

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (11): 1982.doi: 10.7503/cjcu20170063
• Organic Chemistry • Previous Articles Next Articles
TANG Qian1,2,*( ), SU Jinhong2,3, CAO Hongyu1,2, WANG Lihao2,3, SHI Fei2,3, WANG Ailing2,3, GONG Tingting1, JIN Xiaojun2,3, ZHENG Xuefang2,*(
), SU Jinhong2,3, CAO Hongyu1,2, WANG Lihao2,3, SHI Fei2,3, WANG Ailing2,3, GONG Tingting1, JIN Xiaojun2,3, ZHENG Xuefang2,*( )
)
Received:2017-01-26
Online:2017-11-10
Published:2017-10-30
Contact:
TANG Qian,ZHENG Xuefang
E-mail:tangqian@dlu.edu.cn;dlxfzheng@126.com
Supported by:CLC Number:
TrendMD:
TANG Qian, SU Jinhong, CAO Hongyu, WANG Lihao, SHI Fei, WANG Ailing, GONG Tingting, JIN Xiaojun, ZHENG Xuefang. Interaction of Pyrimidine Derivatives with Human Serum Albumin†[J]. Chem. J. Chinese Universities, 2017, 38(11): 1982.
| PDs | IC50/(μmol·L-1) | PDs | IC50/(μmol·L-1) | ||
|---|---|---|---|---|---|
| HCC(7721) | MC(A375) | HCC(7721) | MC(A375) | ||
| A | 23.65 | 9.42 | E | 166.90 | 28.94 |
| B | 88.05 | 81.37 | F | 22.43 | 12.85 |
| C | 9.53 | 17.86 | G | 23.57 | 27.28 |
| D | 16.66 | 34.76 | H | 12.22 | 48.19 |
Table 1 IC50 values of the pyrimidine derivatives against tumor cells
| PDs | IC50/(μmol·L-1) | PDs | IC50/(μmol·L-1) | ||
|---|---|---|---|---|---|
| HCC(7721) | MC(A375) | HCC(7721) | MC(A375) | ||
| A | 23.65 | 9.42 | E | 166.90 | 28.94 |
| B | 88.05 | 81.37 | F | 22.43 | 12.85 |
| C | 9.53 | 17.86 | G | 23.57 | 27.28 |
| D | 16.66 | 34.76 | H | 12.22 | 48.19 |
| PDs | T/K | KSV/ (L·mol-1) | Kq/ (L·mol-1·s-1) | Quenching mechanism | PDs | T/K | KSV/ (L·mol-1) | Kq/ (L·mol-1·s-1) | Quenching mechanism |
|---|---|---|---|---|---|---|---|---|---|
| A | 300 | 3.206×104 | 3.20×1012 | Static quenching | E | 300 | 3.32×103 | 3.32×1011 | Static quenching |
| 310 | 3.17×104 | 3.17×1012 | 310 | 6.80×103 | 6.80×1011 | ||||
| 320 | 3.05×103 | 3.05×1012 | 320 | 6.12×103 | 6.12×1011 | ||||
| B | 300 | 7.05×103 | 7.05×1011 | Static quenching | F | 300 | 5.01×103 | 5.01×1011 | Dynamic quenching |
| 310 | 6.82×103 | 6.82×1011 | 310 | 3.32×103 | 3.32×1011 | ||||
| 320 | 6.36×103 | 6.36×1011 | 320 | 3.69×103 | 3.69×1011 | ||||
| C | 300 | 4.73×104 | 4.73×1012 | Static quenching | G | 300 | 4.91×103 | 4.91×1011 | Dynamic quenching |
