

高等学校化学学报 ›› 2016, Vol. 37 ›› Issue (4): 693.doi: 10.7503/cjcu20150844
收稿日期:2015-11-06
出版日期:2016-04-10
发布日期:2016-03-17
基金资助:
JIANG Junhui, XIA Shengjie, NI Zheming( ), Zhang Lianyang
), Zhang Lianyang
Received:2015-11-06
Online:2016-04-10
Published:2016-03-17
Supported by:摘要:
采用密度泛函理论计算了巴豆醛4种构型的稳定性, 并选取最优构型进一步研究了其Au(111)面上的吸附及选择性加氢机理. 计算结果表明, 具有E-(s)-trans构型的巴豆醛稳定性最高. 当巴豆醛通过CO吸附于Au(111)面的顶位时, 该构型吸附能最大, 吸附模型最稳定; 巴豆醛向Au(111)表面转移电子0.045 e, 且其p轨道与金属表面的d轨道发生较强相互作用, 使得巴豆醛的键级减弱. 此外, 通过分析各基元反应的活化能、 反应热以及构型变化可知, 巴豆醛在Au(111)面上按照2,1-加成机理(部分加氢机理)生成巴豆醇的可能性最大, 且降低温度有利于反应转化率的提高.
中图分类号:
TrendMD:
蒋军辉, 夏盛杰, 倪哲明, 张连阳. 巴豆醛在Au(111)面上的吸附及选择性加氢机理研究. 高等学校化学学报, 2016, 37(4): 693.
JIANG Junhui, XIA Shengjie, NI Zheming, Zhang Lianyang. Adsorption and Selective Hydrogenation Mechanism of Crotonaldehyde on AuSurface. Chem. J. Chinese Universities, 2016, 37(4): 693.
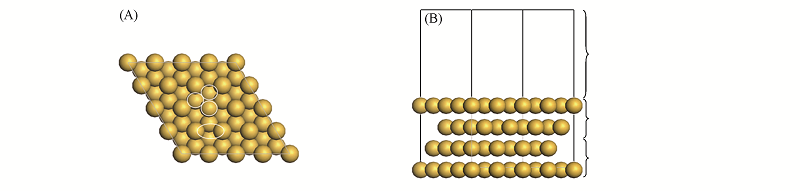
Fig.1 Top(A) and side(B) views of Au(111) surface models(4×4)^The four representative surface sites(Top, Bri, Hcp and Fcc site) are indicated in the top view.
| Initial adsorption site | Final adsorption site | Eads/(kJ·mol-1) | Initial adsorption site | Final adsorption site | Eads/(kJ·mol-1) |
|---|---|---|---|---|---|
| O | C | ||||
| Top | Top | -85.1 | Top-Top | Bri-Top | -84.5 |
| Bri | Bri | -86.8 | Top-Bri | Bri-Bri | -84.5 |
| Hcp | Hcp | -84.1 | Top-Hcp | Top-Hcp | -84.2 |
| Fcc | Fcc | -84.9 | Top-Fcc | Top-Fcc | -84.3 |
| C | Bri-Top | Bri-Top | -84.5 | ||
| Top | Top | -93.7 | Bri-Bri | Bri-Bri | -84.6 |
| Bri | Bri | -90.5 | Bri-Hcp | Bri-Top | -84.0 |
| Hcp | Hcp | -85.0 | Bri-Fcc | Bri-Fcc | -83.8 |
| Fcc | Fcc | -86.7 | Hcp-Top | Hcp-Top | -84.7 |
| C | Hcp-Bri | Hcp-Bri | -84.5 | ||
| Top | Top | -79.7 | Hcp-Hcp | Bri-Fcc | -83.5 |
| Bri | Bri | -80.4 | Hcp-Fcc | Hcp-Fcc | -84.0 |
