiao1,2,LIANG Zhishan3,SONG Zhongqian1,HAN Dongxue1,2,3,*( ),NIU Li1,2,3
),NIU Li1,2,3
Received:2019-12-11
Online:2020-04-10
Published:2020-02-07
Contact:
Dongxue HAN
E-mail:dxhan@gzhu.edu.cn
Supported by:摘要:
在新陈代谢过程中, 机体会产生大量以自由基为主要形式的氧化活性物质, 而抗氧化剂可以通过电子转移的方式捕获并中和自由基, 从而有效抵御自由基引起的细胞损害, 以保障和维护人体健康. 食品作为人体外源性抗氧化剂的重要来源可以有效补充因体内代谢及体液排出而损失的抗氧化物质, 因此对食品中抗氧化物质消除自由基的能力即抗氧化能力的测定和评价具有重要意义. 光电化学技术作为一种简单快捷、 低成本、 低背景且高灵敏度的测定方法, 能够有效克服光学法、 色谱法和电化学法等传统测试手段在抗氧化容量分析中的不足. 本文综述了基于半导体及其复合材料的光电化学传感平台的构建及食品体系抗氧化容量分析的研究进展, 评论了多种检测体系的特点并对其研究前景进行了展望.
中图分类号:
TrendMD:
韩方杰, 代梦娇, 梁芷珊, 宋忠乾, 韩冬雪, 牛利. 光电化学技术应用于抗氧化分析的研究进展. 高等学校化学学报, 2020, 41(4): 591.
HAN Fangjie, DAI Mengjiao, LIANG Zhishan, SONG Zhongqian, HAN Dongxue, NIU Li. Research Progress of Photoelectrochemical Technology Applied in Antioxidant Analysis †. Chem. J. Chinese Universities, 2020, 41(4): 591.
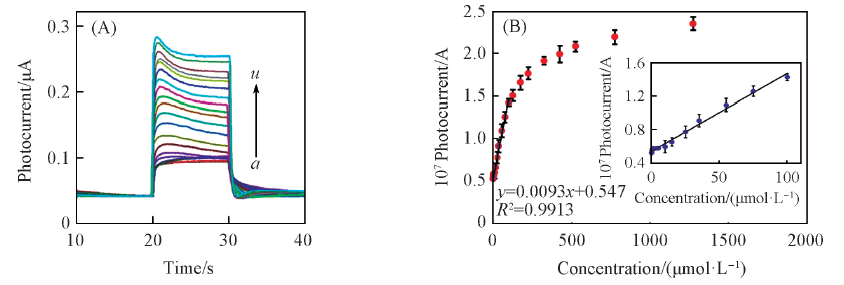
Fig.3 Photocurrent responses of the g-C3N4/ITO electrode to different concentrations of AA(A) and corresponding calibration curve(B) in 0.1 mol/L PBS(pH=7.4)[62](A) c(AA)/(μmol·L-1): a. 0; b. 0.25; c. 0.5; d. 1; e. 3; f. 5; g. 10; h. 15; i. 25; j. 35; k. 55; l. 75; m. 100; n. 125; o. 175; p. 225; q. 325; r. 425; s. 525; t. 775; u. 1275. Inset of (B) shows better illustration of linear part in calibration curve. Error bars correspond to the standard deviations from three independent measurements with RSD equal to 5.2%.Copyright 2018, Elsevier.
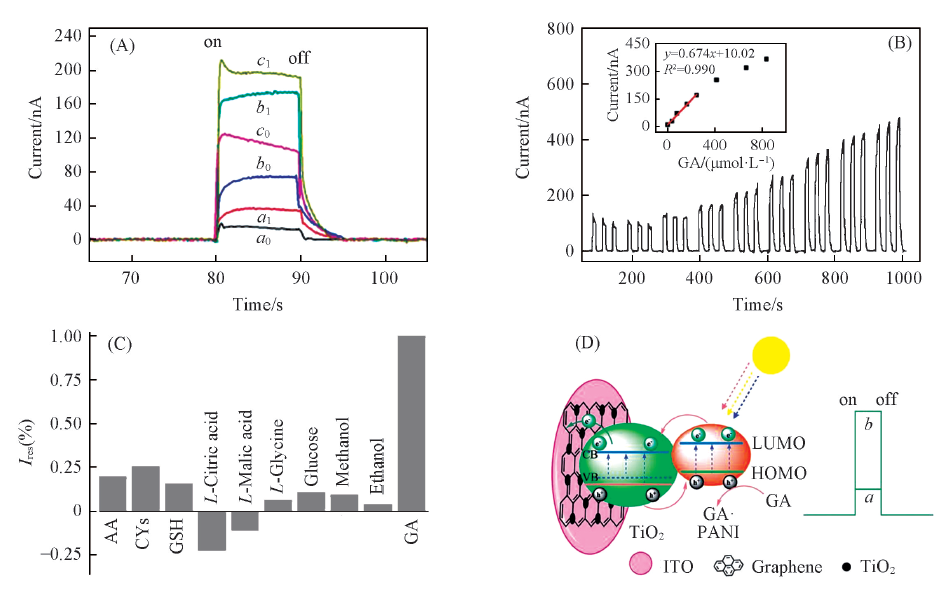
Fig.4 Photocurrent responses of TiO2(a0, a1), rGO-TiO2(b0, b1) and PANI-rGO-TiO2(c0, c1) modified ITO without(a0—c0) and with(a1—c1) 166.8 mmol/L GA(A); photocurrent responses of PANI-rGO-TiO2 modified ITO with different concentrations of GA(B); photocurrent response of a PANI-rGO-TiO2 modified ITO electrode upon the addition of 166.8 mmol/L each of AA, GA, 33.36 mmol/L each of GSH, CYs, L-citric acid, L-malic acid, 0.167 mol/L each of ethanol, methanol, glucose, L-glycine in 0.1 mol/L PBS(pH=7.4) at 0 V under 420 nm light excitation(C) and illustration of photoelectro-chemical process for GA oxidation at PANI-rGO-TiO2 modified ITO(D)[39](B) The photoelectrochemical sensors were applied at 0 V under 420 nm light excitation in 0.1 mol/L PBS(pH=7.4). Inset in (B) is the linear calibration curve. ^Copyright 2013, Royal Society of Chemistry.