| 310 | 4.34×104 | 4.34×1012 | 310 | 5.65×103 | 5.65×1011 | ||||
| 320 | 3.71×104 | 3.71×1012 | 320 | 6.60×103 | 6.60×1011 | ||||
| D | 300 | 4.76×103 | 4.76×1011 | Static quenching | H | 300 | 4.27×103 | 4.27×1011 | Dynamic quenching |
| 310 | 4.40×103 | 4.40×1011 | 310 | 4.42×103 | 4.42×1011 | ||||
| 320 | 4.20×103 | 4.20×1011 | 320 | 4.72×103 | 4.72×1011 |
Table 2 Stern-Volmer quenching constants(KSV), bimolecular quenching constants(Kq) and quenching mechanism
| PDs | T/K | KSV/ (L·mol-1) | Kq/ (L·mol-1·s-1) | Quenching mechanism | PDs | T/K | KSV/ (L·mol-1) | Kq/ (L·mol-1·s-1) | Quenching mechanism |
|---|---|---|---|---|---|---|---|---|---|
| A | 300 | 3.206×104 | 3.20×1012 | Static quenching | E | 300 | 3.32×103 | 3.32×1011 | Static quenching |
| 310 | 3.17×104 | 3.17×1012 | 310 | 6.80×103 | 6.80×1011 | ||||
| 320 | 3.05×103 | 3.05×1012 | 320 | 6.12×103 | 6.12×1011 | ||||
| B | 300 | 7.05×103 | 7.05×1011 | Static quenching | F | 300 | 5.01×103 | 5.01×1011 | Dynamic quenching |
| 310 | 6.82×103 | 6.82×1011 | 310 | 3.32×103 | 3.32×1011 | ||||
| 320 | 6.36×103 | 6.36×1011 | 320 | 3.69×103 | 3.69×1011 | ||||
| C | 300 | 4.73×104 | 4.73×1012 | Static quenching | G | 300 | 4.91×103 | 4.91×1011 | Dynamic quenching |
| 310 | 4.34×104 | 4.34×1012 | 310 | 5.65×103 | 5.65×1011 | ||||
| 320 | 3.71×104 | 3.71×1012 | 320 | 6.60×103 | 6.60×1011 | ||||
| D | 300 | 4.76×103 | 4.76×1011 | Static quenching | H | 300 | 4.27×103 | 4.27×1011 | Dynamic quenching |
| 310 | 4.40×103 | 4.40×1011 | 310 | 4.42×103 | 4.42×1011 | ||||
| 320 | 4.20×103 | 4.20×1011 | 320 | 4.72×103 | 4.72×1011 |
| Sample | τ1/ns | τ2/ns | B1 | B2 | τ/ns | χ2 | k2 | C2/(mol·L-1) |
|---|---|---|---|---|---|---|---|---|
| HSA | 2.24 | 6.62 | -0.30 | 0.81 | 7.25 | 1.13 | 1.51×105 | 1.59×10-6 |
| HSA+A | 2.18 | 6.61 | -0.37 | 0.95 | 7.26 | 1.25 | 1.51×105 | 1.64×10-6 |
| HSA+B | 2.22 | 6.52 | -0.38 | 0.97 | 7.18 | 0.97 | 1.53×105 | 1.64×10-6 |
| HSA+C | 2.06 | 5.58 | -0.24 | 0.59 | 6.20 | 1.02 | 1.79×105 | 1.69×10-6 |
| HSA+D | 2.26 | 6.62 | -0.39 | 0.96 | 7.32 | 1.26 | 1.51×105 | 1.68×10-6 |
| HSA+E | 1.92 | 5.92 | -0.26 | 0.72 | 6.45 | 0.69 | 1.69×105 | 1.57×10-6 |
| HSA+F | 1.89 | 6.00 | -0.36 | 1.05 | 6.50 | 1.69 | 1.67×105 | 1.52×10-6 |
| HSA+G | 2.00 | 5.83 | -0.43 | 1.07 | 6.44 | 1.25 | 1.72×105 | 1.67×10-6 |
