

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (2): 317.doi: 10.7503/cjcu20190474
张丽1,钱明超1,刘雪珂1,高帅涛1,余江1,*( ),谢海深2,王宏斌2,孙风江2,苏向红3
),谢海深2,王宏斌2,孙风江2,苏向红3
收稿日期:2019-09-03
出版日期:2020-02-10
发布日期:2019-12-31
通讯作者:
余江
E-mail:yujiang@mail.buct.edu.cn
基金资助:
ZHANG Li1,QIAN Mingchao1,LIU Xueke1,Gao Shuaitao1,YU Jiang1,*( ),XIE Haishen2,WANG Hongbin2,SUN Fengjiang2,SU Xianghong3
),XIE Haishen2,WANG Hongbin2,SUN Fengjiang2,SU Xianghong3
Received:2019-09-03
Online:2020-02-10
Published:2019-12-31
Contact:
Jiang YU
E-mail:yujiang@mail.buct.edu.cn
Supported by:摘要:
以铁基离子液体(Fe-IL) /聚乙二醇二甲醚(NHD)为脱硫体系, 通过静态吸收反应装置研究了共溶体系吸收氧化H2S的动力学过程. 结果表明, 纯Fe-IL脱硫体系的动力学方程可表示为r=54.62exp(-11720/RT)·
中图分类号:
TrendMD:
张丽,钱明超,刘雪珂,高帅涛,余江,谢海深,王宏斌,孙风江,苏向红. 铁基离子液体/NHD吸收氧化H2S的反应动力学. 高等学校化学学报, 2020, 41(2): 317.
ZHANG Li,QIAN Mingchao,LIU Xueke,Gao Shuaitao,YU Jiang,XIE Haishen,WANG Hongbin,SUN Fengjiang,SU Xianghong. Dynamic Study of Oxidative Desulfurization by Iron-based Ionic Liquids/NHD †. Chem. J. Chinese Universities, 2020, 41(2): 317.
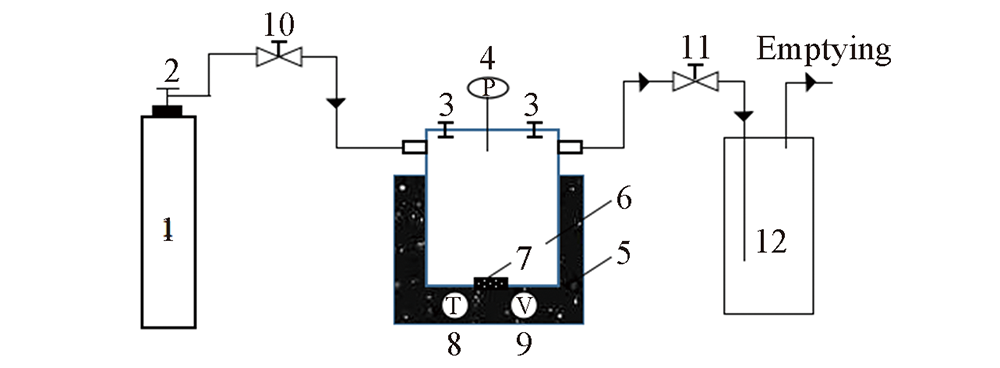
Fig.1 Static absorption reactor 1. H2S gas tank; 2. gas reducing valve; 3. pressure flange; 4. precision pressure gauge; 5. thermostat water bath; 6. high pressure absorption reactor; 7. magneto; 8. thermometer; 9. speed controller; 10. inlet valve; 11. outlet valve; 12. tail gas treatment unit.