| Hcp | Hcp | -81.8 | Fcc-Top | Fcc-Top | -84.4 |
| Fcc | Fcc | -82.6 | Fcc-Bri | Fcc-Bri | -83.5 |
| Fcc-Hcp | Bri-Bri | -84.6 | |||
| Fcc-Fcc | Fcc-Fcc | -83.7 |
Table 1 Adsorption energies of CAL on Au(111) surface
| Initial adsorption site | Final adsorption site | Eads/(kJ·mol-1) | Initial adsorption site | Final adsorption site | Eads/(kJ·mol-1) |
|---|---|---|---|---|---|
| O | C | ||||
| Top | Top | -85.1 | Top-Top | Bri-Top | -84.5 |
| Bri | Bri | -86.8 | Top-Bri | Bri-Bri | -84.5 |
| Hcp | Hcp | -84.1 | Top-Hcp | Top-Hcp | -84.2 |
| Fcc | Fcc | -84.9 | Top-Fcc | Top-Fcc | -84.3 |
| C | Bri-Top | Bri-Top | -84.5 | ||
| Top | Top | -93.7 | Bri-Bri | Bri-Bri | -84.6 |
| Bri | Bri | -90.5 | Bri-Hcp | Bri-Top | -84.0 |
| Hcp | Hcp | -85.0 | Bri-Fcc | Bri-Fcc | -83.8 |
| Fcc | Fcc | -86.7 | Hcp-Top | Hcp-Top | -84.7 |
| C | Hcp-Bri | Hcp-Bri | -84.5 | ||
| Top | Top | -79.7 | Hcp-Hcp | Bri-Fcc | -83.5 |
| Bri | Bri | -80.4 | Hcp-Fcc | Hcp-Fcc | -84.0 |
| Hcp | Hcp | -81.8 | Fcc-Top | Fcc-Top | -84.4 |
| Fcc | Fcc | -82.6 | Fcc-Bri | Fcc-Bri | -83.5 |
| Fcc-Hcp | Bri-Bri | -84.6 | |||
| Fcc-Fcc | Fcc-Fcc | -83.7 |
| Species | Charge/e | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O1 | C2 | C3 | C4 | C5 | H6 | H7 | H8 | H9 | H10 | H11 | Tol | |
| CAL | -0.378 | 0.282 | -0.050 | 0.010 | -0.135 | -0.009 | 0.042 | 0.044 | 0.055 | 0.069 | 0.070 | 0.000 |
| CAL/Au(111) | -0.355 | 0.232 | -0.086 | -0.033 | -0.281 | 0.032 | 0.099 | 0.100 | 0.099 | 0.118 | 0.120 | 0.045 |
Table 2 Mulliken charges of CAL at advantage adsorption site on Au(111) surface
| Species | Charge/e | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O1 | C2 | C3 | C4 | C5 | H6 | H7 | H8 | H9 | H10 | H11 | Tol | |
| CAL | -0.378 | 0.282 | -0.050 | 0.010 | -0.135 | -0.009 | 0.042 | 0.044 | 0.055 | 0.069 | 0.070 | 0.000 |
