

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (5): 729.doi: 10.7503/cjcu20160908
• Articles: Inorganic Chemistry • Previous Articles Next Articles
SUN Jiayin, LU Ke, LIU Guangzhi, LIU Lu, PAN Wenrong, ZHANG Sijing, QIN Wei, WU Genhua*( )
)
Received:2016-12-16
Online:2017-05-10
Published:2017-04-18
Contact:
WU Genhua
E-mail:wugenh@aqtc.edu.cn
Supported by:CLC Number:
TrendMD:
SUN Jiayin, LU Ke, LIU Guangzhi, LIU Lu, PAN Wenrong, ZHANG Sijing, QIN Wei, WU Genhua. Syntheses, Crystal Structures and Luminescent Properties of Two Magnesium(Ⅱ) Supramolecular Architectures Based on Hydrogen Bonds†[J]. Chem. J. Chinese Universities, 2017, 38(5): 729.
| Compound | 1 | 2 | γ/(°) | 105.4980(10) | 78.03(3) |
|---|---|---|---|---|---|
| Formula | C9H12MgN2O8 | C9H22MgN2O13 | V/nm3 | 0.57579(7) | 0.8534(3) |
| Formula weight | 300.52 | 390.60 | Z | 2 | 2 |
| Crystal system | Triclinic | Triclinic | Dc/(g·cm3) | 1.733 | 1.520 |
| Space group | P | P | μ/mm-1 | 0.200 | 0.174 |
| a/nm | 0.68005(5) | 0.68302(14) | F(000) | 312.0 | 412 |
| b/nm | 0.72370(5) | 1.1021(2) | Rint | 0.0165 | 0.0401 |
| c/nm | 1.22063(9) | 1.1713(2) | GOF | 1.068 | 1.050 |
| α/(°) | 92.5300(10) | 87.22(3) | R1, wR2[I>2σ(I)] | 0.0363/0.0939 | 0.0501/0.1255 |
| β(°) | 94.4880(10) | 81.70(3) | R1, wR2(all data) | 0.0449/0.0994 | 0.0649/0.1357 |
Table 1 Crystal data and structure refinement parameters for complexes 1 and 2
| Compound | 1 | 2 | γ/(°) | 105.4980(10) | 78.03(3) |
|---|---|---|---|---|---|
| Formula | C9H12MgN2O8 | C9H22MgN2O13 | V/nm3 | 0.57579(7) | 0.8534(3) |
| Formula weight | 300.52 | 390.60 | Z | 2 | 2 |
| Crystal system | Triclinic | Triclinic | Dc/(g·cm3) | 1.733 | 1.520 |
| Space group | P | P | μ/mm-1 | 0.200 | 0.174 |
| a/nm | 0.68005(5) | 0.68302(14) | F(000) | 312.0 | 412 |
| b/nm | 0.72370(5) | 1.1021(2) | Rint | 0.0165 | 0.0401 |
| c/nm | 1.22063(9) | 1.1713(2) | GOF | 1.068 | 1.050 |
| α/(°) | 92.5300(10) | 87.22(3) | R1, wR2[I>2σ(I)] | 0.0363/0.0939 | 0.0501/0.1255 |
| β(°) | 94.4880(10) | 81.70(3) | R1, wR2(all data) | 0.0449/0.0994 | 0.0649/0.1357 |
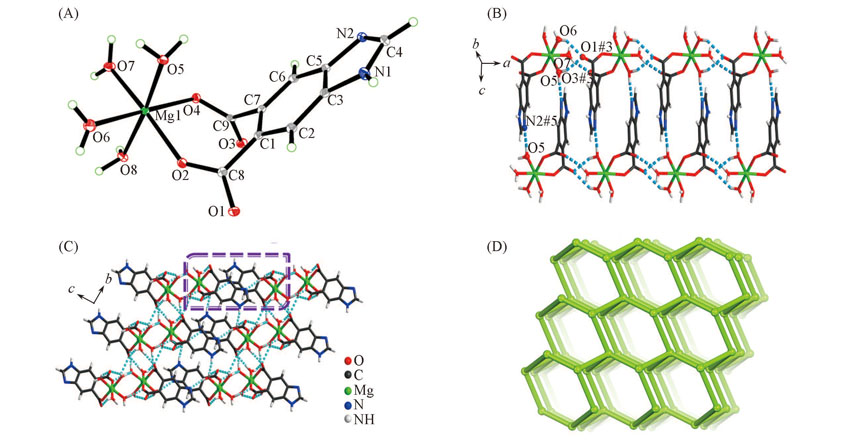
Fig.1 View of the asymmetric unit(A), 1D ladder chain formed by dimeric [Mg(Hbidc)(H2O)4]2 ring along a axis(B), 3D supramolecular framework(C) and topological representation of the 3D H-bonded network displaying a diamondoid network(D) of complex 1The dashed lines represent the H-bonds.