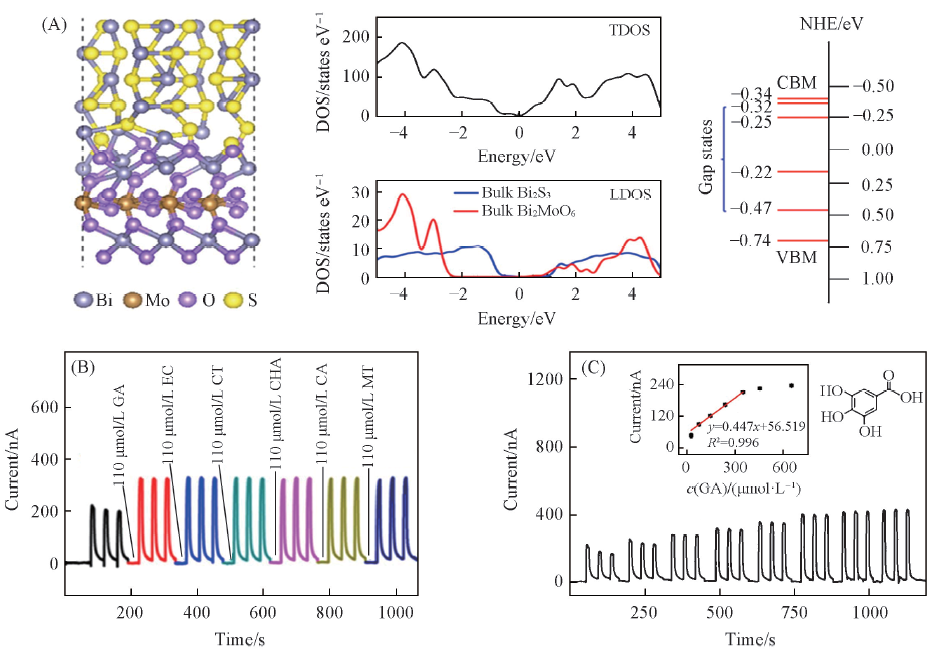
Fig.5 Schematic illustration of the surface contact region between Bi2MoO6 and Bi2S3, TDOS and LDOS of the Bi2MoO6/Bi2S3 heterostructures, the energy levels for valence and conduction bands of Bi2MoO6/Bi2S3(A); photocurrents of GA, EC, CT, CHA, CA and MT on Bi2MoO6/Bi2S3(B) and photocurrent responses of Bi2MoO6/Bi2S3 modified ITO electrode upon different concentrations of GA(C)[42]Inset of (C) is the corresponding liner calibration. The photoelectrochemical sensor was applied at 0 V under 470 nm irradiation in 0.1 mol/L PBS(pH=7.4). Copyright 2017, Elsevier.
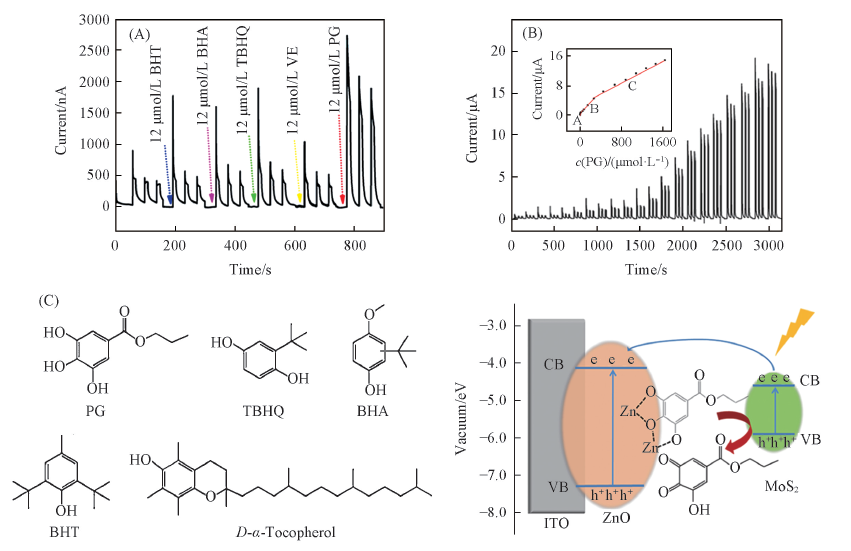
Fig.6 Photocurrents of 12 μmol/L PG, TBHQ, BHA, BHT and VE on MoS2/ZnO heterostructures(A); photocurrent responses of MoS2/ZnO heterostructures modified ITO electrodes upon different concentrations of PG(B) and chemical structures of five antioxidants and proposed mechanism of the MoS2/ZnO-based photoelectrochemical sensor for the detection of PG(C)[66] (B) The inset is the corresponding linear calibration. The preceding photoelectrochemical experiments were applied at 0 V(vs. Ag/AgCl) under 470 nm irradiation in 0.1 mol/L PBS(pH=7.4). Copyright 2019, American Chemical Society.