| HSA+H | 2.05 | 6.09 | -0.52 | 1.29 | 6.72 | 1.80 | 1.64×105 | 1.68×10-6 |
Table 3 Fluorescent lifetime of HSA and HSA+PDs
| Sample | τ1/ns | τ2/ns | B1 | B2 | τ/ns | χ2 | k2 | C2/(mol·L-1) |
|---|---|---|---|---|---|---|---|---|
| HSA | 2.24 | 6.62 | -0.30 | 0.81 | 7.25 | 1.13 | 1.51×105 | 1.59×10-6 |
| HSA+A | 2.18 | 6.61 | -0.37 | 0.95 | 7.26 | 1.25 | 1.51×105 | 1.64×10-6 |
| HSA+B | 2.22 | 6.52 | -0.38 | 0.97 | 7.18 | 0.97 | 1.53×105 | 1.64×10-6 |
| HSA+C | 2.06 | 5.58 | -0.24 | 0.59 | 6.20 | 1.02 | 1.79×105 | 1.69×10-6 |
| HSA+D | 2.26 | 6.62 | -0.39 | 0.96 | 7.32 | 1.26 | 1.51×105 | 1.68×10-6 |
| HSA+E | 1.92 | 5.92 | -0.26 | 0.72 | 6.45 | 0.69 | 1.69×105 | 1.57×10-6 |
| HSA+F | 1.89 | 6.00 | -0.36 | 1.05 | 6.50 | 1.69 | 1.67×105 | 1.52×10-6 |
| HSA+G | 2.00 | 5.83 | -0.43 | 1.07 | 6.44 | 1.25 | 1.72×105 | 1.67×10-6 |
| HSA+H | 2.05 | 6.09 | -0.52 | 1.29 | 6.72 | 1.80 | 1.64×105 | 1.68×10-6 |
| Sample | F0/F | τ0/τ | Sample | F0/F | τ0/τ |
|---|---|---|---|---|---|
| HSA+A | 1.13 | 1.00 | HSA+E | 3.89 | 1.12 |
| HSA+B | 1.28 | 1.01 | HSA+F | 1.11 | 1.12 |
| HSA+C | 2.68 | 1.18 | HSA+G | 1.13 | 1.13 |
| HSA+D | 1.18 | 0.99 | HSA+H | 1.09 | 1.08 |
Table 4 Ratio of parameters of HSA+PDs
| Sample | F0/F | τ0/τ | Sample | F0/F | τ0/τ |
|---|---|---|---|---|---|
| HSA+A | 1.13 | 1.00 | HSA+E | 3.89 | 1.12 |
| HSA+B | 1.28 | 1.01 | HSA+F | 1.11 | 1.12 |
| HSA+C | 2.68 | 1.18 | HSA+G | 1.13 | 1.13 |
| HSA+D | 1.18 | 0.99 | HSA+H | 1.09 | 1.08 |
| PDs | T/K | Ka/(L·mol-1) | n | ΔH/(kJ·mol-1) | ΔS/(J·mol-1·K-1) | ΔG/(kJ·mol-1) | Interaction force |
|---|---|---|---|---|---|---|---|
| A | 300 | 3.41×103 | 1 | -6.62 | -45.6 | -20.3 | Hydrogen bond and Van der |
| 310 | 3.17×103 | 1.01 | -20.76 | Waals’ force | |||
| 320 | 2.89×103 | 1.02 | -21.23 | ||||
| B | 300 | 5.54×103 | 1.06 | -9.58 | 39.92 | -21.56 | Static electricity and |
| 310 | 5.22×103 | 1.06 | -21.96 | hydrophobic interaction | |||
| 320 | 4.37×103 | 1.09 | -22.35 | ||||
| C | 300 | 2.37×104 | 1.16 | -1.37 | 79.18 | -25.12 | Static electricity and |
| 310 | 2.33×104 | 1.15 | -25.92 | hydrophobic interaction | |||
| 320 | 2.29×104 | 1.11 | -26.71 | ||||
| D | 300 | 4.67×103 | 0.87 | -24.32 | -9.98 | -21.33 | Hydrogen bond and |
