

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (12): 2394.doi: 10.7503/cjcu20150367
• Analytical Chemistry • Previous Articles Next Articles
CHEN Linfeng1, ZHANG Jing1, ZHU Yaxian2, ZHANG Yong1,3,*( )
)
Received:2015-05-08
Online:2015-12-10
Published:2015-10-09
Contact:
ZHANG Yong
E-mail:yzhang@xmu.edu.cn
Supported by:CLC Number:
TrendMD:
CHEN Linfeng, ZHANG Jing, ZHU Yaxian, ZHANG Yong. Molecular Interactions of 1-Hydroxypyrene with Catalase Revealed by Spectroscopic Methods Combined with Molecular Docking†[J]. Chem. J. Chinese Universities, 2015, 36(12): 2394.
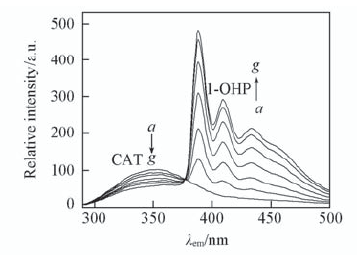
Fig.1 Fluorescence quenching spectra of CAT in the presence of various amounts of 1-OHP(pH=7.00) a—g. 106 c(1-OHP)/(mol·L-1): 0, 1.0, 2.0, 4.0, 6.0, 8.0, 10.0; c(CAT)=2.0×10-6 mol/L; λex=280 nm.
| T/K | 10-4Ksv/ (L·mol-1) | 10-12Kq/ (L·mol-1·s-1) | R2 |
|---|---|---|---|
| 290 | 7.92 | 7.92 | 0.995 |
| 300 | 6.88 | 6.88 | 0.994 |
| 308 | 6.64 | 6.64 | 0.995 |
Table 1 Stern-Volmer quenching constants and the correlation coefficient of 1-OHP-CAT systems at 290, 300 and 308 K
| T/K | 10-4Ksv/ (L·mol-1) | 10-12Kq/ (L·mol-1·s-1) | R2 |
|---|---|---|---|
| 290 | 7.92 | 7.92 | 0.995 |
| 300 | 6.88 | 6.88 | 0.994 |
| 308 | 6.64 | 6.64 | 0.995 |
| 106c(1-OHP)/(mol·L-1) | Lifetime/ns | Amplitude(%) | τAV | χ2 | ||
|---|---|---|---|---|---|---|
| τ1 | τ2 | a1 | a2 | |||
| 0 | 1.94 | 5.29 | 35.47 | 64.53 | 4.10 | 0.980 |
| 2.0 | 1.91 | 5.19 | 34.52 | 65.48 | 4.06 | 1.030 |
| 4.0 | 2.05 | 5.25 | 36.71 | 63.29 | 4.08 | 1.059 |
| 6.0 | 1.70 | 5.05 | 30.22 | 69.79 | 4.04 | 0.913 |
| 8.0 | 1.91 | 5.25 | 35.62 | 64.38 | 4.06 | 1.080 |
Table 2 Fluorescence lifetime constants of 1-OHP-CAT system*
| 106c(1-OHP)/(mol·L-1) | Lifetime/ns | Amplitude(%) | τAV | χ2 | ||
|---|---|---|---|---|---|---|
| τ1 | τ2 | a1 | a2 | |||
| 0 | 1.94 | 5.29 | 35.47 | 64.53 | 4.10 | 0.980 |
| 2.0 | 1.91 | 5.19 | 34.52 | 65.48 | 4.06 | 1.030 |
| 4.0 | 2.05 | 5.25 | 36.71 | 63.29 | 4.08 | 1.059 |
| 6.0 | 1.70 | 5.05 | 30.22 | 69.79 | 4.04 | 0.913 |
| 8.0 | 1.91 | 5.25 | 35.62 | 64.38 | 4.06 | 1.080 |
| T/K | 10-5K/(L·mol-1) | n | R2 |
|---|---|---|---|
| 290 | 2.96 | 1.11 | 0.995 |
| 300 | 1.62 | 1.07 | 0.994 |
| 308 | 0.554 | 0.98 | 0.995 |
Table 3 Binding constants(K) of 1-OHP-CAT interactions
| T/K | 10-5K/(L·mol-1) | n | R2 |
|---|---|---|---|
| 290 | 2.96 | 1.11 | 0.995 |
| 300 | 1.62 | 1.07 | 0.994 |
| 308 | 0.554 | 0.98 | 0.995 |
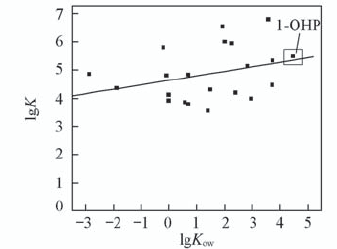
Fig.3 Correlation between the binding constant(lgK) for binding to CAT and the octanol/water partition coefficients lgKow Pearson correlation factor r is 0.316.