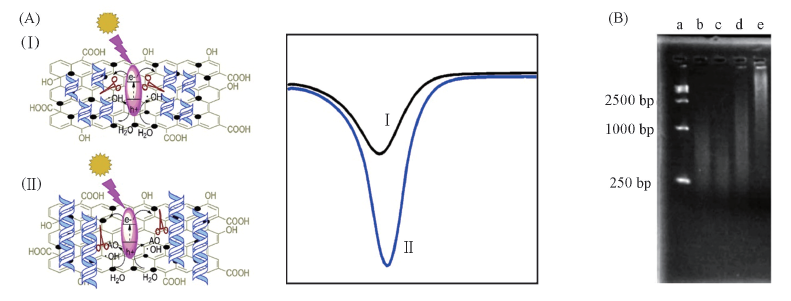
Fig.7 Schematic illustration of photoelectrochemical process for antioxidant capacity sensor designed with GO-TiO2 composites as source of OH radicals and DNA as a molecular probe(A) and agarose gel electrophoresis of different types of DNA(B) [40] Lane a: DNA markers; lanes b and c: DNA illuminated for 20 and 30 min with GOB-TiO2; lane d: after addition of gallic acid, DNA was illuminated 30 min with GOB-TiO2; lane e: DNA was illuminated 30 min without GOB-TiO2. Copyright 2013, Royal Society of Chemistry.
| Sample | DNA sensor for GA/(mg·g-1) | F-C method for GA/(mg·g-1) | DPPH method for trolox/(mg·g-1) |
|---|---|---|---|
| B1 | 47.45 | 60.29 | 141.20 |
| B2 | 66.61 | 62.94 | 201.10 |
| B3 | 41.86 | 78.40 | 191.50 |
| B4 | 24.34 | 18.55 | 49.90 |
| B5 | 38.39 | 39.82 | 86.30 |
Table 1 Detection of antioxidant capacity by three different methods[40]
| Sample | DNA sensor for GA/(mg·g-1) | F-C method for GA/(mg·g-1) | DPPH method for trolox/(mg·g-1) |
|---|---|---|---|
| B1 | 47.45 | 60.29 | 141.20 |
| B2 | 66.61 | 62.94 | 201.10 |
| B3 | 41.86 | 78.40 | 191.50 |
| B4 | 24.34 | 18.55 | 49.90 |
| B5 | 38.39 | 39.82 | 86.30 |
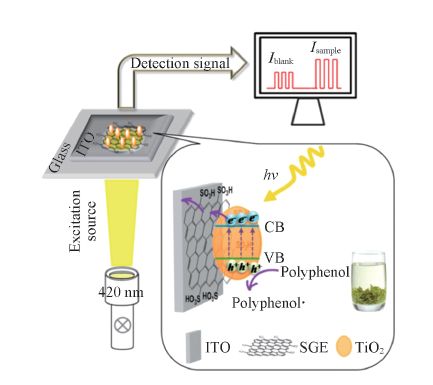
Fig.8 Schematic illustration of photoelectrochemical process for tea polyphenols oxidation at SGE-TiO2 modified ITO electrode[37] Copyright 2014, American Chemistry Society.
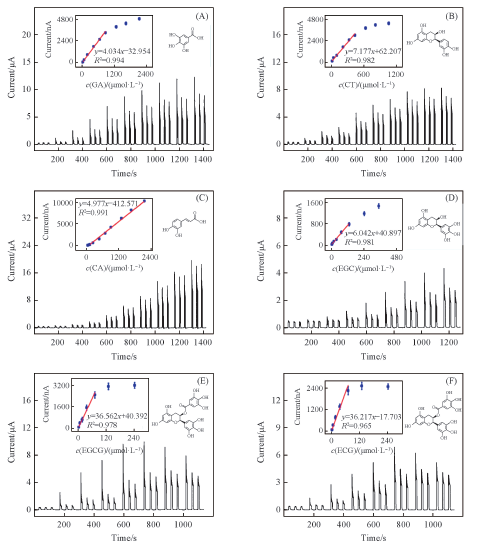
Fig.9 Photocurrent responses of SGE-TiO2 modified ITO electrode upon different concentrations of GA(A), CT(B), CA(C), EGC(D), EGCG(E) and ECG(F), respectively [37]Inset in each graph is the corresponding liner calibration curve. The photoelectrochemical sensors were applied at 0 V under 420 nm light excitation in 0.1 mol/L PBS(pH=7.4).Copyright 2014, American Chemistry Society.