| CAL/Au(111) | -0.355 | 0.232 | -0.086 | -0.033 | -0.281 | 0.032 | 0.099 | 0.100 | 0.099 | 0.118 | 0.120 | 0.045 |
| Mechanism | Reaction | Ea/ (kJ·mol-1) | ΔE/ (kJ·mol-1) | Mechanism | Reaction | Ea/ (kJ·mol-1) | ΔE/ (kJ·mol-1) |
|---|---|---|---|---|---|---|---|
| A1 | CAL*+H*→MS2*+* | 35.1 | -43.8 | C1 | CAL*+H*→MS2*+* | 35.1 | -43.8 |
| MS2*+H*→COL*+* | 150.7 | -47.5 | MS2*+H*→ENOL*+* | 312.2 | -89.6 | ||
| A2 | CAL*+H*→MS1*+* | 70.3 | -10.2 | C2 | CAL*+H*→MS3*+* | 288.1 | -0.4 |
| MS1*+H*→COL*+* | 28.4 | -81.0 | MS3*+H*→ENOL*+* | 42.6 | -133.0 | ||
| B1 | CAL*+H*→MS4*+* | 240.2 | -32.5 | CAL+*→CAL* | -93.7 | ||
| MS4*+H*→BAL*+* | 301.7 | -124.7 | COL*→COL +* | 51.6 | |||
| B2 | CAL*+H*→MS3*+* | 288.1 | -0.4 | ENOL*→ENOL +* | 56.2 | ||
| MS3*+H*→BAL*+* | 220.3 | -156.9 | BAL*→BAL +* | 60.9 |
Table 3 Activation energy(Ea) and reaction energy(ΔE) of main elementary reactions for the partial hydrogenation of CAL on Au(111) surface
| Mechanism | Reaction | Ea/ (kJ·mol-1) | ΔE/ (kJ·mol-1) | Mechanism | Reaction | Ea/ (kJ·mol-1) | ΔE/ (kJ·mol-1) |
|---|---|---|---|---|---|---|---|
| A1 | CAL*+H*→MS2*+* | 35.1 | -43.8 | C1 | CAL*+H*→MS2*+* | 35.1 | -43.8 |
| MS2*+H*→COL*+* | 150.7 | -47.5 | MS2*+H*→ENOL*+* | 312.2 | -89.6 | ||
| A2 | CAL*+H*→MS1*+* | 70.3 | -10.2 | C2 | CAL*+H*→MS3*+* | 288.1 | -0.4 |
| MS1*+H*→COL*+* | 28.4 | -81.0 | MS3*+H*→ENOL*+* | 42.6 | -133.0 | ||
| B1 | CAL*+H*→MS4*+* | 240.2 | -32.5 | CAL+*→CAL* | -93.7 | ||
| MS4*+H*→BAL*+* | 301.7 | -124.7 | COL*→COL +* | 51.6 | |||
| B2 | CAL*+H*→MS3*+* | 288.1 | -0.4 | ENOL*→ENOL +* | 56.2 | ||
| MS3*+H*→BAL*+* | 220.3 | -156.9 | BAL*→BAL +* | 60.9 |
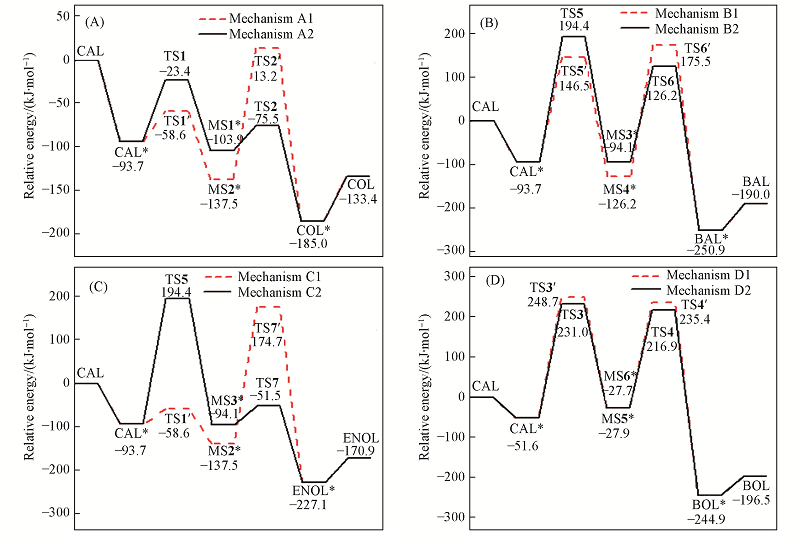
Fig.6 Sketch for potential relative energy of reaction mechanisms on Au(111) surface^ (A) Mechanism A; (B) mechanism B; (C) mechanism C; (D) mechanism D.
