

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (3): 516.doi: 10.7503/cjcu20141007
• Physical Chemistry • Previous Articles Next Articles
DONG Lu1, YI Zhongsheng1,*( ), WU Zhiwei1, WANG Haiyang1, ZHANG Aiqian2
), WU Zhiwei1, WANG Haiyang1, ZHANG Aiqian2
Received:2014-11-17
Online:2015-03-10
Published:2015-01-30
Contact:
YI Zhongsheng
E-mail:yzs@glut.edu.cn
Supported by:CLC Number:
TrendMD:
DONG Lu, YI Zhongsheng, WU Zhiwei, WANG Haiyang, ZHANG Aiqian. Mechanism Study on the Interaction Between 2'-Hydroxy-2,4-dibromo Diphenyl Ethers and Human Serum Albumin Based on Spectroscopic Methods and Computional Simulations†[J]. Chem. J. Chinese Universities, 2015, 36(3): 516.
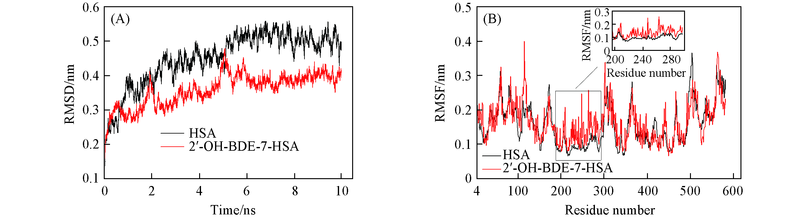
Fig.1 Root-mean-square displacement(RMSD)(A)and root mean square fluctuation(RMSF)(B) of free HSA and 2'-OH -BDE-7-HSA complexes from 10 ns of MD simulations
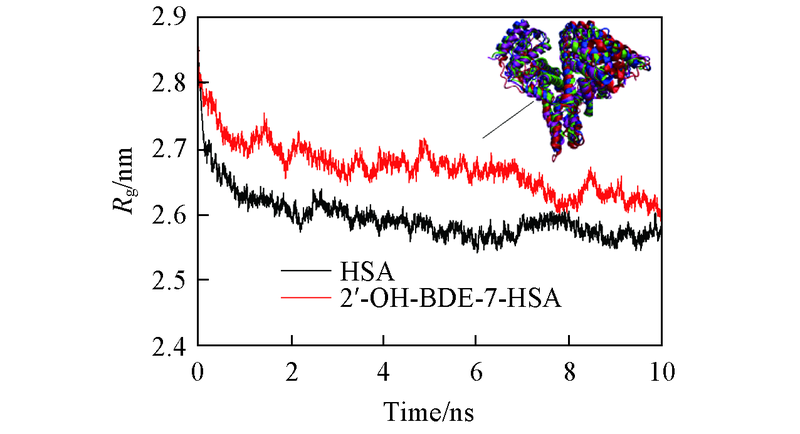
Fig.2 Rg of free HSA and 2'-OH-BDE-7-HSA complexes structural superposition of HSA at specific time from 10 ns MD MD time/ns: 0(red); 3(purple); 7(blue); 10(green).
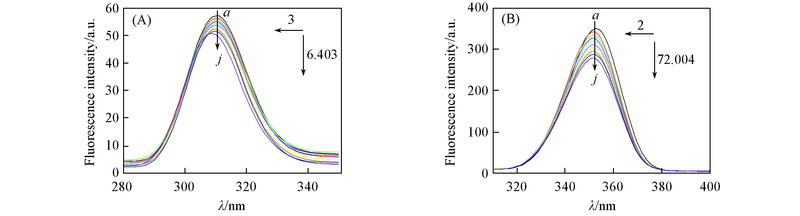
Fig.4 Synchronous fluorescence spectra of 2'-OH-BDE-7-HSA (A) Δλ=15 nm; (B) Δλ=60 nm. cHSA=1.0 × 10-6 mol/L; 109c2'-OH-BDE-7/(mol·L-1): a—j. 0—9. Left arrow: blue shift; the maximum absorption wavelength move to short wavelength; number value: blue shift value; down arrow: intensity of quenching.