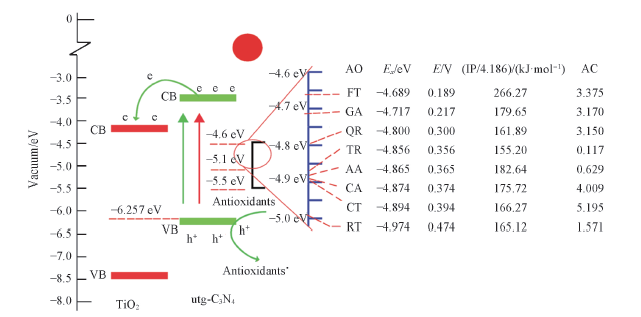
Fig.10 Mechanism of the photoelectrochemical sensor for the detection of the antioxidant capacity[38]AO: antioxidant; Ex(eV): the redox potential of the antioxidants with respect to a vacuum; E(V): the redox potential of the antioxidants(vs. Ag/AgCl); IP(kJ/mol): ionization potential of the antioxidants; AC: antioxidant capacity obtained from the slope of the standard calibration curve of each antioxidant. Copyright 2014, Royal Society of Chemistry.
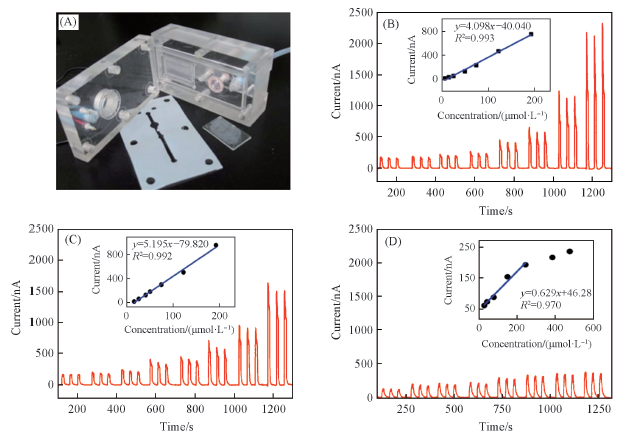
Fig.11 Photograph of the thin layer photoelectrochemical flow cell(A), concentration-dependent photocurrent of CA(B), CT(C) and AA(D)[38]Insets in (B)—(D) are the linear curves of CA, CT and AA, respectively. Copyright 2014, Royal Society of Chemistry.
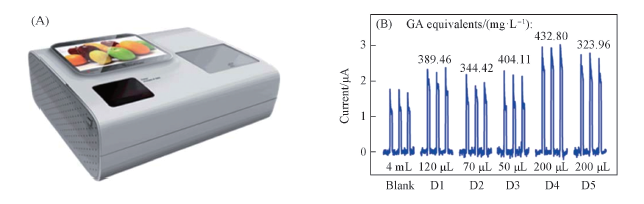
Fig.12 Photograph of the integrated PEC platform(A) and photocurrent responses of the BiMo0.015V0.985O4 modified ITO electrode in the PEC platform without(blank: PBS solution) and with a certain volume of juice into 4 mL PBS(0.1 mol/L, pH=7.4)(B)[35]Copyright 2015, Royal Society of Chemistry.
| Practical sample | Antioxidant capacity/(mg·L-1) | ||
|---|---|---|---|
| PEC sensor | F-C method | DPPH method | |
| Mango | 356.38±6.80 | 369.73±2.63 | 267.61±1.46 |
| Grape | 229.40±1.13 | 247.71±0.61 | 172.73±2.92 |
| Apple | 257.45±4.95 | 252.01±2.43 | 176.86±2.53 |
| Mangosteen | 265.54±6.63 | 308.44±5.27 | 182.01±3.86 |
| Pitaye | 104.18±3.14 | 129.27±0.38 | 83.76±0.73 |
| Orange | 445.59±9.12 | 468.67±1.52 | 281.66±1.82 |
| Leechee | 540.48±1.01 | 564.39±3.04 | 325.50±3.16 |
| Kiwi fruit | 601.12±5.38 | 611.73±4.56 | 344.83±11.38 |
Table 2 Antioxidant capacities of fresh fruits detected by different methods(n=3)[35]
| Practical sample | Antioxidant capacity/(mg·L-1) | ||
|---|---|---|---|
| PEC sensor | F-C method | DPPH method | |
| Mango | 356.38±6.80 | 369.73±2.63 | 267.61±1.46 |
| Grape | 229.40±1.13 | 247.71±0.61 | 172.73±2.92 |
| Apple | 257.45±4.95 | 252.01±2.43 | 176.86±2.53 |
| Mangosteen | 265.54±6.63 | 308.44±5.27 | 182.01±3.86 |
| Pitaye | 104.18±3.14 | 129.27±0.38 | 83.76±0.73 |
| Orange | 445.59±9.12 | 468.67±1.52 | 281.66±1.82 |
| Leechee | 540.48±1.01 | 564.39±3.04 | 325.50±3.16 |
| Kiwi fruit | 601.12±5.38 | 611.73±4.56 | 344.83±11.38 |
| Practical sample | Antioxidant capacity/(mg·g-1 or mg·L-1) | ||
|---|---|---|---|
| PEC sensor | F-C method | DPPH method | |
| T1 | 18.78±0.80 | 35.82±0.25 | 54.84±0.24 |
| T2 | 73.33±1.72 | 106.56±0.53 | 144.03±0.42 |
| T3 | 108.63±1.91 | 120.87±0.70 | 161.18±0.85 |
| T4 | 52.11±1.51 | 71.60±0.23 | 106.90±0.73 |