| D—H…A | d(D—H)/nm | d(H…A)/nm | d(D…A)/nm | ∠(DHA)/(°) |
|---|---|---|---|---|
| O6—H6'…O8#1 | 0.078(4) | 0.234(4) | 0.2952(3) | 135(4) |
| O8—H8'…O4#2 | 0.083(3) | 0.189(3) | 0.2713(2) | 172(3) |
| O7—H7'…O3#3 | 0.086(4) | 0.195(4) | 0.2759(2) | 157(3) |
| O6—H6…O3#4 | 0.082(3) | 0.245(3) | 0.2916(2) | 118(3) |
| O6—H6…O1#3 | 0.082(3) | 0.216(3) | 0.2864(3) | 143(3) |
| O5—H5'…O1#3 | 0.085(4) | 0.197(3) | 0.2767(2) | 154(3) |
| O7—H7…O3#2 | 0.090(3) | 0.184(3) | 0.2733(2) | 172(3) |
| O8—H8…O2#1 | 0.087(4) | 0.188(4) | 0.2722(2) | 164(3) |
| O5—H5…N2#5 | 0.088(4) | 0.187(4) | 0.2729(2) | 167(3) |
| N1—H1…O5#6 | 0.079(3) | 0.214(3) | 0.2919(3) | 166(3) |
Table 2 Bond lengths and bond angles of hydrogen bonds for complex 1*
| D—H…A | d(D—H)/nm | d(H…A)/nm | d(D…A)/nm | ∠(DHA)/(°) |
|---|---|---|---|---|
| O6—H6'…O8#1 | 0.078(4) | 0.234(4) | 0.2952(3) | 135(4) |
| O8—H8'…O4#2 | 0.083(3) | 0.189(3) | 0.2713(2) | 172(3) |
| O7—H7'…O3#3 | 0.086(4) | 0.195(4) | 0.2759(2) | 157(3) |
| O6—H6…O3#4 | 0.082(3) | 0.245(3) | 0.2916(2) | 118(3) |
| O6—H6…O1#3 | 0.082(3) | 0.216(3) | 0.2864(3) | 143(3) |
| O5—H5'…O1#3 | 0.085(4) | 0.197(3) | 0.2767(2) | 154(3) |
| O7—H7…O3#2 | 0.090(3) | 0.184(3) | 0.2733(2) | 172(3) |
| O8—H8…O2#1 | 0.087(4) | 0.188(4) | 0.2722(2) | 164(3) |
| O5—H5…N2#5 | 0.088(4) | 0.187(4) | 0.2729(2) | 167(3) |
| N1—H1…O5#6 | 0.079(3) | 0.214(3) | 0.2919(3) | 166(3) |
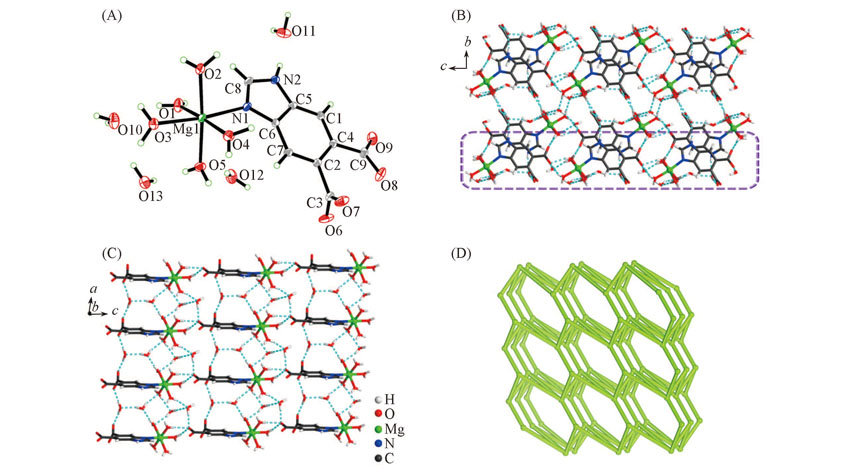
Fig.2 View of the asymmetric unit(A), 3D supramolecular framework along the bc plane(B), 2D supramolecular plane trapped tetramer water cyclic (H2O)4(C) and topological representation of the 3D H-bonded network displaying an sqp network(D) of complex 2The dashedlines represent the H-bonds.