| Variable | Dynamic region | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| Concentration of Fe(Ⅲ) | + | - | + | + | ? | ? | + | + |
| Volume fraction of H2S | + | + | + | + | ? | ? | + | + |
| Phase interfacial area | + | + | + | + | + | + | + | - |
| Volume of desulfurization liquid | - | - | - | - | + | + | + | + |
| Coefficient of mass transfer in liquid phase | + | - | - | - | ? | + | + | - |
| Coefficient of mass transfer in gas phase | + | - | + | + | ? | ? | + | - |
| Second-order reaction rate constant | - | - | + | + | ? | ? | - | + |
Table 1 Determination method of dynamic region*
| Variable | Dynamic region | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| Concentration of Fe(Ⅲ) | + | - | + | + | ? | ? | + | + |
| Volume fraction of H2S | + | + | + | + | ? | ? | + | + |
| Phase interfacial area | + | + | + | + | + | + | + | - |
| Volume of desulfurization liquid | - | - | - | - | + | + | + | + |
| Coefficient of mass transfer in liquid phase | + | - | - | - | ? | + | + | - |
| Coefficient of mass transfer in gas phase | + | - | + | + | ? | ? | + | - |
| Second-order reaction rate constant | - | - | + | + | ? | ? | - | + |
| No. | Liquid-phase volume/mL | ||
|---|---|---|---|
| 60 | 80 | 100 | |
| 1 | 1.51 | 1.49 | 1.48 |
| 2 | 1.54 | 1.47 | 1.49 |
| 3 | 1.52 | 1.49 | 1.48 |
Table 2 Effect of liquid-phase volume on H2S absorption rate(mol·m-2·min-1)*
| No. | Liquid-phase volume/mL | ||
|---|---|---|---|
| 60 | 80 | 100 | |
| 1 | 1.51 | 1.49 | 1.48 |
| 2 | 1.54 | 1.47 | 1.49 |
| 3 | 1.52 | 1.49 | 1.48 |
| No. | KL/(r·min-1) | |
|---|---|---|
| 100 | 150 | |
| 1 | 1.48 | 1.50 |
| 2 | 1.49 | 1.52 |
| 3 | 1.48 | 1.52 |
Table 3 Effect of kL on H2S absorption rate(mol·m-2·min-1)*
| No. | KL/(r·min-1) | |
|---|---|---|
| 100 | 150 | |
| 1 | 1.48 | 1.50 |
| 2 | 1.49 | 1.52 |
| 3 | 1.48 | 1.52 |
| No. | Mass ratio of Fe-IL/NHD | |||
|---|---|---|---|---|
| 8:1 | 6:1 | 4:1 | 1:0 | |
| 1 | 1.35 | 1.41 | 1.48 | 1.29 |
| 2 | 1.33 | 1.42 | 1.49 | 1.28 |
| 3 | 1.32 | 1.42 | 1.48 | 1.29 |
Table 4 Effect of different mass ratios of Fe-IL/NHD on H2S absorption rate(mol·m-2·min-1)*
| No. | Mass ratio of Fe-IL/NHD | |||
|---|---|---|---|---|
| 8:1 | 6:1 | 4:1 | 1:0 | |
| 1 | 1.35 | 1.41 | 1.48 | 1.29 |
| 2 | 1.33 | 1.42 | 1.49 | 1.28 |
| 3 | 1.32 | 1.42 | 1.48 | 1.29 |
| Mass ratio of Fe-IL/NHD | b | R2 |
|---|---|---|
| 1:0 | 1.07 | 0.9779 |
| 8:1 | 1.15 | 0.9771 |
| 6:1 | 1.39 | 0.9813 |
| 4:1 | 1.56 | 0.9999 |
Table 5 Ftting relation of lnr with lncFe(Ⅲ)
| Mass ratio of Fe-IL/NHD | b | R2 |
|---|---|---|
