

Chem. J. Chinese Universities ›› 2020, Vol. 41 ›› Issue (1): 111.doi: 10.7503/cjcu20190311
• Analytical Chemistry • Previous Articles Next Articles
WANG Lianping1,LI Qingjie2,LIU Xiaoyan3,REN Yueying1,*( ),YANG Xiuwei3,*(
),YANG Xiuwei3,*( )
)
Received:2019-05-30
Online:2020-01-10
Published:2019-11-26
Contact:
Yueying REN,Xiuwei YANG
E-mail:381717169@qq.com;xwyang@bjmu.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Lianping,LI Qingjie,LIU Xiaoyan,REN Yueying,YANG Xiuwei. Screening of Cholinesterase Inhibitors in Fructus Evodiae Alkaloids Based on UFLC-MS/molecular Simulation †[J]. Chem. J. Chinese Universities, 2020, 41(1): 111.
| Compound | Precursor, m/z | Product, m/z | Dwell time/ms | Q1/V | CE/V | Q3/V |
|---|---|---|---|---|---|---|
| Dehydroevodiamine | 302.05 | 286.00 | 40.5 | -12.0 | -34.0 | -30.0 |
| 302.05 | 287.05 | 40.5 | -12.0 | -17.0 | -30.0 | |
| Evocarpine | 340.10 | 173.10 | 26.0 | -27.0 | -21.0 | -28.0 |
| 340.10 | 186.10 | 26.0 | -27.0 | -11.0 | -23.0 | |
| Rutaecarpine | 288.05 | 273.10 | 26.0 | -12.0 | -32.0 | -29.0 |
| 288.05 | 115.05 | 26.0 | -12.0 | -55.0 | -20.0 | |
| Wuchuyuamide Ⅰ | 352.10 | 158.10 | 26.0 | -27.0 | -21.0 | -28.0 |
| 352.10 | 334.05 | 26.0 | -27.0 | -11.0 | -23.0 | |
| Dihydroevocarpine | 342.05 | 173.10 | 40.5 | -12.0 | -23.0 | -25.0 |
| 342.05 | 186.10 | 40.5 | -12.0 | -21.0 | -16.0 | |
| Evodiamine | 304.10 | 134.00 | 40.5 | -12.0 | -23.0 | -25.0 |
| 304.10 | 161.00 | 40.5 | -12.0 | -21.0 | -16.0 | |
| 1-Methyl-2-nonyl-4(1H)-quinolone | 286.05 | 186.10 | 40.5 | -30.0 | -38.0 | -19.0 |
| 286.05 | 131.05 | 40.5 | -30.0 | -49.0 | -23.0 | |
| 1-Methyl-2-[Z-6-undecenyl]-4(H)-quinolone | 312.20 | 186.10 | 26.0 | -24.0 | -37.0 | -19.0 |
| 312.20 | 173.05 | 26.0 | -24.0 | -34.0 | -18.0 | |
| 1-Methyl-2-pentadecadienyl-4(H)-quinolone | 366.00 | 186.10 | 26.0 | -30.0 | -41.0 | -19.0 |
| 366.00 | 173.10 | 26.0 | -30.0 | -37.0 | -17.0 | |
| 1-Methyl-2-pentadeceny-4(H)-quinolone | 368.30 | 186.10 | 26.0 | -25.0 | -37.0 | -18.0 |
| 368.30 | 173.10 | 26.0 | -25.0 | -42.0 | -19.0 |
| Compound | Precursor, m/z | Product, m/z | Dwell time/ms | Q1/V | CE/V | Q3/V |