| D1 | 387.19±0.62 | 420.30±1.07 | 203.64±2.39 |
| D2 | 345.31±1.24 | 386.24±6.77 | 193.31±2.30 |
| D3 | 396.16±4.34 | 437.52±1.32 | 318.36±7.59 |
| D4 | 429.88±4.24 | 459.78±2.97 | 325.78±2.39 |
| D5 | 316.78±0.98 | 337.46±2.21 | 185.21±1.53 |
| D6 | 393.10±0.98 | 435.85±1.14 | 250.63±4.08 |
| D7 | 457.50±3.01 | 492.08±1.14 | 566.88±3.28 |
| D8 | 712.10±13.04 | 770.78±1.02 | 731.51±1.55 |
| D9 | 641.11±9.59 | 701.33±3.70 | 728.08±1.37 |
| D10 | 436.60±4.37 | 487.50±0.41 | 522.16±4.07 |
| D11 | 296.41±0.76 | 310.27±1.70 | 164.06±2.06 |
| D12 | 352.02±5.05 | 388.18±0.62 | 198.06±2.06 |
| D13 | 391.22±1.42 | 432.17±1.03 | 232.09±4.07 |
| Practical sample | Antioxidant capacity/(mg·g-1 or mg·L-1) | ||
| PEC sensor | F-C method | DPPH method | |
| D14 | 176.01±0.76 | 137.02±0.52 | 45.29±1.63 |
| D15 | 58.07±0.36 | 34.43±0.19 | 27.96±0.73 |
| D16 | 59.29±0.27 | 40.32±0.18 | 31.07±0.48 |
Table 3 Antioxidant capacities of commercial teas(T, mg/g) and drinks(D, mg/L) detected by different methods(n=3)[35]
| Practical sample | Antioxidant capacity/(mg·g-1 or mg·L-1) | ||
|---|---|---|---|
| PEC sensor | F-C method | DPPH method | |
| T1 | 18.78±0.80 | 35.82±0.25 | 54.84±0.24 |
| T2 | 73.33±1.72 | 106.56±0.53 | 144.03±0.42 |
| T3 | 108.63±1.91 | 120.87±0.70 | 161.18±0.85 |
| T4 | 52.11±1.51 | 71.60±0.23 | 106.90±0.73 |
| D1 | 387.19±0.62 | 420.30±1.07 | 203.64±2.39 |
| D2 | 345.31±1.24 | 386.24±6.77 | 193.31±2.30 |
| D3 | 396.16±4.34 | 437.52±1.32 | 318.36±7.59 |
| D4 | 429.88±4.24 | 459.78±2.97 | 325.78±2.39 |
| D5 | 316.78±0.98 | 337.46±2.21 | 185.21±1.53 |
| D6 | 393.10±0.98 | 435.85±1.14 | 250.63±4.08 |
| D7 | 457.50±3.01 | 492.08±1.14 | 566.88±3.28 |
| D8 | 712.10±13.04 | 770.78±1.02 | 731.51±1.55 |
| D9 | 641.11±9.59 | 701.33±3.70 | 728.08±1.37 |
| D10 | 436.60±4.37 | 487.50±0.41 | 522.16±4.07 |
| D11 | 296.41±0.76 | 310.27±1.70 | 164.06±2.06 |
| D12 | 352.02±5.05 | 388.18±0.62 | 198.06±2.06 |
| D13 | 391.22±1.42 | 432.17±1.03 | 232.09±4.07 |
| Practical sample | Antioxidant capacity/(mg·g-1 or mg·L-1) | ||
| PEC sensor | F-C method | DPPH method | |
| D14 | 176.01±0.76 | 137.02±0.52 | 45.29±1.63 |
| D15 | 58.07±0.36 | 34.43±0.19 | 27.96±0.73 |
| D16 | 59.29±0.27 | 40.32±0.18 | 31.07±0.48 |
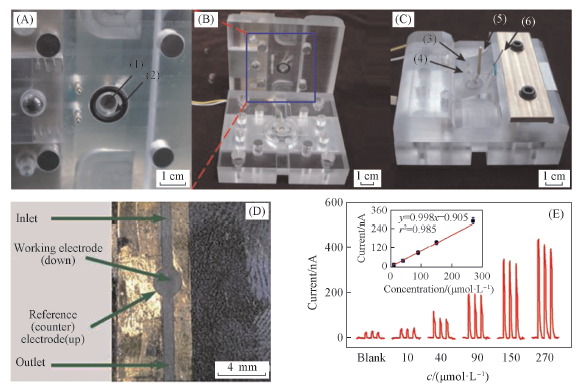
Fig.13 Inside(A), the whole instrument(B), one part of the two-EPCS(C), scheme of microfluidic chip(D) and profile of concentration vs. photocurrent of GA on the microfluidic chip(E)[41](1) The counter electrode(d=8.5 mm); (2) the working electrode(d=10.0 mm); (3) the inlet; (4) the outlet; (5) the lead wire of counter electrode; (6) the lead wire of working electrode. Copyright 2015, Elsevier.