| 310 | 4.57×103 | 1.02 | -21.23 | Van der Waals’ force | |||
| 320 | 2.57×103 | 1.03 | -21.13 | ||||
| E | 300 | 1.17×105 | 0.86 | -32.18 | 9.78 | -29.25 | Static electricity and |
| 310 | 9.07×104 | 0.90 | -29.15 | hydrophobic interaction | |||
| 320 | 5.25×104 | 0.98 | -29.05 |
Table 5 Thermodynamic parameters of PDs-HSA binding process
| PDs | T/K | Ka/(L·mol-1) | n | ΔH/(kJ·mol-1) | ΔS/(J·mol-1·K-1) | ΔG/(kJ·mol-1) | Interaction force |
|---|---|---|---|---|---|---|---|
| A | 300 | 3.41×103 | 1 | -6.62 | -45.6 | -20.3 | Hydrogen bond and Van der |
| 310 | 3.17×103 | 1.01 | -20.76 | Waals’ force | |||
| 320 | 2.89×103 | 1.02 | -21.23 | ||||
| B | 300 | 5.54×103 | 1.06 | -9.58 | 39.92 | -21.56 | Static electricity and |
| 310 | 5.22×103 | 1.06 | -21.96 | hydrophobic interaction | |||
| 320 | 4.37×103 | 1.09 | -22.35 | ||||
| C | 300 | 2.37×104 | 1.16 | -1.37 | 79.18 | -25.12 | Static electricity and |
| 310 | 2.33×104 | 1.15 | -25.92 | hydrophobic interaction | |||
| 320 | 2.29×104 | 1.11 | -26.71 | ||||
| D | 300 | 4.67×103 | 0.87 | -24.32 | -9.98 | -21.33 | Hydrogen bond and |
| 310 | 4.57×103 | 1.02 | -21.23 | Van der Waals’ force | |||
| 320 | 2.57×103 | 1.03 | -21.13 | ||||
| E | 300 | 1.17×105 | 0.86 | -32.18 | 9.78 | -29.25 | Static electricity and |
| 310 | 9.07×104 | 0.90 | -29.15 | hydrophobic interaction | |||
| 320 | 5.25×104 | 0.98 | -29.05 |
| HSA+PDs | J/(cm3·L·mol-1) | R0/nm | E | r/nm |
|---|---|---|---|---|
| HSA+A | 1.55×10-14 | 2.64 | 0.12 | 3.68 |
| HSA+B | 1.43×10-14 | 2.60 | 0.22 | 3.21 |
| HSA+C | 2.95×10-14 | 2.94 | 0.65 | 2.65 |
| HSA+D | 1.41×10-14 | 2.60 | 0.16 | 3.42 |
| HSA+E | 2.54×10-14 | 2.86 | 0.74 | 2.41 |
Table 6 Energy transfer parameters between PDs and HSA
| HSA+PDs | J/(cm3·L·mol-1) | R0/nm | E | r/nm |
|---|---|---|---|---|
| HSA+A | 1.55×10-14 | 2.64 | 0.12 | 3.68 |
| HSA+B | 1.43×10-14 | 2.60 | 0.22 | 3.21 |
| HSA+C | 2.95×10-14 | 2.94 | 0.65 | 2.65 |
| HSA+D | 1.41×10-14 | 2.60 | 0.16 | 3.42 |
| HSA+E | 2.54×10-14 | 2.86 | 0.74 | 2.41 |
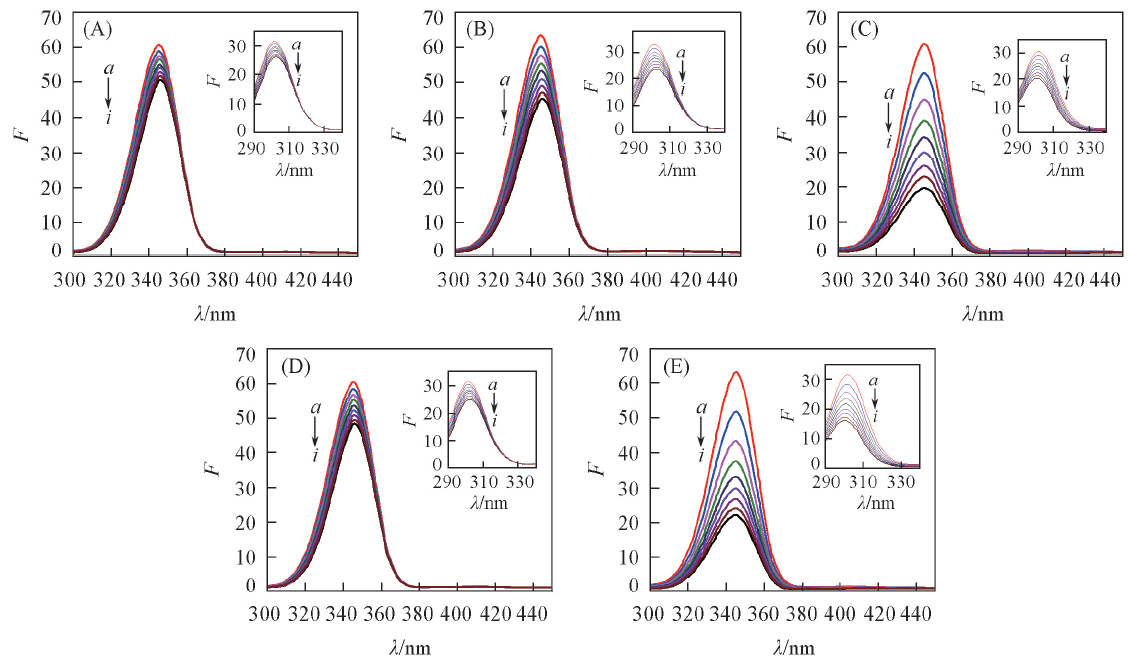
Fig.5 Synchronous fluorescent spectra showing the interaction between PDs and HSAc(HSA)=5 μmol/L, c(PDs)/(μmol·L-1) from a to i: 0, 5, 10, 15, 20, 25, 30, 35, 40.(A)—(E) PDs A—E, respectively, Δλ=60 nm; insets: Δλ=15 nm.