| Mechanism | Reaction | Ea/ (kJ·mol-1) | ΔE/ (kJ·mol-1) | Mechanism | Reaction | Ea/ (kJ·mol-1) | ΔE/ (kJ·mol-1) |
|---|---|---|---|---|---|---|---|
| D1 | CAL+*→CAL* | -93.7 | D2 | CAL*+H*→MS1*+* | 70.3 | -10.2 | |
| CAL*+H*→MS1*+* | 70.3 | -10.2 | MS1*+H*→COL*+* | 28.4 | -81.0 | ||
| MS1*+H*→COL*+* | 28.4 | -81.0 | COL*+H*→MS5*+* | 282.6 | 23.7 | ||
| COL*+H*→MS6*+* | 300.3 | 23.9 | MS5*+H*→BOL*+* | 244.8 | -217.0 | ||
| MS6*+H*→BOL*+* | 263.1 | -217.2 | BOL*→BOL+* | 48.4 |
Table 4 Activation energy(Ea) and reaction energy(ΔE) of main elementary reactions for the full hydrogenation of CAL on Au(111) surface
| Mechanism | Reaction | Ea/ (kJ·mol-1) | ΔE/ (kJ·mol-1) | Mechanism | Reaction | Ea/ (kJ·mol-1) | ΔE/ (kJ·mol-1) |
|---|---|---|---|---|---|---|---|
| D1 | CAL+*→CAL* | -93.7 | D2 | CAL*+H*→MS1*+* | 70.3 | -10.2 | |
| CAL*+H*→MS1*+* | 70.3 | -10.2 | MS1*+H*→COL*+* | 28.4 | -81.0 | ||
| MS1*+H*→COL*+* | 28.4 | -81.0 | COL*+H*→MS5*+* | 282.6 | 23.7 | ||
| COL*+H*→MS6*+* | 300.3 | 23.9 | MS5*+H*→BOL*+* | 244.8 | -217.0 | ||
| MS6*+H*→BOL*+* | 263.1 | -217.2 | BOL*→BOL+* | 48.4 |
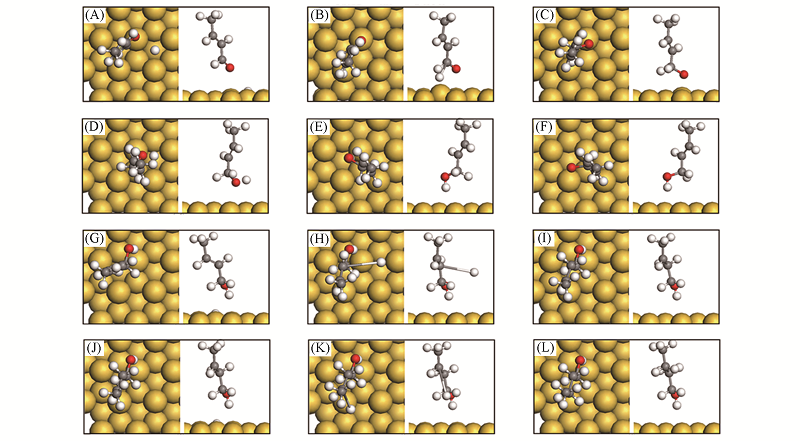
Fig.8 Structure change for reactants of mechanisms A2 and D2 on Au(111) surface^(A)—(C) CAL*+H*→MS1*+*; (D)—(F) MS1*+H*→COL*+*; (G)—(I) COL*+H*→MS5*+*; (J)—(L) MS5*+H*→BOL*+*. (A) IS1; (B) TS1; (C) MS1; (D) IS2; (E) TS2; (F) COL; (G) IS3; (H) TS3; (I) MS5; (J) IS4; (K) TS4; (L) BOL.