| [1] | Sohal R S .,Weindruch R., Science, 1996, ( 273), 59— 63 |
| [2] | Harman D ., Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences, 1981, ( 78), 7124— 7128 |
| [3] | Halliwell B ., J. Neurochem., 1992, ( 59), 1609— 1623 |
| [4] | Perera R. M., Bardeesy N., Nature, 2011, ( 475), 43— 44 |
| [5] | Mittler R ., Trends Plant Sci., 2002, ( 7), 405— 410 |
| [6] | Velioglu Y. S., Mazza G., Gao L., Oomah B. D., J. Agric. Food Chem., 1998, ( 46), 4113— 4117 |
| [7] | Eberhardt M. V ., Lee C. Y., Liu R. H., Nature, 2000, ( 405), 903— 904 |
| [8] | Bedner M., Duewer D . L.,Anal. Chem., 2011, ( 83), 6169— 6176 |
| [9] | Merken H M., .Beecher G. R., J. Agric. Food Chem., 2000, ( 48), 577— 599 |
| [10] | Pukalskas A ., van Beek T. A.,de Waard P., J. Chromatogr. A, 2005, ( 1074), 81— 88 |
| [11] | Prior R L .,Wu X. L.,Schaich K., J. Agric. Food Chem., 2005, ( 53), 4290— 4302 |
| [12] | Ozyurek M., Guclu K., Apak R ., TRAC-Trends in Anal. Chem., 2011, ( 30), 652— 664 |
| [13] | Ozyurek M., Gungor N., Baki S., Guclu K., Apak R ., Anal. Chem., 2012, ( 84), 8052— 8059 |
| [14] | Kilmartin P. A., Zou H. L.,Waterhouse A. L., J. Agric. Food Chem., 2001, ( 49), 1957— 1965 |
| [15] | Crevillen A. G ., Avila M.,Pumera M.,Gonzalez M. C.,Escarpa A., Anal. Chem., 2007, ( 79), 7408— 7415 |
| [16] | Liu J., Su B., Lagger G., Tacchini P., Girault H. H., Anal. Chem., 2006, ( 78), 6879— 6884 |
| [17] | Liu J F .,Roussel C.,Lagger G.,Tacchini P.,Girault H. H., Anal. Chem., 2005, ( 77), 7687— 7694 |
| [18] | Koleva ,II, Niederlander H. A. G.,Van Beek T. A., Anal. Chem., 2000, ( 72), 2323— 2328 |
| [19] | Tsao R., Yang R., Christopher J., Zhu Y., Zhu.H H ., J. Agric. Food Chem., 2003, ( 51), 6347— 6353 |
| [20] | Li H., Li J., Xu Q., Hu X ., Anal. Chem., 2011, ( 83), 9681— 9686 |
| [21] | Pardo-Yissar V., Katz E., Wasserman J., Willner I., J. Am. Chem. Soc., 2003, (125),622—623 |
| 22 | Sheeney-Haj-Khia L., Basnar B.,Willner I Angew. Chem. Int. Ed., 2005, ( 44), 78— 83 |
| [23] | Xin Y. M., Li Z. Z., Wu W. L., Fu B. H., Wu H. J., Zhang Z. H., Biosens. Bioelectron., 2017, 87, 396— 403 |
| [24] | Li X., Yu J. G., Jaroniec M., Chem. Soc. Rev., 2016, 45, 2603— 2636 |
| [25] | Wang Q., Ruan Y., Zhao W., Lin P., Xu J., Chen H ., Anal. Chem., 2018, ( 90), 3759— 3765 |
| [26] | Yu L., Zhu Y., Liu Y., Qu P., Xu M., Shen Q., Zhao W., Anal. Chem., 2018, 90, 10803— 10811 |
| [27] | Zhan W W .,Kuang Q., Zhou J. Z.,Kong X. J.,Xie Z. X.,Zheng L. S., J. Am. Chem. Soc., 2013, ( 135), 1926— 1933 |
| [28] | Zhang .S S.,Zhang S. Q.,Peng B. Y.,Wang H. J.,Yu H.,Wang H. H.,Peng F., Electrochem. Commun., 2014, ( 40), 24— 27 |
| [29] | Tang J., Kong B., Wang Y.C .,Xu M.,Wang Y. L.,Wu H., Zheng G. F., Nano Letter, 2013, ( 13), 5350— 5354 |
| [30] | Tu W. W ., Dong Y. T., Lei J. P., Ju H. X., Anal. Chem., 2010, ( 82), 8711— 8716 |