| T/K | ΔG/ (kJ·mol-1) | ΔS/ (J·mol-1·K-1) | ΔH/ (kJ·mol-1) |
|---|---|---|---|
| 290 | -30.60 | -118.87 | -65.07 |
| 300 | -29.41 | -118.87 | -65.07 |
| 308 | -28.34 | -118.87 | -65.07 |
Table 4 Thermodynamic parameters of 1-OHP-CAT interaction
| T/K | ΔG/ (kJ·mol-1) | ΔS/ (J·mol-1·K-1) | ΔH/ (kJ·mol-1) |
|---|---|---|---|
| 290 | -30.60 | -118.87 | -65.07 |
| 300 | -29.41 | -118.87 | -65.07 |
| 308 | -28.34 | -118.87 | -65.07 |
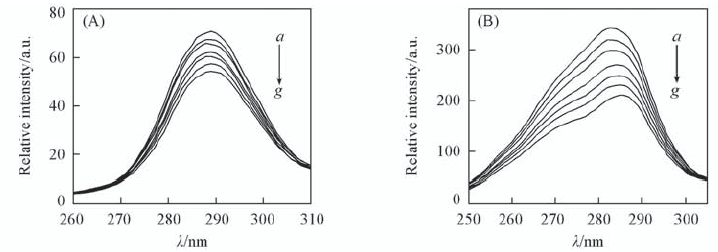
Fig.6 Synchronous fluorescence spectra of 1-OHP-CAT system(pH=7.00) (A) Δλ=15 nm; (B) Δλ=60 nm. c(CAT)=2.0×10-6 mol/L; a—g. 106c(1-OHP)/(mol·L-1): 0, 1.0, 2.0, 4.0, 6.0, 8.0, 10.0.
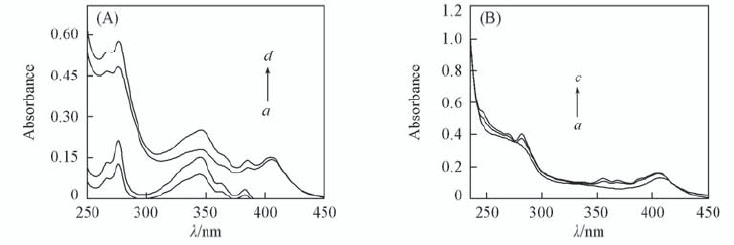
Fig.7 UV-Vis absorption spectra(A) and difference spectra(B) of CAT in the absence and presence of different concentrations of 1-OHP(pH=7.00) (A) a. c(1-OHP)=6×10-6 mol/L; b. c(1-OHP)=1.0×10-5 mol/L; c. c(CAT)=1.0×10-6 mol/L, c(1-OHP)=6×10-6 mol/L; d. c(CAT)=1.0×10-6 mol/L, c(1-OHP)=1.0×10-5 mol/L; (B) a. c(CAT)=1.0×10-6 mol/L; b. difference spectrum of c-a in (A); c. difference spectrum of d-b in (A).