|---|---|---|---|---|---|---|
| Dehydroevodiamine | 302.05 | 286.00 | 40.5 | -12.0 | -34.0 | -30.0 |
| 302.05 | 287.05 | 40.5 | -12.0 | -17.0 | -30.0 | |
| Evocarpine | 340.10 | 173.10 | 26.0 | -27.0 | -21.0 | -28.0 |
| 340.10 | 186.10 | 26.0 | -27.0 | -11.0 | -23.0 | |
| Rutaecarpine | 288.05 | 273.10 | 26.0 | -12.0 | -32.0 | -29.0 |
| 288.05 | 115.05 | 26.0 | -12.0 | -55.0 | -20.0 | |
| Wuchuyuamide Ⅰ | 352.10 | 158.10 | 26.0 | -27.0 | -21.0 | -28.0 |
| 352.10 | 334.05 | 26.0 | -27.0 | -11.0 | -23.0 | |
| Dihydroevocarpine | 342.05 | 173.10 | 40.5 | -12.0 | -23.0 | -25.0 |
| 342.05 | 186.10 | 40.5 | -12.0 | -21.0 | -16.0 | |
| Evodiamine | 304.10 | 134.00 | 40.5 | -12.0 | -23.0 | -25.0 |
| 304.10 | 161.00 | 40.5 | -12.0 | -21.0 | -16.0 | |
| 1-Methyl-2-nonyl-4(1H)-quinolone | 286.05 | 186.10 | 40.5 | -30.0 | -38.0 | -19.0 |
| 286.05 | 131.05 | 40.5 | -30.0 | -49.0 | -23.0 | |
| 1-Methyl-2-[Z-6-undecenyl]-4(H)-quinolone | 312.20 | 186.10 | 26.0 | -24.0 | -37.0 | -19.0 |
| 312.20 | 173.05 | 26.0 | -24.0 | -34.0 | -18.0 | |
| 1-Methyl-2-pentadecadienyl-4(H)-quinolone | 366.00 | 186.10 | 26.0 | -30.0 | -41.0 | -19.0 |
| 366.00 | 173.10 | 26.0 | -30.0 | -37.0 | -17.0 | |
| 1-Methyl-2-pentadeceny-4(H)-quinolone | 368.30 | 186.10 | 26.0 | -25.0 | -37.0 | -18.0 |
| 368.30 | 173.10 | 26.0 | -25.0 | -42.0 | -19.0 |
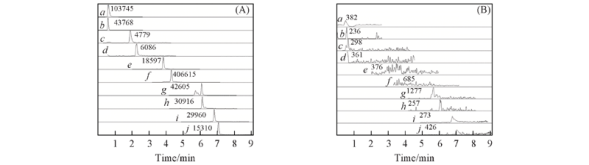
Fig.2 Specificity of 10 compounds in fructus evodiae (A) Blank plasma samples added the standard compounds; (B) blank plasma samples. a—j. dehydroevodiamine, wuchuyuamide Ⅰ, evodiamine, rutaecarpine, 1-methyl-2-nonyl-4(1H)-quinolone, 1-methyl-2-[Z-6-undecenyl]-4(H)-quinolone, evocarpine, 1-methyl-2-pentadecadienyl-4(H)-quinolone, dihydroevocarpine, 1-methyl-2-pentadeceny-4(H)-quinolone, respectively.
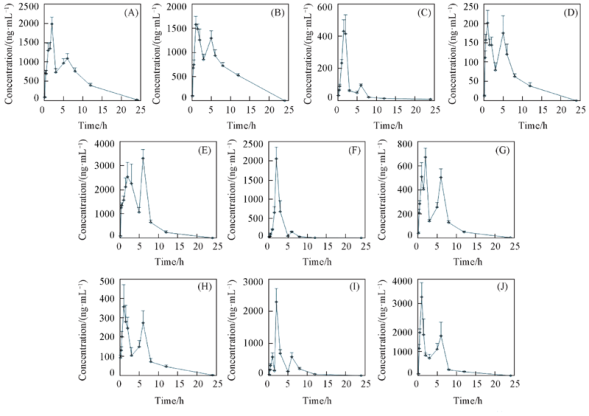
Fig.3 Blood concentration-time curves of 10 compounds (A) Dehydroevodiamine; (B) evocarpine; (C) rutaecarpine; (D) wuchuyuamide Ⅰ; (E) dihydroevocarpine; (F) evodiamine; (G) 1-methyl-2-nonyl-4(1H)-quinolone; (H) 1-methyl-2-[Z-6-undecenyl]-4-(H)-quinolone; (I) 1-methyl-2-pentadecadinenyl-4(H)-quinolone; (J) 1-methyl-2-pentadecenyl-4(H)-quinolone.