| [31] | Zhao W W., .Ma Z. Y., Yan D. Y., Xu J. J., Chen H. Y.,Anal. Chem., 2012, ( 84), 10518— 10521 |
| [32] | Y Li .J ., Ma M. J., Zhu J. J., Anal. Chem., 2012, ( 84), 10492— 10499 |
| [33] | Zhao .W W ., Yu P. P., Shan Y., Wang J., Xu J. J., Chen H. Y., Anal. Chem., 2012, ( 84), 5892— 5897 |
| [34] | W Zhao .W., Tian C. Y., Xu J. J.,Chen H. Y., Chem. Commun., 2012, ( 48), 895 |
| [35] | Wang L N., .Han D. X., Ni S., MW. G., Wang W., Niu L.,Chem. Sci., 2015, ( 6), 6632— 6638 |
| [36] | Ma W.G, Wang L. N., Zhang N., Han D. X., Dong X. D., Niu L ., Anal. Chem., 2015, ( 87), 4844— 4850 |
| [37] | Wang L.N ., W. G. Ma, S. Y. Gan, D. X. Han, Q. X. Zhang, L. Niu, Anal. Chem., 2014, ( 86), 10171— 10178 |
| [38] | Ma W.G ., D Han. X., Zhou M., Sun H., Wang L. N., Dong X. D., Niu L., Chem. Sci., 2014, ( 5), 3946— 3951 |
| [39] | Ma W G.. Han D. X., Gan S. Y., Zhang N., Liu S. W., Wu T. S., Zhang Q. X., Dong X. D., Niu L., Chem. Commun., 2013, ( 49), 7842— 7844 |
| [40] | W Ma. G ., Han D. X., Zhang N., Li F. H., Wu T. S., Dong X. D., Niu L., Analyst, 2013, ( 138), 2335— 2342 |
| [41] | Han D.X ., Ma W. G., Wang L. N., Ni S., Zhang N., Wang W., Dong X. D., Niu L., Biosens. Bioelectron., 2015, ( 75), 458— 464 |
| [42] | Wang L., Liu Z., Wang D., Ni S., Han D., Wang W., Niu L ., Biosens. Bioelectron. 2017, ( 94), 107— 114 |
| [43] | Jiang D., Du X., Zhou L., Li H., Wang K ., Anal. Chem., 2017, ( 89), 4525— 4531 |
| [44] | Zhang Z.J., Wang W. Z, Wang, L., Sun S. M., ACS Appl. Mater. Interf., 2012, ( 4), 593— 597 |
| [45] | Li H P. .,Liu J Y., Hou W. G., Du ., Zhang R., Appl. Catal. B: Environ., 2014, ( 160), 89— 97 |
| [46] | Zhao K., Yan X., Gu Y., Kang Z., Bai Z., Cao S., Liu Y., Zhang X., Zhang Y ., Small, 2016, 12( 2), 245— 251 |
| [47] | Kang Z., Yan X., Wang Y., Bai Z., L., Zhang Z., Lin P., Zhang X., Yuan H., Zhang X., Zhang Y., Scientific Reports, 2015, 5, 7882— 7888 |
| [48] | Li Z., Zhang J., Li Y., Zhao S., Zhang P., Zhang Y., Bi J., Liu G., Yue Z., Biosens. Bioelectron., 2018, 99, 251— 258 |
| [49] | Bouayed J., Bohn T ., Oxidative Medicine and Cellular Longevity, 2010, ( 3), 228— 237 |
| [50] | Kang Q., Wang X., Ma X., Kong L., Zhang P., Shen D., Sens. Actuators B, 2016, 230, 231— 241 |
| [51] | Monteiro T., Tanaka A., Damos F., Luz R ., Food Chem., 2017, ( 227) 16— 21 |
| [52] | Monteiro T., Neto S., Damos F., Luz R., J. Electroanal. Chem., 2016, 774, 36— 41 |
| [53] | Watjen W., Michels G., Steffan B.R .,Niering P., Chovolou Y., Kampkotter A., Tran-Thi Q H., Proksch P., Kahl R., .,Journal of Nutrition, 2005, ( 135), 525— 531 |
| [54] | Galati G., Lin A., Sultan A M., O’Brien P J., .,Free Radical Biol. Med., 2006, ( 40), 570— 580 |
| [55] | Robaszkiewicz A., Balcerczyk A., Bartosz G ., Cell Biol. Int., 2007, ( 31), 1245— 1250 |
| [56] | De Marchi U., Biasutto L., Garbisa S., Toninello A., Zoratti M., Biochim. Biophys. Acta-Bioenerg., 2009, 1787, 1425— 1432 |