| [1] | Li X. H., Zheng W. L., Pan H. Y., J. Catal., 2013, 300, 9—19 |
| [2] | Galletti A. M. R., Toniolo L., Antonetti C., Evangelisti C., Forte C., Appl. Catal. A-Gen., 2012, 447, 49—59 |
| [3] | Hong X., Li B., Wang Y. J., Lu J. Q., Hu G. S., Luo M. F., Appl. Surf. Sci., 2013, 270, 388—394 |
| [4] | Nholler H., Lin W. M., J. Catal., 1984, 85, 25—30 |
| [5] | Fang C., Chen Y. J., Mao H., Zhao J., Jiang Y. F., Zhao S. L., Ma J., Chem. J. Chinese Universities, 2015, 36(1), 124—130 |
| (方超, 陈亚君, 毛卉, 赵俊, 蒋云福, 赵仕林, 马骏. 高等学校化学学报, 2015, 36(1), 124—130) | |
| [6] | Hisahiro H., Tomomi N., Takashi H., Toshio S., Green Chem., 2011, 13, 1133—1137 |
| [7] | Rodrigues E. L., Bueno J. M. C., Appl. Catal. A-Gen., 2004, 257, 201—211 |
| [8] | Tian Z. B., Li Q. Y., Li Y., Ai S. Y., Catal. Commun., 2015, 61, 97—101 |
| [9] | Aoun M., Benamar A., Chater M., Chinese J. Catal., 2011, 32, 1185—1190(Aoun M., Benamar A., Chater M., 催化学报, 2011,32, 1185—1190) |
| [10] | Bailie J.E., Hutching G. J.,Chem. Commun., 1999, (21), 2151—2152 |
| [11] | Bailie J. E., Abdullah H. A., Anderson J. A., Rochester C. H., Richardson N. V., Hodge N., Zhang J. G., Burrows A., Kiely C. J., Hutchings G. J., Phys. Chem. Chem. Phys., 2001, 3, 4113—4121 |
| [12] | Mohr C., Hofmeister H., Radnik J., Claus P., J. Am. Chem. Soc., 2003, 125, 1905—1911 |
| [13] | Zhao J., Ni J., Xu J. H., Xu J. T., Cen J., Li X. N., Catal. Commun., 2014, 54, 72—76 |
| [14] | Pan W., Ma W. G., Yang X. D., Zheng J. Y., Song B. Q., Niu Y. Z., Gu J., Hu D. B., Yang Q., Zhu H. J., Chem. J. Chinese Universities, 2015, 36(2), 325—329 |
| (潘威, 马文广, 杨晓东, 郑昀晔, 宋碧清, 牛永志, 古吉, 胡栋宝, 杨芹, 朱华结. 高等学校化学学报, 2015, 36(2), 325—329) | |
| [15] | Gholizadeh R., Yu Y. X., Appl. Surf. Sci., 2015, 357, 1187—1195 |
| [16] | Liu T. T., Lu X., Zhang M. T., Chem. Res. Chinese Universities, 2014, 30(4), 656—660 |
| [17] | Stefanov B. I., Topalian Z., Granqvist C. G., Osterlund L., J. Mol. Catal. A-Chem., 2014, 381, 77—88 |
| [18] | Shi W., Zhang L. Y., Ni Z. M., Xiao X. C., Xia S. J., RSC Adv., 2014, 4, 27003—27012 |
| [19] | Cao X. M., Burch R., Hardacre C., Hu P., J. Phys. Chem. C, 2011, 115, 19819—19827 |
| [20] | Yu Y. X.,ACS Appl. Mater. Interfaces, 2014, 6, 16267—16275 |
| [21] | Yu Y. X., J. Mater. Chem. A, 2014, 2, 8910—8917 |
| [22] | Ge Q., Jenkins S. J., King D. A., Chem. Phys. Lett., 2000, 327, 125—130 |
| [23] | Ni Z. M., Shi W., Xia M. Y., Xue J. L., Chem. J. Chinese Universities, 2013, 34(10), 2353—2362 |