| [1] | Chinese Pharmacopoeia Commission, Chinese Pharmacopoeia , Part 1, the Medicine Science and Technology Press of China, Beijing, 2015, 171— 172 |
| ( 中华人民共和国国家药典委员会. 中华人民共和国药典, 一部, 北京: 中国医药科技出版社, 2015, 171— 172) | |
| [2] | Gao X. M. , Chinese Materia Medica, China Press of Traditional Chinese Medicine, Beijing, 2002, 278— 279 |
| ( 高学敏. 中药学, 北京: 中国中医药出版社, 2002, 278— 279) | |
| [3] | Han H. W., Cao X. M.,Peng H.,Zhang Z. R., Chinese J. Basic Med. Tradit. Chin. Med., 2012,18(8), 898— 899 |
| ( 韩红伟, 曹秀梅, 彭红, 张尊如 . 中国中医基础医学杂志, 2012,18(8), 898— 899) | |
| [4] | Lin B. X., Cai L. R.,Huang G. W., Clin. J. Tradit. Chin. Med., 2018,30(7), 1340— 1342 |
| ( 林宝惜, 蔡玲端, 黄冠伟. 中医药临床杂志, 2018,30(7), 1340— 1342) | |
| [5] | Liu X. T., J. Practi. Tradit. Chin. Med, 2018,34(10), 1261— 1262 |
| ( 刘显涛. 实用中医药杂志, 2018,34(10), 1261— 1262) | |
| [6] | Lou L. L., Xu S. S.,Fang D. S., Chinese J. Traditional Chinese Medicine, 2017,35(8), 2154— 2157 |
| ( 楼璐璐, 徐姗姗, 方淡思. 中华中医药学刊, 2017,35(8), 2154— 2157) | |
| [7] | Guo Y. R., Lin M. L., Lin M. Z., Huang P. F., Guangming J. Chinese Medicine, 2018,33(1), 147— 149 |
| ( 郭月蓉, 林美兰, 林明珠, 黄培芳. 光明中医, 2018,33(1), 147— 149) | |
| [8] | Liu S. S., Geng Y. J., Xia Y. Y., Liu J. Y., Wei G. L., Si D. Y., Acta Pharmaceutica Sinica, 2019,54(4), 695— 700 |
| ( 刘珊珊, 耿雅杰, 夏媛媛, 刘静媛, 魏广力, 司端运. 药学学报, 2019,54(4), 695— 700) | |
| [9] |
Kryger G., Silman I., Sussman J. L ., . Structure, 1999,7(3), 297— 307
doi: 10.1016/s0969-2126(99)80040-9 URL pmid: 10368299 |
| [10] | Yang X. Y., Zhao T. T.,Dong K. K.,Zhu X. L., J. Nanjing Normal Univrsity(Natural Science Edition), 2017,40(3), 123— 127 |
| ( 杨雪雨, 赵腾腾, 董珂珂, 朱小蕾. 南京师大学报(自然科学版), 2017,40(3), 123— 127) | |
| [11] | Trott O., Olson A. J ., J. Computat. Chem., 2010,31(2), 455— 461 |
| [12] | Diaz-Rubio L., Hernandez-Martinez R., Estolano-Cobian A ., Applied. Sciences-Basel, 2019,9(3), 410— 429 |
| [13] |
Salentin S., Schreiber S., Haupt V. J ., . Nucleic Acids Research, 2015,43(W1), W443— W447
doi: 10.1093/nar/gkv315 URL pmid: 25873628 |
| [14] |
Nicolet Y., Lockridge O., Masson P ., J. Biolog. Chem., 2003,278(42), 41141— 41147
doi: 10.1074/jbc.M210241200 URL pmid: 12869558 |
| [15] |
Chen X. Y., Zhan Y.,Zhong D. F., Chinese Pharmaceutical J., 2014,49(13), 1176— 1180
doi: 10.11669/cpj.2014.13.020 URL |
|
( 陈笑艳, 詹燕, 钟大放. 中国药学杂志, 2014,49(13), 1176— 1180)
doi: 10.11669/cpj.2014.13.020 URL |
|
| [16] | Zhang J. P., Guo C., China Pharmacy, 2008,19(31), 2469— 2471 |
| ( 张剑萍, 郭澄. 中国药房, 2008,19(31), 2469— 2471) | |