| 1:0 | 1.07 | 0.9779 |
| 8:1 | 1.15 | 0.9771 |
| 6:1 | 1.39 | 0.9813 |
| 4:1 | 1.56 | 0.9999 |
| [1] | Zhang Q., Hou Y. C., Ren S. H., Zhang K., Wu W. Z., Chem. Eng., 2019,7(12), 10931— 10936 |
| [2] | Debski B., Hanel A., Aranowski R., Stolte S., Markiewicz M., Veltzke T., Cichowska-Kopczynska I ., J. Mol. Lid., 2019,291, 110477 |
| [3] | Zheng W. T., Wu D. S., Feng X., Hu J. L., Zhang F., Wu Y. T., Hu X. B., J. Mol. Lid., 2018,263, 209— 217 |
| [4] |
Paramanik M., Singh R., Mukhopadhyay S., Ghosh S. K., J. Fluorine Chem., 2015,178, 47— 55
doi: 10.1016/j.jfluchem.2015.06.022 URL |
| [5] |
Ruan C. X., Sun Z. L., Lu S. S., Li L. F., Lou J., Sun W., Russ. J. Electrochem., 2014,50(2), 129— 135
doi: 10.1134/S1023193513020158 URL |
| [6] |
Ma Y. Q., Liu X. P., Wang R., J. Hazard. Mater., 2017,331, 109— 116
doi: 10.1016/j.jhazmat.2017.02.036 URL |
| [7] |
Liu X. P., Qang R., Fuel Process. Technol., 2017,160, 78— 85
doi: 10.1016/j.fuproc.2017.02.024 URL |
| [8] |
Lv B. H., Jing G. H., Qian Y. H., Zhou Z. M., Chem. Eng. J., 2016,289, 212— 218
doi: 10.1016/j.cej.2015.12.096 URL |
| [9] | Kazmi B., Haider J., Qyyum M. A., Saeed S., Kazmi M. R., Int. J. Greenhouse Gas Control, 2019,87, 88— 99 |
| [10] |
Dai F., Chen X. J., He B., Liu R. X., Zhang S. J., Cat. Sci. Technol., 2018,8(17), 4515— 4525
doi: 10.1039/C8CY01023D URL |
| [11] |
Ding W. J., Zhu W. S., Xiong J., Yang L., Wei A. M., Zhang M., Li H. M., Chem. Eng. J., 2015,266, 213— 221
doi: 10.1016/j.cej.2014.12.040 URL |
| [12] | Xie M. Y., Li P. P., Guo H. F., Gao L. X., Yu J., Chinese J. Chem. Eng., 2012,1, 140— 145 |
| [13] |
Lee S. H., Hu S. H., You C. Y., Koo Y. M., Korean J. Chem. Eng., 2007,24, 436— 437
doi: 10.1007/s11814-007-0075-x URL |
| [14] | He Y., Yu J., Chen L. B., CIESC J., 2010,61(4), 963— 968 |
| ( 何义, 余江, 陈灵波 . 化工学报, 2010,61(4), 963— 968) | |
| [15] | Wang J. H., Chen J. Q., Yan H. Z., Chem. Res., 2012,23(1), 9— 13 |
| ( 王建宏, 陈家庆, 阎红昭 . 化学研究, 2012,23(1), 9— 13) | |
| [16] |
Wang J. H., Zhang W. D., Energy Fuels, 2014,28(9), 5930— 5935
doi: 10.1021/ef500527w URL |
| [17] | Guo Z. H., Zhang T. T., Sun L. L., He Y., Yu J., Fresenius Environ. Bulletin, 2015,24(8A), 2587— 2592 |
| [18] |
Guo Z. H., Zhang T. T., Liu T. T., Du J., Jia B., Gao S. J., Yu J., Environ. Sci. Technol., 2015,49(9), 5697— 5703
doi: 10.1021/es505728f URL |
| [19] | Hu J. C., Gao L. X., Liu W. H., Zhao Y. L., Gao S., Pan X. P., Guo Z. H., Yu J., CIESC J., 2016,67(S1), 347— 352 |
| ( 胡锦超, 高丽霞, 刘伟海, 赵永禄, 高尚, 潘兴朋, 郭智慧, 余江 . 化工学报, 2016,67(S1), 347— 352) | |