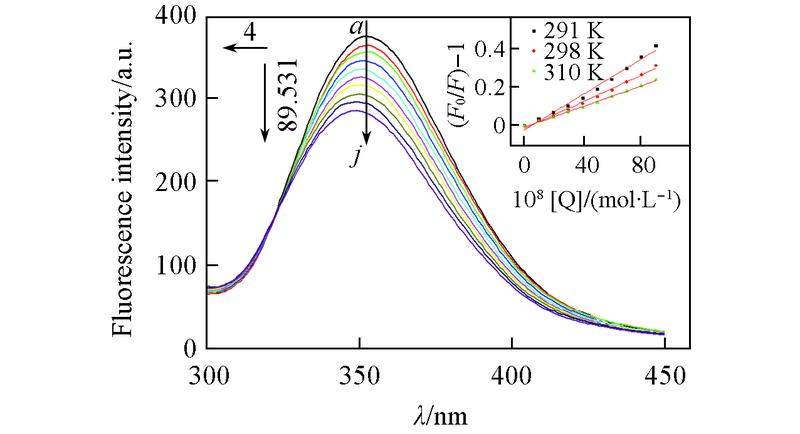
Fig.5 Fluorescence spectra of HSA in the presence of 2'-OH-BDE-7 Inset: Stern-Vomer curves at different temperatures.cHSA=1.0 × 10-6 mol/L; 109c2'-OH-BDE-7/(mol·L-1): a—j. 0—9. Left arrow: blue shift; the maximum absorption wavelength move to short wavelength; number value: blue shift value; down arrow: intensity of quenching.
| T/K | 106KSV/(L·mol-1) | 10-14Kq/(L·mol-1·s-1) | r |
|---|---|---|---|
| 291 | 4.58 | 4.58 | 0.9976 |
| 298 | 3.44 | 3.44 | 0.9970 |
| 310 | 2.71 | 2.71 | 0.9989 |
Table 1 Quenching constant of 2'-OH-BDE-7-HSA interaction at different temperatures
| T/K | 106KSV/(L·mol-1) | 10-14Kq/(L·mol-1·s-1) | r |
|---|---|---|---|
| 291 | 4.58 | 4.58 | 0.9976 |
| 298 | 3.44 | 3.44 | 0.9970 |
| 310 | 2.71 | 2.71 | 0.9989 |
| Site | -lgKd | ΔG/(kJ·mol-1) | Hydrogen bond |
|---|---|---|---|
| Ⅰ | 3.6267 | -20.71 | Ala291 |
| Ⅱ | 3.4474 | -19.68 |
Table 2 Docking results of Surflex-Dock at sites I and Ⅱ
| Site | -lgKd | ΔG/(kJ·mol-1) | Hydrogen bond |
|---|---|---|---|
| Ⅰ | 3.6267 | -20.71 | Ala291 |
| Ⅱ | 3.4474 | -19.68 |

Fig.7 Molecular docking and detailed view of the interactions of 2'-OH-BDE-7 and HSA (A) 2'-OH-BDE-7 located at site Ⅰ of HSA; (B) HSA amino acids residues around 0.5 nm of 2'-OH-BDE-7; (C) electrostatic potential around 2'-OH-BDE-7; (D) combine distance between Trp214 and 2'-OH-BDE-7, r=0.427 nm.