| [17] | Wang Y. P., Fu T.,Xu J. B.,Li J.,Wang Z. C., Journal of Hunan University of Science and Engineering, 2015,36(5), 64— 69 |
| ( 王玉平, 付桃, 徐巾卜, 李佳, 王宗成. 湖南科技学院学报, 2015,36(5), 64— 69) | |
| [18] | Yang D. Q., Yang X., Modern Agrochemicals, 2015,14(2), 14— 18 |
| ( 杨德秋, 杨潇 . 现代农药, 2015,14(2), 14— 18) | |
| [19] | Deng P. Y., Wang H.,Yuan H. Z.,Wang G. J.,Li C. K., Pharmaceutical J. Chinese People’s Liberation Army, 2017,33(6), 538— 541 |
| ( 邓培渊, 王辉, 袁洪哲, 王广军, 李长看. 解放军药学学报, 2017,33(6), 538— 541) | |
| [20] | Yang X. Y., Wang X.,Dong K. K.,Zhu X. L., J. Nanjing Normal University(Natural Science Edition), 2017,40(2), 144— 148 |
| ( 杨雪雨, 王璇, 董珂珂, 朱小蕾. 南京师大学报(自然科学版), 2017,40(2), 144— 148) | |
| [21] | Wang Y. J., Zhao D. X.,Yang Z. D., J. Mole. Sci., 2012,28(2), 115— 122 |
| ( 王永杰, 赵东霞, 杨忠志 . 分子科学学报, 2012,28(2), 115— 122) | |
| [22] | Wu H., Li X. D., Peng D. T.,Jiao J. S.,Li Y. F.,Yu P. L.,Ji Y., Chin J. Contemp Neurol Neurosurg, 2017,17(12), 933— 936 |
| ( 吴昊, 李旭东, 彭丹涛, 焦劲松, 李延峰, 于普林, 纪勇. 中国现代神经疾病杂志, 2017,17(12), 933— 936) | |
| [23] | Meng X. Y., Chu H. Y.,Zheng Q. C., Sciencepaper Online, 2009,4(9), 627— 631 |
| ( 孟烜宇, 楚慧郢, 郑清川 . 中国科技论文在线, 2009,4(9), 627— 631) |
| [1] | LEI Xiaotong, JIN Yiqing, MENG Xuanyu. Prediction of the Binding Site of PIP2 in the TREK-1 Channel Based on Molecular Modeling [J]. Chem. J. Chinese Universities, 2021, 42(8): 2550. |
| [2] | SHUAI Die, ZHAO Meijuan, CHEN Bingnian, WANG Li. Inhibitory Effect of Four Kinds of Keegin-type Phosphomolybdate on Tyrosinase and Melanin Formation and Its Antioxidant Activities [J]. Chem. J. Chinese Universities, 2021, 42(12): 3579. |
| [3] | YANG Ju, SU Lijiao, LI Canhua, LU Jiajia, YANG Junli, GU Jie, YANG Li, YANG Lijuan. Host-guest Complexation Behavior of Nardosinone and Water-soluble Phosphate Salt Pillar[6]arene [J]. Chem. J. Chinese Universities, 2021, 42(10): 3099. |
| [4] | ZHANG Aiqin, WANG Man, SHEN Gangyi, JIN Jun. Interactions Between Polybrominated Diphenyl Ethers and Human Serum Albumin Using SPR and Molecular Docking [J]. Chem. J. Chinese Universities, 2020, 41(9): 2054. |
| [5] | WANG Xiaoxia, MA Litong, NIE Zhihua, WANG Zhengde, CUI Jinlong, ZHAO Wenyuan, SAI Huazheng. Interaction Between Fulvic Acid and Pepsin Investigated by Multispectral and Molecular Docking Simulation † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1840. |