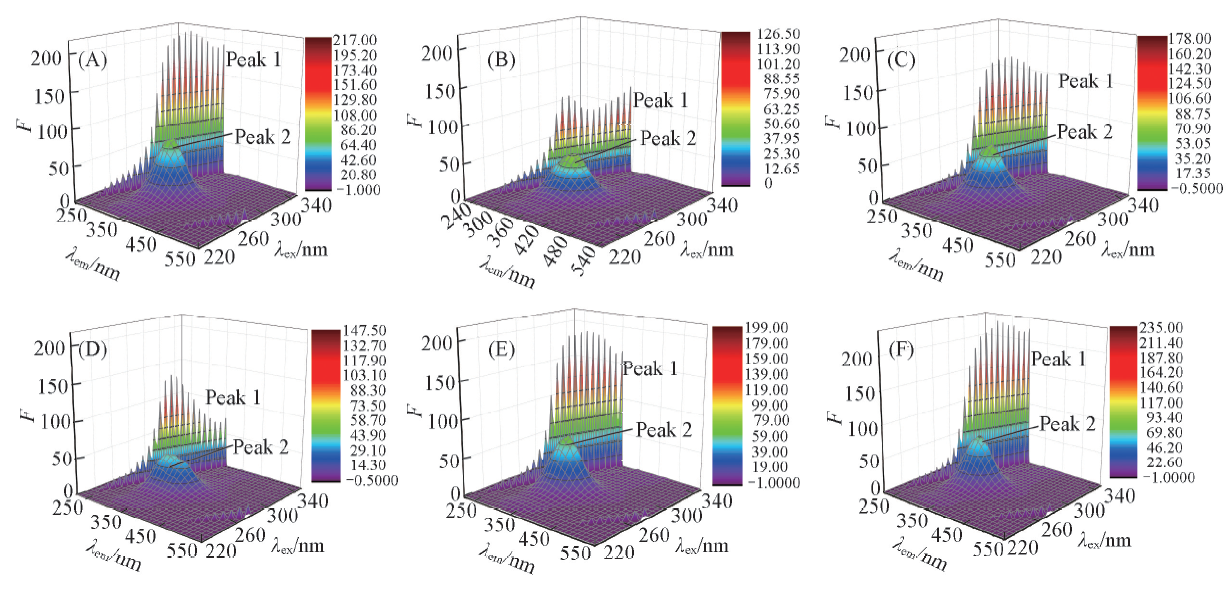
Fig.6 Three-dimensional fluorescence spectra of free HSA and the PDs-HSA systemc(HSA)=5 μmol/L, c(PDs)=20 μmol/L. (A) HSA; (B) HSA+A; (C) HSA+B; (D) HSA+C; (E) HSA+D; (F) HSA+E.