| D—H…A | d(D—H)/nm | d(H…A)/nm | d(D…A)/nm | ∠(DHA)/(°) |
|---|---|---|---|---|
| O2—H17…O12#1 | 0.090(4) | 0.179(5) | 0.2683(3) | 176(4) |
| O4—H16…O2 | 0.086(4) | 0.205(4) | 0.2907(3) | 169(3) |
| O4—H15…O9#2 | 0.085(3) | 0.202(3) | 0.2807(3) | 153(3) |
| N2—H2…O3#3 | 0.086 | 0.196 | 0.2811(3) | 170.9 |
| O9—H12…O10#4 | 0.073(3) | 0.193(3) | 0.2661(3) | 173(3) |
| O5—H8…O12#5 | 0.075(4) | 0.205(4) | 0.2756(3) | 158(4) |
| O6—H5…O13#6 | 0.077(4) | 0.200(4) | 0.2776(3) | 180(4) |
| O7—H9…O12#5 | 0.086(4) | 0.203(4) | 0.2830(3) | 154(3) |
| O5—H11…O13#7 | 0.088(4) | 0.188(4) | 0.2753(3) | 173(3) |
| O6—H6…O2 | 0.080(4) | 0.202(4) | 0.2790(3) | 162(3) |
| O8—H13…O11#4 | 0.087(4) | 0.177(4) | 0.2637(3) | 172(4) |
| O8—H3…O4 | 0.071(4) | 0.209(4) | 0.2805(3) | 177(4) |
| O9—H14…O1#8 | 0.093(5) | 0.175(5) | 0.2678(3) | 171(4) |
| O7—H10…O4#9 | 0.088(4) | 0.197(4) | 0.2841(3) | 175(3) |
| O3—H21…O10#5 | 0.088(4) | 0.187(4) | 0.2738(3) | 168(3) |
| O3—H22…O11#1 | 0.089(4) | 0.184(4) | 0.2716(3) | 170(4) |
| O1—H19…O3#10 | 0.085(5) | 0.201(5) | 0.2824(4) | 159(4) |
| O1—H20…O8 | 0.080(5) | 0.215(5) | 0.2923(3) | 162(4) |
Table 3 Bond lengths and bond angles of hydrogen bonds for complex 2*
| D—H…A | d(D—H)/nm | d(H…A)/nm | d(D…A)/nm | ∠(DHA)/(°) |
|---|---|---|---|---|
| O2—H17…O12#1 | 0.090(4) | 0.179(5) | 0.2683(3) | 176(4) |
| O4—H16…O2 | 0.086(4) | 0.205(4) | 0.2907(3) | 169(3) |
| O4—H15…O9#2 | 0.085(3) | 0.202(3) | 0.2807(3) | 153(3) |
| N2—H2…O3#3 | 0.086 | 0.196 | 0.2811(3) | 170.9 |
| O9—H12…O10#4 | 0.073(3) | 0.193(3) | 0.2661(3) | 173(3) |
| O5—H8…O12#5 | 0.075(4) | 0.205(4) | 0.2756(3) | 158(4) |
| O6—H5…O13#6 | 0.077(4) | 0.200(4) | 0.2776(3) | 180(4) |
| O7—H9…O12#5 | 0.086(4) | 0.203(4) | 0.2830(3) | 154(3) |
| O5—H11…O13#7 | 0.088(4) | 0.188(4) | 0.2753(3) | 173(3) |
| O6—H6…O2 | 0.080(4) | 0.202(4) | 0.2790(3) | 162(3) |
| O8—H13…O11#4 | 0.087(4) | 0.177(4) | 0.2637(3) | 172(4) |
| O8—H3…O4 | 0.071(4) | 0.209(4) | 0.2805(3) | 177(4) |
| O9—H14…O1#8 | 0.093(5) | 0.175(5) | 0.2678(3) | 171(4) |
| O7—H10…O4#9 | 0.088(4) | 0.197(4) | 0.2841(3) | 175(3) |
| O3—H21…O10#5 | 0.088(4) | 0.187(4) | 0.2738(3) | 168(3) |
| O3—H22…O11#1 | 0.089(4) | 0.184(4) | 0.2716(3) | 170(4) |
| O1—H19…O3#10 | 0.085(5) | 0.201(5) | 0.2824(4) | 159(4) |