| [57] | Lima D. R. S., M. Cossenza, Garcia C. G., Portugal C. C., Marques F. F. d. C., Paes-de-Carvalho R., Pereira Netto A. D., Anal. Methods, 2016, 8, 5441— 5447 |
| [58] | Liu J., Chen Y., Wang W., Feng J., Liang M., Ma S., Chen X., J. Agric. Food Chem., 2016, 64, 371— 380 |
| [59] | Jothi L., Neogi S., Jaganathan S. K., Nageswaran G., Biosens. Bioelectron., 2018, 105, 236— 242 |
| [60] | Noroozifar M., Khorasani-Motlagh M., Akbari R., Bemanadi Parizi M., Biosens. Bioelectron., 2011, 28, 56— 63 |
| [61] | Chen H., Li W., Zhao P., Nie Z., Yao S ., Electrochimica Acta, 2015, ( 178), 407— 413 |
| [62] | Mazhabi R. M .,Ge L.,Q Jiang.,H Wang X.,.,Biosens. Bioelectron., 2018, ( 107), 54— 61 |
| [63] | Naczk M., Shahidi F ., J. Pharm. Biomed. Anal., 2006, ( 41), 1523— 1542 |
| [64] | Cui M., Huang J., Wang Y., Wu Y., Luo X., Biosens. Bioelectron., 2015, 68, 563— 569 |
| [65] | Andre C., Castanheira I., Cruz J., Paseiro P., Sanches-Silva A ., Trends Food Sci. Technol. 2010, ( 21), 229— 246 |
| [66] | Han F. J., Song Z. Q., Nawaz M. H., Dai M. J., Han D. F., Han L. P., Fan Y. Y., Xu J. N., Han D. X., and Niu L., Anal. Chem., 2019, 91, 10657— 10662 |
| [67] | Vikraman A., Rasheed Z., Rajith L., Lonappan L., Krishnapillai K., Food Anal. Methods, 2013, 6, 775— 780 |
| [68] | Ni S., Han F. J., Wang W., Han D. F., Bao Y., Han D. X., Wang H. Y., Niu L., Sen. Actuators B, 2018, 259, 963— 971 |
| [1] | 李玉龙, 谢发婷, 管燕, 刘嘉丽, 张贵群, 姚超, 杨通, 杨云慧, 胡蓉. 基于银离子与DNA相互作用的比率型电化学传感器用于银离子的检测[J]. 高等学校化学学报, 2022, 43(8): 20220202. |
| [2] | 王君旸, 刘争, 张茜, 孙春燕, 李红霞. DNA银纳米簇在功能核酸荧光生物传感器中的应用[J]. 高等学校化学学报, 2022, 43(6): 20220010. |
| [3] | 刘家琪, 李天保. BiVO4/CuBi2O4薄膜光电极的制备及光电性能[J]. 高等学校化学学报, 2022, 43(4): 20220017. |
| [4] | 魏闯宇, 陈艳丽, 姜建壮. 基于乙硫基取代的三层酞菁铕二聚体修饰ITO电极构筑电化学多巴胺和尿酸传感器[J]. 高等学校化学学报, 2022, 43(1): 20210582. |
| [5] | 赵凌云, 黄汉雄, 罗杜宇, 苏逢春. 复合材料柔软性对倒金字塔微结构阵列传感器性能的影响[J]. 高等学校化学学报, 2021, 42(9): 2953. |
| [6] | 黄罗仪, 翁约约, 黄旭慧, 王朝杰. 车前草中黄酮类成分结构和性质的理论研究[J]. 高等学校化学学报, 2021, 42(9): 2752. |
| [7] | 潘晓君, 鲍容容, 潘曹峰. 可穿戴柔性触觉传感器的研究进展[J]. 高等学校化学学报, 2021, 42(8): 2359. |
| [8] | 蔡雅倩, 张家怀, 刘方哲, 李海潮, 石建平, 关爽. Hofmeister效应辅助的蛋白质基水凝胶应变传感器[J]. 高等学校化学学报, 2021, 42(8): 2609. |
| [9] | 胡皓程, 李文利, 张嘉宁, 刘宇博. 黑木耳寡糖的提取、 结构表征及生物活性[J]. 高等学校化学学报, 2021, 42(8): 2465. |
| [10] | 唐定, 衷水平. Bi1-xFexVO4薄膜光阳极的制备及光电化学性能[J]. 高等学校化学学报, 2021, 42(8): 2509. |
| [11] | 杨依然, 姚华, 闫江红, 孙志恒, 张余, 房雪晴, 李绪文, 金永日. 薤中新的甾体皂苷类化学成分[J]. 高等学校化学学报, 2021, 42(6): 1742. |
| [12] | 吴杨仪, 陈建平, 艾益静, 汪庆祥, 高飞, 高凤. 2-(2-羟基-3-甲氧基苯基)-C60的合成及在花椰菜花叶病毒启动子DNA传感检测中的应用[J]. 高等学校化学学报, 2021, 42(6): 1754. |
| [13] | 徐梦祎, 黄雪雯, 李小杰, 魏玮, 刘晓亚. “串珠状”复合纳米组装体修饰丝网印刷电极构建的生物传感器[J]. 高等学校化学学报, 2021, 42(6): 1768. |
| [14] | 王杰, 李莹, 邵亮, 白阳, 马忠雷, 马建中. 聚乙烯醇/聚吡咯复合导电水凝胶应变传感器的制备及性能[J]. 高等学校化学学报, 2021, 42(3): 929. |
| [15] | 沙卉雯, 马维廷, 周晓娟, 宋卫星. 激光诱导三维网状石墨烯的一步法制备及应用[J]. 高等学校化学学报, 2021, 42(2): 607. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||