| System | Rayleigh scattering peak(Peak 1) | Fluorescence peak(Peak 2) | |||||
|---|---|---|---|---|---|---|---|
| Peak position λex/λem(nm/nm) | Stokes Δλ/nm | Fluorescence intensity | Peak position λex/λem(nm/nm) | Stokes Δλ/nm | Fluorescence intensity | ||
| HSA | 290/290 | 0 | 134.15 | 290/334 | 44 | 75.80 | |
| HSA+A | 290/290 | 0 | 100.69 | 290/338 | 48 | 51.63 | |
| HSA+B | 290/290 | 0 | 9.22 | 290/334 | 44 | 66.10 | |
| HSA+C | 290/290 | 0 | 112.33 | 290/328 | 38 | 41.25 | |
| HSA+D | 290/290 | 0 | 97.86 | 290/335 | 45 | 67.90 | |
| HSA+E | 290/290 | 0 | 125.45 | 290/335 | 45 | 71.71 | |
Table 7 Three-dimensional fluorescence spectral characteristics of HSA and HSA+PDs system
| System | Rayleigh scattering peak(Peak 1) | Fluorescence peak(Peak 2) | |||||
|---|---|---|---|---|---|---|---|
| Peak position λex/λem(nm/nm) | Stokes Δλ/nm | Fluorescence intensity | Peak position λex/λem(nm/nm) | Stokes Δλ/nm | Fluorescence intensity | ||
| HSA | 290/290 | 0 | 134.15 | 290/334 | 44 | 75.80 | |
| HSA+A | 290/290 | 0 | 100.69 | 290/338 | 48 | 51.63 | |
| HSA+B | 290/290 | 0 | 9.22 | 290/334 | 44 | 66.10 | |
| HSA+C | 290/290 | 0 | 112.33 | 290/328 | 38 | 41.25 | |
| HSA+D | 290/290 | 0 | 97.86 | 290/335 | 45 | 67.90 | |
| HSA+E | 290/290 | 0 | 125.45 | 290/335 | 45 | 71.71 | |
| [1] | Kratochwil N. A., Huber W., Muller F., Kansy M., Gerber P. R., Biochem. Pharmacol., 2002, 64(9), 1355-1374 |
| [2] | Ahmad B., Parveen S., Khan R. H., Biomacromolecules,2006, 7(4), 1350-1356 |
| [3] | Kelarakis A., Castelletto V., Krysmann M. J., Havredaki V., Viras K., Hamley I. W., Biomacromolecules,2008, 9(5), 1366-1371 |
| [4] | Ruiz J., Vicente C., de Haro C., Bautista D., Inorg. Chem., 2013, 52( 2), 974-982 |
| [5] | Han Y. C., Fan Y. R., Huang X., Wang Y. L., Chem. J. Chinese Universities,2010, 31(6), 1014-1023 |
| (韩玉淳, 樊艳茹, 黄旭, 王毅琳. 中国科学: 化学, 2014, 44(6), 1014-1023) | |
| [6] | Wen J. Y., Lü B. B., Zhang Y., Wang J. M., Ying X., Wang H., Ji L. N., Liu H. Y., Chem. J. Chinese Universities,2010, 31(6), 1033-1041 |
| (闻金燕, 吕标彪, 张阳, 王家敏, 应晓, 王慧, 计亮年, 刘海洋. 高等学校化学学报, 2015, 36(6), 1033-1041) | |
| [7] | Wang C., Wu Q. H., Wang Z., Zhao J., Anal. Sci., 2006, 22(3), 435-438 |
| [8] | Maciᶏzek-Jurczyk M., Sułkowska A., Bojko B., Równicka-Zubik J., Szkudlarek-Hasnik A., Zubik-Skupień I., Góra A., Dubas M., Korzonek-Szlacheta I., Wielkoszyński T., Zurawiński W., Sosada K., Spectrochim. Acta A Mol. Biomol. Spectrosc., 2012, 89(4), 270-275 |
| [9] | Maciᶏzek-Jurczyk M., Sułkowska A., Bojko B., Równicka-Zubik J., Sułkowski W. W., Spectrochim. Acta A Mol. Biomol. Spectrosc., 2011, 82(1), 189-190 |
| [10] | Gomha S. M., Zaki Y. H., Abdelhamid A. O., Molecules,2015, 20(12), 21826-21839 |
| [11] | Ghoneim K. M., El-Telbany F., Youssef K. M., J. Indian Chem. Soc., 1986, 63, 914-917 |