| O1—H20…O8 | 0.080(5) | 0.215(5) | 0.2923(3) | 162(4) |
| Atom | Charge | Atom | Charge | Atom | Charge | Atom | Charge | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H3bidc | 1 | H3bidc | 1 | H3bidc | 1 | H3bidc | ||||
| Mg1 | 1.491 | O4 | -0.732 | -0.578 | C7 | -0.026 | 0.026 | H5' | 0.539 | ||
| O5 | -1.077 | N1 | -0.575 | -0.541 | C4 | 0.079 | 0.166 | H6 | 0.541 | ||
| O6 | -1.018 | N2 | -0.515 | -0.405 | C8 | 0.568 | 0.539 | H6' | 0.519 | ||
| O7 | -1.049 | C1 | -0.058 | 0.026 | C9 | 0.604 | 0.529 | H7 | 0.508 | ||
| O8 | -1.080 | C2 | -0.349 | -0.191 | H2 | 0.283 | 0.193 | H7' | 0.525 | ||
| O1 | -0.627 | -0.519 | C3 | 0.234 | 0.255 | H3 | 0.309 | H8 | 0.535 | ||
| O2 | -0.737 | -0.553 | C5 | 0.204 | 0.214 | H1 | 0.523 | 0.352 | H8' | 0.544 | |
| O3 | -0.662 | -0.451 | C6 | -0.331 | -0.221 | H5 | 0.538 | H4 | 0.290 | 0.191 | |
Table 4 Mulliken atomic charges of compound 1 and H3bidc ligand
| Atom | Charge | Atom | Charge | Atom | Charge | Atom | Charge | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H3bidc | 1 | H3bidc | 1 | H3bidc | 1 | H3bidc | ||||
| Mg1 | 1.491 | O4 | -0.732 | -0.578 | C7 | -0.026 | 0.026 | H5' | 0.539 | ||
| O5 | -1.077 | N1 | -0.575 | -0.541 | C4 | 0.079 | 0.166 | H6 | 0.541 | ||
| O6 | -1.018 | N2 | -0.515 | -0.405 | C8 | 0.568 | 0.539 | H6' | 0.519 | ||
| O7 | -1.049 | C1 | -0.058 | 0.026 | C9 | 0.604 | 0.529 | H7 | 0.508 | ||
| O8 | -1.080 | C2 | -0.349 | -0.191 | H2 | 0.283 | 0.193 | H7' | 0.525 | ||
| O1 | -0.627 | -0.519 | C3 | 0.234 | 0.255 | H3 | 0.309 | H8 | 0.535 | ||
| O2 | -0.737 | -0.553 | C5 | 0.204 | 0.214 | H1 | 0.523 | 0.352 | H8' | 0.544 | |
| O3 | -0.662 | -0.451 | C6 | -0.331 | -0.221 | H5 | 0.538 | H4 | 0.290 | 0.191 | |
| Atom | Charge | Atom | Charge | Atom | Charge | Atom | Charge | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | H3bidc | 2 | H3bidc | 2 | H3bidc | 2 | H3bidc | ||||
| Mg | 1.510 | O12 | -0.751 | -0.578 | C9 | 0.572 | 0.539 | H12 | 0.522 | ||
| O1 | -0.997 | O13 | -0.656 | -0.553 | H1 | 0.289 | 0.191 | H13 | 0.520 | ||
| O2 | -0.989 | N1 | -0.587 | -0.405 | H4 | 0.376 | 0.193 | H14 | 0.538 | ||
| O3 | -1.029 | N2 | -0.551 | -0.541 | H7 | 0.274 | 0.163 | H16 | 0.518 | ||
| O4 | -1.023 | C1 | 0.077 | 0.166 | H2 | 0.507 | 0.352 | H18 | 0.466 | ||