| Energy-ranked result | Conformation data | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Binding energy/(kJ·mol-1) | -35.09 | -34.71 | -33.75 | -33.75 | -32.95 |
| Ligand efficiency/(kJ·mol-1/non-hydrogen atom) | -2.05 | -2.05 | -1.96 | -1.96 | -1.92 |
| Inhibition constant/(nmol·L-1) | 706.77 | 830.92 | 1220 | 1220 | 1670 |
| Intermolecular energy/(kJ·mol-1) | -36.34 | -35.92 | -35.00 | -35.00 | -34.20 |
Table 5 Energy-ranked results of five 1-OHP-CAT binding conformations
| Energy-ranked result | Conformation data | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Binding energy/(kJ·mol-1) | -35.09 | -34.71 | -33.75 | -33.75 | -32.95 |
| Ligand efficiency/(kJ·mol-1/non-hydrogen atom) | -2.05 | -2.05 | -1.96 | -1.96 | -1.92 |
| Inhibition constant/(nmol·L-1) | 706.77 | 830.92 | 1220 | 1220 | 1670 |
| Intermolecular energy/(kJ·mol-1) | -36.34 | -35.92 | -35.00 | -35.00 | -34.20 |
| [1] | Xu Q., Lu Y. N., Jing L. Y., Cai L. J., Zhu X. F., Xie J., Hu X. Y., Spectrochim. Acta A,2014, 123(7), 327—335 |
| [2] | Gönenç A., Hacışşevki A., Aslan S., Torun M., Şimşek B., Eur. J. Intern. Med., 2012, 23(4), 350—354 |
| [3] | Shen H. Z., Huang Y., Wang R., Zhu D., Li W., Shen G. F., Wang B., Zhang Y. Y., Chen Y. C., Lu Y., Chen H., Li T. C., Sun K., Li B. G., Liu W. X., Liu J. F., Tao S., Environ. Sci. Technol., 2013, 47(12), 6415—6424 |
| [4] | Jongeneelen F. J., Ann. Occup. Hyg., 2001, 45(1), 3—13 |
| [5] | Singh V. K., Patel D. K., Ram S., Mathur N., Siddiqui M., Clin. Biochem., 2008, 41(3), 152—161 |
| [6] | Kim K. H., Jahan S. A., Kabir E., Brown R. J. C., Environ. Int., 2013, 60(5), 71—80 |
| [7] | Kim K. B., Lee B. M., Cancer Lett., 1997, 113(1), 205—212 |
| [8] | Kamal A., Qamar K., Gulfraz M., Anwar M. A., Malik R. N., Chemosphere, 2015, 120, 59—67 |
| [9] | Freitas F., Brucker N., Durgante J., Bubols G., Bulcão R., Moro A., Charão M., Baierle M., Nascimento S., Gauer B., Sauer E., Zimmer M., Thiesen F., Castro I., Saldiva P., Garcia S. C., Inter. J. Env. Res. Pub. Heal., 2014, 11(9), 9024—9037 |
| [10] | Hussain T., Al-Attas O. S., Al-Daghri N. M., Mohammed A. A., de Rosas E., Ibrahim S., Vinodson B., Ansari M. G., El-Din K. I. A., Mol. Cell. Biochem., 2014, 391(1/2), 127—136 |
| [11] | Aksmann A., Pokora W., Baścik-Remisiewicz A., Dettlaff-Pokora A., Wielgomas B., Dziadziuszko M., Tukaj Z., Ecotox. Environ. Safe., 2014, 110, 31—40 |
| [12] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery Jr. J. A., Peralta J. E., Ogliaro F., Bearpark M. J., Heyd J., Brothers E. N., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A. P., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam N. J., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas Ö., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Gaussian Inc., Wallingford, CT, USA, 2009 |
| [13] | Morris G. M., Goodsell D. S., Halliday R. S., Huey R., Hart W. E., Belew R. K., Olson A. J., J. Comput. Chem., 1998, 19(14), 1639—1662 |
| [14] | DeLano W. L., The PyMOL Molecular Graphics System, DeLano Scientific, San Carlos, CA, USA, 2002 |