| [20] | Liu K., Liu H. J., Guo Q., Chem. Enterprise Management, 2019, ( 11), 185— 186 |
| ( 刘凯, 刘海菊, 郭琦 . 化工管理, 2019, ( 11), 185— 186) | |
| [21] |
Rayer A. V., Henni A., Tontiwachwuthikul P., Can. J. Chem. Eng., 2012,90(3), 576— 583
doi: 10.1002/cjce.20615 URL |
| [22] |
Schmidt K. A. G., Mather A. E., Can. J. Chem. Eng., 2001,79(6), 946— 960
doi: 10.1002/cjce.v79:6 URL |
| [23] | Lin M. H., Guo S. C., Chem. Eng., 2000, ( 3), 58— 61 |
| ( 林民鸿, 郭淑翠 . 化学工程, 2000, ( 3), 58— 61) | |
| [24] | Xu B ., Chem. Enterprise Management, 2016, ( 18), 87 |
| ( 徐斌 . 化工管理, 2016, ( 18), 87) | |
| [25] | Feng S., Anhui Chem. Industry, 2018,44(1), 49— 52 |
| ( 冯升 . 安徽化工, 2018,44(1), 49— 52) | |
| [26] |
Karamanev D. G., Nikolov L. N., Mamatarkova V., Minerals Eng., 2002,15(5), 341— 345
doi: 10.1016/S0892-6875(02)00026-2 URL |
| [27] | Chen J., Luo W. L., Li H., CIESC J., 2014,65(1), 12— 21 |
| ( 陈健, 罗伟亮, 李晗 . 化工学报, 2014,65(1), 12— 21) | |
| [28] | Shen Y. T., Zhang H. T., Fang D. Y., Li T., J. East China University Sci. Technol.(Nat. Sci. Ed.), 2016,42(4), 446— 453 |
| ( 沈叶婷, 张海涛, 房鼎业, 李涛 . 华东理工大学学报(自然科学版), 2016,42(4), 446— 453) |
| [1] | 崔伟, 赵德银, 白文轩, 张晓东, 余江. CO2在非质子溶剂与铁基离子液体复合体系中的吸收[J]. 高等学校化学学报, 2022, 43(8): 20220120. |
| [2] | 高志伟, 李军委, 史赛, 付强, 贾钧儒, 安海龙. 基于分子动力学模拟的TRPM8通道门控特性分析[J]. 高等学校化学学报, 2022, 43(6): 20220080. |
| [3] | 曾晛阳, 赵熹, 黄旭日. 细胞松弛素B对葡萄糖/质子共转运蛋白GlcPSe的抑制机理[J]. 高等学校化学学报, 2022, 43(4): 20210822. |
| [4] | 刘嘉欣, 闵杰, 许华杰, 任海生, 谈宁馨. 基于反应力场分子模拟的乙烯燃烧自由基与氮气相互作用研究[J]. 高等学校化学学报, 2022, 43(4): 20210834. |
| [5] | 孟祥龙, 杨歌, 郭海玲, 刘晨光, 柴永明, 王纯正, 郭永梅. 纳米分子筛的合成及硫化氢吸附性能[J]. 高等学校化学学报, 2022, 43(3): 20210687. |
| [6] | 陈瀚翔, 边绍菊, 胡斌, 李武. LiCl-NaCl-KCl-H2O溶液体系渗透压的分子动力学模拟[J]. 高等学校化学学报, 2022, 43(3): 20210727. |
| [7] | 孙翠红, 吕立强, 刘迎, 王妍, 杨静, 张绍文. 硝酸异丙酯与Cl原子、 OH和NO3自由基反应的机理及动力学[J]. 高等学校化学学报, 2022, 43(2): 20210591. |
| [8] | 胡波, 朱昊辰. 双层氧化石墨烯纳米体系中受限水的介电常数[J]. 高等学校化学学报, 2022, 43(2): 20210614. |
| [9] | 唐元晖, 李春玉, 林亚凯, 张春晖, 刘泽, 余立新, 王海辉, 王晓琳. 链段刚性对非溶剂致相分离成膜过程影响的耗散粒子动力学模拟[J]. 高等学校化学学报, 2022, 43(10): 20220169. |
| [10] | 张伶育, 张继龙, 曲泽星. RDX分子内振动能量重分配的动力学研究[J]. 高等学校化学学报, 2022, 43(10): 20220393. |
| [11] | 张想, 谢旭岚, 熊力堃, 彭扬. 海胆针状金纳米颗粒用于电催化二氧化碳还原[J]. 高等学校化学学报, 2021, 42(9): 2824. |
| [12] | 雷晓彤, 金怡卿, 孟烜宇. 基于分子模拟方法预测PIP2在双孔钾通道TREK-1上结合位点的研究[J]. 高等学校化学学报, 2021, 42(8): 2550. |
| [13] | 李聪聪, 刘明皓, 韩佳睿, 朱镜璇, 韩葳葳, 李婉南. 基于分子动力学模拟的VmoLac非特异性底物催化活性的理论研究[J]. 高等学校化学学报, 2021, 42(8): 2518. |
| [14] | 安丰, 胡茜茜, 谢代前. 三原子分子非绝热传能动力学的研究进展[J]. 高等学校化学学报, 2021, 42(7): 2103. |
| [15] | 李维唐, 任佳骏, 帅志刚. 含时密度矩阵重正化群的理论与应用[J]. 高等学校化学学报, 2021, 42(7): 2085. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||