| [12] | Vishwas D., Suryawanshi Prashant V. A., Anil H. G., Shivajirao R. P., Govind B. K., J. Photochem. Photobiol. B: Biology,2013, 118, 1-8 |
| [13] | Zhao P. L., You W. W., Duan A. N., Chem. J. Chinese Universities,2010, 31(5), 580-587 |
| (赵培亮, 游文玮, 段安娜. 药学学报, 2012, 47(5), 580-587) | |
| [14] | Yasumoto M., Yamawaki I., Marunaka T., Hashimoto S., J. Med. Chem., 1978, 21(8), 738-741 |
| [15] | Masao T. A. D. A., Bull. Chem. Soc. Japan, 1975, 48(11), 3427-3428 |
| [16] | Mohammad H., RezaY., Asghar T. K., Farhad P., AliKhalafi N., Chem. J. Chinese Universities,2010, 31, 374-379 |
| [17] | Arica B., Calis S., Kas H., Sargon M., Hincal A., Int. J. Pharm., 2002, 242(1/2), 267-269 |
| [18] | Wang X. D., Chi G. C., Chen R. Y., Chin. J. Synth. Chem., 2001, 9(1), 1-2 |
| [19] | Rodgers K. R., Curr. Opin. Chem. Biol., 1999, 3(2), 158-167 |
| [20] | Seetharamappa J., Kamat B. P., Chem. Pharm. Bull., 2004, 52, 1053-1057 |
| [21] | Yang Y., Hu Q., Fan Y., Shen H., Chem. J. Chinese Universities,2010, 31(2), 432-436 |
| [22] | Kandagal P. B., Seetharamappa J., Ashoka S., Shaikh S. M., Manjunatha D. H., Int. J. Biol. Macromol., 2006, 39(4/5), 234-239 |
| [23] | Ray D., Paul B. K., Guchhait N., Chem. J. Chinese Universities,2010, 31, 18-27 |
| [24] | Sharma A. S., Ilanchelian M., Chem. J. Chinese Universities,2010, 31(30), 9461-9476 |
| [25] | Wei P., Fei D., Jiang Y. T., Peng Y. K., J. Agric. Food Chem., 2014, 62, 2271-2283 |
| [26] | Ganesh B. M., Shateesh B., Sudhir K., Santosh K. H., Chem. J. Chinese Universities,2010, 31, 20944-20950 |
| [27] | Shahabadi N., Khorshidi A., Moghadam N. H., Chem. J. Chinese Universities,2010, 31, 627-632 |
| [28] | Wang R., Wang X., Li Z., Xie Y., Yang L., Shi J., Chang J., Spectrochim Acta A: Mol. Biomol. Spectrosc., 2014, 132, 786-794 |
| [29] | Ma J., Zheng X.F., Tang Q., Yang Y. J., Sun X., Gao D. B.,Chem. J. Chinese Universities, 2008, 29(2), 258-263 |
| (马静, 郑学仿, 唐乾, 杨彦杰, 孙霞, 高大彬. 高等学校化学学报, 2008, 29(2), 258-263) | |
| [30] | Wang Y. Q., Zhang H. M., Zhang G. C., Liu S. X., Zhou Q. H., Fei Z. H., Liu Z. T., Int. J. Biol. Macromol., 2007, 41(3), 243-250 |
| [31] | Ross P. D., Subramanian S., Biochemistry,1981, 20(11), 3096-3102 |
| [32] | Bourassa P., Dubeau S., Maharvi G. M., Fauq A. H., Thomas T. J., Tajmir-Riahi H. A., Eur. J. Med. Chem., 2011, 46(9), 4344-4353 |
| [33] | Molina-Bolívar J. A., Galisteo-González C. R. F., Medina-O’ D. M., Parra A., J. Mol. Liq., 2015, 208, 304-313 |
| [34] | Xie X., Lü W., Chen X., J. Hazard. Mater., 2013, 248-249, 347-354 |
| [35] | Han X. L., Tian F. F., Ge Y. S, Jiang F. L., Lai L., Li D. W., Yu Q. L., Wang J., Lin C., Liu Y., Int. J. Biol. Macromol., 2012, 109, 1-11 |
| [36] | Li X., Li C., Jiang J. H., Gu H. W., Wei D. L., YE L. J., Hu J. L., Xiao S. X., Zhang H., Li X., Li Q. G., Chem. J. Chinese Universities,2010, 31(2), 166-171 |