| [15] | Yang B. J., Hao F., Li J. R., Chen D. L., Liu R. T., J. Photoch. Photobio. B,2013, 128, 35—42 |
| [16] | Chi Z. X., Liu R. T., Zhang H., Sci. Total Environ., 2010, 408(22), 5399—5404 |
| [17] | Dong L., Yi Z. S., Wu Z. W., Wang H. Y., Zhang A. Q., Chem. J. Chinese Universities,2015, 36(3), 516—522 |
| (董露, 易忠胜, 伍智蔚, 王海洋, 张爱茜. 高等学校化学学报,2015, 36(3), 516—522) | |
| [18] | Du C. R., Luo X., Wei J. R., He T. T., Zheng X. Y., Lin C. W., Chem. Res. Chinese Universities,2013, 29(5), 854—860 |
| [19] | Ware W. R., J. Phys. Chem., 1962, 66(3), 455—458 |
| [20] | Xu X., Qian Y., Wu P., Zhang H., Cai C., J. Colloid Interface Sci., 2015, 445, 102—111 |
| [21] | Seidel C. A., Schulz A., Sauer M. H., J. Phys. Chem., 1996, 100(13), 5541—5553 |
| [22] | Cao Z. Z., Liu R. T., Yang B. J., Spectrochim. Acta A,2013, 115, 457—463 |
| [23] | Sun H. Y., Yang B. J., Cui E. Q., Liu R. T., Spectrochim. Acta A,2014, 132(21), 692—699 |
| [24] | Teng Y., Zhang H., Liu R. T., Mol. BioSyst., 2011, 7(11), 3157—3163 |
| [25] | Morris J.J., Bruneau P. P., Prediction of Physicochemical Properties, Wiley-VCH, Weinheim, 2000, 10 |
| [26] | Wang M. Y., Zhang C., Li J., Li Z. X., Gong M. X., Chem. J. Chinese Universities,2013, 35(2), 309—313 |
| (王满元, 张超, 李静, 李朝霞, 龚慕辛. 高等学校化学学报,2013, 35(2), 309—313) | |
| [27] | Chi Z. X., Liu R. T., Yang B. J., Zhang H., J. Hazard. Mater., 2010, 180(1), 741—747 |
| [28] | Huang S., Qiu H. N., Lu S. Y., Zhu F. W., Xiao Q., J. Hazard. Mater., 2015, 285, 18—26 |
| [29] | RossP. D., Subramanian S., Biochemistry, 1981, 20(11), 3096—3102 |
| [30] | Hu Y. D., Da L. J., Spectrochim. Acta A,2014, 121(5), 230—237 |
| [31] | Li D. J., Ji B. M., Jin J., J. Lumin., 2008, 128(9), 1399—1406 |
| [32] | Zhang J. X., Yin Z. N., Wu W., Wang Z. X., He R., Wu Z. X., Chem. Res. Chinese Universities,2012, 28(6), 963—970 |
| [33] | Huang Y., Wang J., Guo G. Y., Tao Z., Xue S. F., Zhu Q. J., Zhou Q. D., Chem. J. Chinese Universities,2013, 34(2), 375—380 |
| (黄英, 王娟, 郭改英, 陶朱, 薛赛凤, 祝黔江, 周清娣. 高等学校化学学报,2013, 34(2), 375—380) | |
| [34] | Wei X. L., Ge Z. Q., Carbon, 2013, 60, 401—409 |
| [35] | Lavery R., Sacquin-Mora S., J. Bioscience,2007, 32(1), 891—898 |
| [36] | Di Giulio R. T., Washburn P. C., Wenning R. J., Winston G. W., Jewell C. S., Environ. Toxicol. Chem., 1989, 8(12), 1103—1123 |
| [37] | Rodriguez-Ariza A., Peinado J., Pueyo C., Lopez-Barea J., Can. J. Fish. Aquat. Sci., 1993, 50(12), 2568—2573 |
| [38] | Zhu X. F., Lu Y. N., Wang J. D., Xu Q., Anal. Methods,2013, 5(21), 6037—6044 |
| [1] | LEI Xiaotong, JIN Yiqing, MENG Xuanyu. Prediction of the Binding Site of PIP2 in the TREK-1 Channel Based on Molecular Modeling [J]. Chem. J. Chinese Universities, 2021, 42(8): 2550. |
| [2] | SHUAI Die, ZHAO Meijuan, CHEN Bingnian, WANG Li. Inhibitory Effect of Four Kinds of Keegin-type Phosphomolybdate on Tyrosinase and Melanin Formation and Its Antioxidant Activities [J]. Chem. J. Chinese Universities, 2021, 42(12): 3579. |
| [3] | YANG Ju, SU Lijiao, LI Canhua, LU Jiajia, YANG Junli, GU Jie, YANG Li, YANG Lijuan. Host-guest Complexation Behavior of Nardosinone and Water-soluble Phosphate Salt Pillar[6]arene [J]. Chem. J. Chinese Universities, 2021, 42(10): 3099. |