| T/K | 107Ka/(L·mol-1) | n | r |
|---|---|---|---|
| 291 | 2.57 | 1.11 | 0.9959 |
| 298 | 1.98 | 1.11 | 0.9982 |
| 310 | 0.83 | 1.07 | 0.9994 |
Table 3 Binding constants(Ka), number of binding sites(n) of 2'-OH-BDE-7-HSA system at different temperatures
| T/K | 107Ka/(L·mol-1) | n | r |
|---|---|---|---|
| 291 | 2.57 | 1.11 | 0.9959 |
| 298 | 1.98 | 1.11 | 0.9982 |
| 310 | 0.83 | 1.07 | 0.9994 |
| T/K | ΔH/(kJ·mol-1) | ΔG/(kJ·mol-1) | ΔS/(J·mol-1·K-1) |
|---|---|---|---|
| 291 | -41.44 | ||
| 298 | -45.79 | -41.34 | -14.92 |
| 310 | -41.16 |
Table 4 Thermodynamic parameters of 2'-OH-BDE-7-HSA system at different temperatures
| T/K | ΔH/(kJ·mol-1) | ΔG/(kJ·mol-1) | ΔS/(J·mol-1·K-1) |
|---|---|---|---|
| 291 | -41.44 | ||
| 298 | -45.79 | -41.34 | -14.92 |
| 310 | -41.16 |
| Compound | 2'-OH-BDE-7-HSA | +Ibuprofen | +Warfarin |
|---|---|---|---|
| 106Ka /(L·mol-1) | 9.90 | 9.23 | 3.67 |
Table 5 Binding constant of experiments of 2'-OH-BDE-7-HSA system(T=298 K)
| Compound | 2'-OH-BDE-7-HSA | +Ibuprofen | +Warfarin |
|---|---|---|---|
| 106Ka /(L·mol-1) | 9.90 | 9.23 | 3.67 |
| [1] | Athanasiadou M., Marsh G., Athanassiadis I., Asplund L., Bergman Å., J. Mass Spectrom., 2006, 41(6), 790—801 |
| [2] | Cantón R. F., Scholten D. E., Marsh G., de Jong P. C., van den Berg M., Toxicol Appl. Pharm., 2008, 227(1), 68—75 |
| [3] | Qiu X., Bigsby R. M., Hites R. A., Environ. Health Persp., 2009, 117(1), 93—98 |
| [4] | Rahman F., Langford K. H., Scrimshaw M. D., Lester J. N., Sci. Total Environt., 2001, 275(1), 1—17 |
| [5] | Sun J., Liu J., Liu Q., Ruan T., Yu M., Wang Y., Wang T., Jiang G., Chemosphere., 2013, 90(9), 2388—2395 |
| [6] | Zhang Y., Fu S., Liu X., Li Z., Dong Y., J. Environ. Sci., 2013, 25(12), 2443—2450 |
| [7] | Zhang L., Li J., Zhao Y., Li X., Wen S., Shen H., Wu Y., J. Arg. Food Chem., 2013, 61(26), 6544—6551 |
| [8] | Lian Y. Q., Chen X. G., Cai Z. W., Environ Chem., 2011, 30(1), 346—350 |
| (赖永权, 陈学国, 蔡宗苇. 环境化学, 2011, 30(1), 346—350) | |
| [9] | Sudlow G., Birkett D., Wade D., Mol. Pharmacol., 1975, 11(6), 824—832 |
| [10] | Gumbart J., Wang Y., Aksimentiev A., Tajkhorshid E., Schulten K., Curr. Opini. Struc. Biol., 2005, 15(4), 423—431 |
| [11] | Fani N., Bordbar A. K., Ghayeb Y., Spectrochim Acta A,2013, 103, 11—17 |
| [12] | Shahabadi N., Khorshidi A., Moghadam N. H., Spectrochim Acta A,2013, 114, 627—632 |
| [13] | Yao Q., Yu X., Zheng T., Liu H., Yang Y., Yi P., Spectrochim Acta A,2013, 113, 447—451 |
| [14] | Neamtu S., Tosa N., Bogdan M., J. Pharmaceut Biomed., 2013, 85, 277—282 |
| [15] | Wang M. Y., Zhang C., Li J., Li C. X, Gong M. X., Chem. J. Chinese Universities,2014, 35(2), 309—313 |
| (王满元, 张超, 李静, 李朝霞, 龚慕辛. 高等学校化学学报, 2014, 35(2), 309—313) | |
| [16] | Xu J.G., Wang B. Z., Fluorescence Analysis, 3rd Ed., Science Press, Beijing, 2006, 64—79 |
| (许金钩, 王尊本. 荧光分析法, 第3版, 北京, 科学出版社, 2006, 64—79) | |
| [17] | Ghalandari B., Divsalar A., Saboury A. A., Haertlé T., Parivar K., Bazl R., Eslami M. M., Amanlou M., Spectrochim Acta A,2014, 118, 1038—1046 |
| [18] | Tabassum S., Al-Asbahy W. M., Afzal M., Arjmand F., J. Photoch. Photobio. B,2012, 114, 132—139 |
| [19] | Fani N., Bordbar A. K., Ghayeb Y., J. Lumin., 2013, 141, 166—172 |
| [20] | Sun Y. T., Zhang Y. P., Bi S. Y., Sun Y., Liu H., Zhai Y. J, Zhang H. Q., Chem. J. Chinese Universities,2009, 30(6), 1095—1100 |