| [37] | Maryam S., Hassan M. T., Ali Akbar S., J. Lumin., 2015, 167, 391-398 |
| [38] | Shen H., Gu Z., Jian K., Qi J., J. Pharm. Biomed. Anal., 2013, 75, 86-93 |
| [39] | Zhang J. L., Gu H. M., Zhang X. H., Carbohyd. Res., 2014, 384, 102-111 |
| [40] | Tu B., Wang Y., Mi R., Ouyang Y., Hu Y. J., Spectrochim Acta A: Mol. Biomol. Spectrosc., 2015, 149, 536-543 |
| [41] | Ge Y. S., Tai S. X., Xu Z. Q., Lai L., Tian F. F., Li D. W., Jiang F. L., Liu Y., Gao Z. N., Langmuir,2012, 28, 5913-5920 |
| [42] | Cheng H., Liu H., Zhang Y., J. Lumin., 2009, 129, 1196-1203 |
| [43] | Nicolott O., Catto M., Giangreco I., Eur. J. Med. Chem., 2012, 58, 368-376 |
| [1] | ZHANG Aiqin, WANG Man, SHEN Gangyi, JIN Jun. Interactions Between Polybrominated Diphenyl Ethers and Human Serum Albumin Using SPR and Molecular Docking [J]. Chem. J. Chinese Universities, 2020, 41(9): 2054. |
| [2] | FANG Fang,XUE Liangmin,CONG Jing,TIAN Chao,WANG Xiaowei,LIU Junyi,ZHANG Zhili. Synthesis and Anti-tumor Activity Evaluation of a Series of 2- or 4-Substituted Pyrido[3,2-d]pyrimidines as Nonclassical Antifolates † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2111. |
| [3] | YANG Meiling, QIN Dongdong, SONG Yumin. Syntheses and Anticoagulant Action of Rare Earth Ternary omplexes with Warfarin and Ferulic Acid† [J]. Chem. J. Chinese Universities, 2015, 36(5): 821. |
| [4] | WANG Cong-Xia, YE Ling*, YAN Fang-Fei, WANG Nan, YU Pei-Lin. Spectroscopic Studies on the Interaction Between Rifabutin and Human Serum Albumin [J]. Chem. J. Chinese Universities, 2007, 28(12): 2280. |
| [5] | LI Hui-Qing, JIANG Zhi-Qin, Wang Xin, PAN Yang, WANG Feng, YU Shu-Qin . Electron Transfer Laser Flash Photolysis Between Nucleosides and Probe Triplet N-(2’-Hydroxyethyl)-1,8-naphthalimide [J]. Chem. J. Chinese Universities, 2004, 25(11): 2134. |
| [6] | Fang Hao-Jie, OU-YANG Bin, ZHU Cheng-Zhu, DONG Wen-Bo, ZHANG Ren-Xi, PAN Xun-Xi, HOU Hui-Qi . Laser Flash Photolysis of CS2-HNO2 Aqueous Solution Complex System [J]. Chem. J. Chinese Universities, 2004, 25(10): 1893. |
| [7] | XU Ye-Ping, SONG Qin-Hua, YU Shu-Qin, CHEN Cong-Xiang, MA Xing-Xiao, LIN Wei-Zhen, YAO Si-De. Reaction of Electron Transfer from Methionine-containing Dipeptides to Triplet 4-Nitroquinoline 1-Oxide [J]. Chem. J. Chinese Universities, 2000, 21(7): 1067. |
| [8] | SONG Qin-Hua, CHU Gao-Sheng, LIN WeiZhen, YAO SiDe. Physical Chemical Processes of Acetone Triplet with Aromatic Amino Acid-containing Peptides [J]. Chem. J. Chinese Universities, 2000, 21(12): 1880. |
| [9] | Zhang Wensheng, Li Renli, Zhang Jundong, Martin Poe. Studies on the Synthesis of 5-(Substituted Phenyl) Thio-2,4- Diaminopyrimidines and Their QSAR of the Inhibition to E. coli DHFR [J]. Chem. J. Chinese Universities, 1988, 9(10): 1029. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||