| [4] | ZHANG Aiqin, WANG Man, SHEN Gangyi, JIN Jun. Interactions Between Polybrominated Diphenyl Ethers and Human Serum Albumin Using SPR and Molecular Docking [J]. Chem. J. Chinese Universities, 2020, 41(9): 2054. |
| [5] | WANG Lianping,LI Qingjie,LIU Xiaoyan,REN Yueying,YANG Xiuwei. Screening of Cholinesterase Inhibitors in Fructus Evodiae Alkaloids Based on UFLC-MS/molecular Simulation † [J]. Chem. J. Chinese Universities, 2020, 41(1): 111. |
| [6] | WANG Xiaoxia, MA Litong, NIE Zhihua, WANG Zhengde, CUI Jinlong, ZHAO Wenyuan, SAI Huazheng. Interaction Between Fulvic Acid and Pepsin Investigated by Multispectral and Molecular Docking Simulation † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1840. |
| [7] | LIU Zhongcheng, LIU Shifang, ZHANG Su, YANG Yanlei, LI Fei, ZHANG Nan, YUAN Xin, ZHANG Yanfen. Structure Prediction and Screening of Oligonucleotide Aptamers Target Cε3-Cε4 Protein† [J]. Chem. J. Chinese Universities, 2019, 40(1): 83. |
| [8] | XIN Meiling, CHU Zhenhua, LI Yu. Molecular Modification of Polychlorinated Biphenyl Dihydroxy Derivatives Through Molecular Docking Associated with CoMSIA/HQSAR Models† [J]. Chem. J. Chinese Universities, 2018, 39(2): 299. |
| [9] | WANG Yan, CHEN Ping, WANG Yunfei, LIU Guiying, YANG Xi, SU Ying, LI Junyang, LIU Weiwei, LIN Lie. Spectral Characterization of the Interaction Between Methamphetamine and Serum Albumin† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2507. |
| [10] | DUAN Yongbin, YIN Yan, MENG Fanli, ZHAO Lianhua, LIU Yukun, YUAN Zhe, FENG Yangbo. Design, Synthesis and Biological Evaluation of Benzothiazoles as Highly Potent ROCK Inhibitors Through Molecular Docking and Free Energy Calculations† [J]. Chem. J. Chinese Universities, 2017, 38(9): 1568. |
| [11] | WANG Song, GUAN Shanshan, WAN Yongfeng, SHAN Yaming, ZHANG Hao. Molecular Dynamics Simulation Study on the Binding Modes of Angiotensin-converting Enzyme with Inhibitory Peptides† [J]. Chem. J. Chinese Universities, 2017, 38(7): 1216. |
| [12] | TANG Qian, SU Jinhong, CAO Hongyu, WANG Lihao, SHI Fei, WANG Ailing, GONG Tingting, JIN Xiaojun, ZHENG Xuefang. Interaction of Pyrimidine Derivatives with Human Serum Albumin† [J]. Chem. J. Chinese Universities, 2017, 38(11): 1982. |
| [13] | ZHANG Jing, CHEN Linfeng, ZHU Yaxian, ZHANG Yong. Effects of Hydroxypropyl-β-cyclodextrin(HPCD) on the Interaction of 1-Hydroxypyrene with Bovine Serum Albumin† [J]. Chem. J. Chinese Universities, 2017, 38(1): 28. |
| [14] | ZHANG Jing, CHEN Weixiao, ZHANG Wei, DUAN Ying, ZHU Yuxiu, ZHU Yaxian, ZHANG Yong. Interaction of 1-Hydroxypyrene with BSA Using Fluorescence Anisotropy and Synchronous Fluorescence Analysis Methods† [J]. Chem. J. Chinese Universities, 2015, 36(8): 1511. |
| [15] | ZHAO Bin, HUO Jingqian, XING Jihong, QI Meng, ZHANG Jinlin, DONG Jingao. Homologous Modeling of Transketolase AtTKL1 and Its Combination with α-Terthienyl in Arabidopsis Thaliana† [J]. Chem. J. Chinese Universities, 2015, 36(4): 682. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||