| (孙艳涛, 张玉璞, 毕淑云, 孙晔, 刘贺, 翟玉娟, 张寒琦. 高等学校化学学报, 2009, 30(6), 1095—1100) | |
| [21] | Ye Z. W., Ying Y., Yang X. L., Zheng Z. Q., Shi J. N., Sun Y. F., Huang P., J. Incl. Phenom. Macrocycl. Chem., 2014, 78, 405—413 |
| [1] | GAO Zhiwei, LI Junwei, SHI Sai, FU Qiang, JIA Junru, AN Hailong. Analysis of Gating Characteristics of TRPM8 Channel Based on Molecular Dynamics [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220080. |
| [2] | HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614. |
| [3] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [4] | LI Congcong, LIU Minghao, HAN Jiarui, ZHU Jingxuan, HAN Weiwei, LI Wannan. Theoretical Study of the Catalytic Activity of VmoLac Non-specific Substrates Based on Molecular Dynamics Simulations [J]. Chem. J. Chinese Universities, 2021, 42(8): 2518. |
| [5] | LEI Xiaotong, JIN Yiqing, MENG Xuanyu. Prediction of the Binding Site of PIP2 in the TREK-1 Channel Based on Molecular Modeling [J]. Chem. J. Chinese Universities, 2021, 42(8): 2550. |
| [6] | ZENG Yonghui, YAN Tianying. Vibrational Density of States Analysis of Proton Hydration Structure [J]. Chem. J. Chinese Universities, 2021, 42(6): 1855. |
| [7] | LI Mengshuo, ZHANG Jing, LIU Dan, ZHU Yaxian, ZHANG Yong. Interactions of Pyrene with Human Serum Albumin and Bovine Serum Albumin: Microenvironmental Polarity Differences at Binding Sites [J]. Chem. J. Chinese Universities, 2021, 42(3): 731. |
| [8] | LIU Aiqing, XU Wensheng, XU Xiaolei, CHEN Jizhong, AN Lijia. Molecular Dynamics Simulation of Polymer/rod Nanocomposite [J]. Chem. J. Chinese Universities, 2021, 42(3): 875. |
| [9] | QI Renrui, LI Minghao, CHANG Hao, FU Xueqi, GAO Bo, HAN Weiwei, HAN Lu, LI Wannan. Theoretical Study on the Unbinding Pathway of Xanthine Oxidase Inhibitors Based on Steered Molecular Dynamics Simulation [J]. Chem. J. Chinese Universities, 2021, 42(3): 758. |
| [10] | SHUAI Die, ZHAO Meijuan, CHEN Bingnian, WANG Li. Inhibitory Effect of Four Kinds of Keegin-type Phosphomolybdate on Tyrosinase and Melanin Formation and Its Antioxidant Activities [J]. Chem. J. Chinese Universities, 2021, 42(12): 3579. |
| [11] | YANG Ju, SU Lijiao, LI Canhua, LU Jiajia, YANG Junli, GU Jie, YANG Li, YANG Lijuan. Host-guest Complexation Behavior of Nardosinone and Water-soluble Phosphate Salt Pillar[6]arene [J]. Chem. J. Chinese Universities, 2021, 42(10): 3099. |
| [12] | ZHANG Aiqin, WANG Man, SHEN Gangyi, JIN Jun. Interactions Between Polybrominated Diphenyl Ethers and Human Serum Albumin Using SPR and Molecular Docking [J]. Chem. J. Chinese Universities, 2020, 41(9): 2054. |
| [13] | MA Xiangying, LIAO Yanjun, QIN Fanghong, YIN Yuanhao, HUANG Zaiyin, CHEN Qifeng. Study on the Photocatalytic Performance of Carbon Doped g-C3N4 Based on in situ Photomicrocalorimeter-fluorescence Spectrometry [J]. Chem. J. Chinese Universities, 2020, 41(11): 2526. |
| [14] | WANG Lianping,LI Qingjie,LIU Xiaoyan,REN Yueying,YANG Xiuwei. Screening of Cholinesterase Inhibitors in Fructus Evodiae Alkaloids Based on UFLC-MS/molecular Simulation † [J]. Chem. J. Chinese Universities, 2020, 41(1): 111. |
| [15] | QU Siying, XU Qin. Different Roles of Some Key Residues in the S4 Pocket of Coagulation Factor Xa for Rivaroxaban Binding † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1918. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||