| (倪哲明, 施炜, 夏明玉, 薛继龙. 高等学校化学学报, 2013, 34(10), 2353—2362) | |
| [24] | Zhao X. D., Song L. Z., Fu J., Tang P., Liu F., Surf. Sci., 2011, 605, 1005—1015 |
| [25] | Zhang L.Y., Jiang J.H., Shi W., Xia S. J., Ni Z. M., Xiao X. C., RSC Adv., 2015, 5, 34319—34326 |
| [26] | Haubrich J., Loffreda D., Delbecq F., Sautet P., Krupski K., Becker C., Wandeltt K., J. Phys. Chem. C, 2009, 113, 13947—13967 |
| [27] | Xiao X. C., Shi W., Ni Z. M., Acta Phys-Chim. Sinica, 2014, 30, 1456—1464 |
| (肖雪春, 施炜, 倪哲明. 物理化学学报, 2014, 30, 1456—1464) | |
| [28] | Delbecq D., Sautet P., J. Catal., 1995, 152, 217—236 |
| [29] | Xie Y., Yu H. T., Chem. Res. Chinese Universities, 2014, 30(5), 794—799 |
| [30] | Mullken R. S., J. Chem. Phys., 1955, 23, 1833—1840 |
| [31] | Loffreda D., Delbecq F., Vigne F., Sautet P., J. Am. Chem. Soc., 2006, 128, 1316—1323 |
| [32] | Peter C., Appl. Catal. A-Gen., 2005, 291, 222—229 |
| [1] | 何鸿锐, 夏文生, 张庆红, 万惠霖. 羟基氧化铟团簇与二氧化碳和甲烷作用的密度泛函理论研究[J]. 高等学校化学学报, 2022, 43(8): 20220196. |
| [2] | 姜宏斌, 代文臣, 张娆, 徐晓晨, 陈捷, 杨光, 杨凤林. Co3O4/UiO-66@α-Al2O3陶瓷膜对VOCs废气的分离催化性能[J]. 高等学校化学学报, 2022, 43(6): 20220025. |
| [3] | 戴卫, 侯华, 王宝山. 七氟异丁腈负离子结构与反应活性的理论研究[J]. 高等学校化学学报, 2022, 43(6): 20220044. |
| [4] | 郝宏蕾, 孟繁雨, 李若钰, 李迎秋, 贾明君, 张文祥, 袁晓玲. 生物质基氮掺杂多孔炭材料的制备及对水中亚甲基蓝的吸附性能[J]. 高等学校化学学报, 2022, 43(6): 20220055. |
| [5] | 黄汉浩, 卢湫阳, 孙明子, 黄勃龙. 石墨炔原子催化剂的崭新道路:基于自验证机器学习方法的筛选策略[J]. 高等学校化学学报, 2022, 43(5): 20220042. |
| [6] | 王红宁, 黄丽, 清江, 马腾洲, 蒋伟, 黄维秋, 陈若愚. 香蒲基生物炭的活化及对VOCs吸附的应用[J]. 高等学校化学学报, 2022, 43(4): 20210824. |
| [7] | 孟祥龙, 杨歌, 郭海玲, 刘晨光, 柴永明, 王纯正, 郭永梅. 纳米分子筛的合成及硫化氢吸附性能[J]. 高等学校化学学报, 2022, 43(3): 20210687. |
| [8] | 陈潇禄, 袁珍闫, 仲迎春, 任浩. 机械球磨制备三苯胺基PAF-106s及C2烃吸附性质[J]. 高等学校化学学报, 2022, 43(3): 20210771. |
| [9] | 靳科研, 白璞, 李小龙, 张佳楠, 闫文付. 新型Mg-Al吸附剂去除压水堆核电厂废水中高浓度硼[J]. 高等学校化学学报, 2022, 43(2): 20210516. |
| [10] | 谭乐见, 仲宣树, 王锦, 刘宗建, 张爱英, 叶霖, 冯增国. β-环糊精的低临界溶解温度现象及其在有序纳米孔道片晶制备中的应用[J]. 高等学校化学学报, 2022, 43(11): 20220405. |
| [11] | 刘洋, 李旺昌, 张竹霞, 王芳, 杨文静, 郭臻, 崔鹏. Sc3C2@C80与[12]CPP纳米环之间非共价相互作用的理论研究[J]. 高等学校化学学报, 2022, 43(11): 20220457. |
| [12] | 郑美琪, 毛方琪, 孔祥贵, 段雪. 类水滑石材料在核废水处理领域的应用[J]. 高等学校化学学报, 2022, 43(10): 20220456. |
| [13] | 王园月, 安梭梭, 郑旭明, 赵彦英. 5-巯基-1, 3, 4-噻二唑-2-硫酮微溶剂团簇的光谱和理论计算研究[J]. 高等学校化学学报, 2022, 43(10): 20220354. |
| [14] | 田晓康, 张青松, 杨舒淋, 白洁, 陈冰洁, 潘杰, 陈莉, 危岩. 微生物发酵诱导多孔材料: 制备方法和应用[J]. 高等学校化学学报, 2022, 43(10): 20220216. |
| [15] | 程媛媛, 郗碧莹. ·OH自由基引发CH3SSC |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||