| [6] | LIU Zhongcheng, LIU Shifang, ZHANG Su, YANG Yanlei, LI Fei, ZHANG Nan, YUAN Xin, ZHANG Yanfen. Structure Prediction and Screening of Oligonucleotide Aptamers Target Cε3-Cε4 Protein† [J]. Chem. J. Chinese Universities, 2019, 40(1): 83. |
| [7] | XIN Meiling, CHU Zhenhua, LI Yu. Molecular Modification of Polychlorinated Biphenyl Dihydroxy Derivatives Through Molecular Docking Associated with CoMSIA/HQSAR Models† [J]. Chem. J. Chinese Universities, 2018, 39(2): 299. |
| [8] | WANG Yan, CHEN Ping, WANG Yunfei, LIU Guiying, YANG Xi, SU Ying, LI Junyang, LIU Weiwei, LIN Lie. Spectral Characterization of the Interaction Between Methamphetamine and Serum Albumin† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2507. |
| [9] | DUAN Yongbin, YIN Yan, MENG Fanli, ZHAO Lianhua, LIU Yukun, YUAN Zhe, FENG Yangbo. Design, Synthesis and Biological Evaluation of Benzothiazoles as Highly Potent ROCK Inhibitors Through Molecular Docking and Free Energy Calculations† [J]. Chem. J. Chinese Universities, 2017, 38(9): 1568. |
| [10] | WANG Song, GUAN Shanshan, WAN Yongfeng, SHAN Yaming, ZHANG Hao. Molecular Dynamics Simulation Study on the Binding Modes of Angiotensin-converting Enzyme with Inhibitory Peptides† [J]. Chem. J. Chinese Universities, 2017, 38(7): 1216. |
| [11] | TANG Qian, SU Jinhong, CAO Hongyu, WANG Lihao, SHI Fei, WANG Ailing, GONG Tingting, JIN Xiaojun, ZHENG Xuefang. Interaction of Pyrimidine Derivatives with Human Serum Albumin† [J]. Chem. J. Chinese Universities, 2017, 38(11): 1982. |
| [12] | ZHAO Bin, HUO Jingqian, XING Jihong, QI Meng, ZHANG Jinlin, DONG Jingao. Homologous Modeling of Transketolase AtTKL1 and Its Combination with α-Terthienyl in Arabidopsis Thaliana† [J]. Chem. J. Chinese Universities, 2015, 36(4): 682. |
| [13] | DONG Lu, YI Zhongsheng, WU Zhiwei, WANG Haiyang, ZHANG Aiqian. Mechanism Study on the Interaction Between 2'-Hydroxy-2,4-dibromo Diphenyl Ethers and Human Serum Albumin Based on Spectroscopic Methods and Computional Simulations† [J]. Chem. J. Chinese Universities, 2015, 36(3): 516. |
| [14] | ZHANG Min, ZHANG Yi, LI Chengtao, QIN Jiaxiang, Gao Rang, MA Xiaoning, QIU Jianhui. N435 Enzymatic Degradation Difference and Molecular Modeling of Hydrophilic Modified PBS-based Copolymer† [J]. Chem. J. Chinese Universities, 2015, 36(3): 568. |
| [15] | CHEN Linfeng, ZHANG Jing, ZHU Yaxian, ZHANG Yong. Molecular Interactions of 1-Hydroxypyrene with Catalase Revealed by Spectroscopic Methods Combined with Molecular Docking† [J]. Chem. J. Chinese Universities, 2015, 36(12): 2394. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||