| O5 | -1.048 | C2 | 0.230 | 0.214 | H3 | 0.520 | H19 | 0.503 | |||
| O6 | -1.030 | C3 | 0.230 | 0.255 | H5 | 0.525 | H20 | 0.511 | |||
| O7 | -1.039 | C4 | -0.354 | -0.191 | H6 | 0.529 | H21 | 0.521 | |||
| O8 | -1.067 | C5 | -0.040 | 0.026 | H8 | 0.511 | H22 | 0.516 | |||
| O9 | -1.085 | C6 | -0.044 | 0.026 | H9 | 0.521 | |||||
| O10 | -0.669 | -0.519 | C7 | -0.342 | -0.221 | H10 | 0.521 | ||||
| O11 | -0.676 | -0.451 | C8 | 0.589 | 0.529 | H11 | 0.521 | ||||
Table 5 Mulliken atomic charges of compound 2 and H3bidc ligand
| Atom | Charge | Atom | Charge | Atom | Charge | Atom | Charge | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | H3bidc | 2 | H3bidc | 2 | H3bidc | 2 | H3bidc | ||||
| Mg | 1.510 | O12 | -0.751 | -0.578 | C9 | 0.572 | 0.539 | H12 | 0.522 | ||
| O1 | -0.997 | O13 | -0.656 | -0.553 | H1 | 0.289 | 0.191 | H13 | 0.520 | ||
| O2 | -0.989 | N1 | -0.587 | -0.405 | H4 | 0.376 | 0.193 | H14 | 0.538 | ||
| O3 | -1.029 | N2 | -0.551 | -0.541 | H7 | 0.274 | 0.163 | H16 | 0.518 | ||
| O4 | -1.023 | C1 | 0.077 | 0.166 | H2 | 0.507 | 0.352 | H18 | 0.466 | ||
| O5 | -1.048 | C2 | 0.230 | 0.214 | H3 | 0.520 | H19 | 0.503 | |||
| O6 | -1.030 | C3 | 0.230 | 0.255 | H5 | 0.525 | H20 | 0.511 | |||
| O7 | -1.039 | C4 | -0.354 | -0.191 | H6 | 0.529 | H21 | 0.521 | |||
| O8 | -1.067 | C5 | -0.040 | 0.026 | H8 | 0.511 | H22 | 0.516 | |||
| O9 | -1.085 | C6 | -0.044 | 0.026 | H9 | 0.521 | |||||
| O10 | -0.669 | -0.519 | C7 | -0.342 | -0.221 | H10 | 0.521 | ||||
| O11 | -0.676 | -0.451 | C8 | 0.589 | 0.529 | H11 | 0.521 | ||||
| [1] | Liu J., Chen L., Cui H., Zhang J., Zhang L., Su C. Y., Chem. Soc. Rev., 2014, 43, 6011—6061 |
| [2] | Hu Z., Deibert B. J., Li J., Chem. Soc. Rev., 2014, 43, 5815—5840 |
| [3] | He C., Liu D., Lin W., Chem. Rev., 2015, 115, 11079—11108 |
| [4] | Li J. R., Kuppler R. J., Zhou H. C., Chem. Soc. Rev., 2009, 38, 1477—1504 |
| [5] | Talin A. A., Centrone A., Ford A. C., Foster M. E., Stavila V., Haney P., Kinney R. A., Szalai V., El Gabaly F., Yoon H. P., Leonard F., Allendorf M. D., Science, 2014, 343, 66—69 |
| [6] | Sun Y. X., Sun W. Y., Chinese Chem. Lett., 2014, 25, 823—828 |
| [7] | Liu L. L., Ren Z. G., Zhu L. W., Wang H. F., Yan W. Y., Lang J. P., Cryst. Growth Des., 2011, 11, 3479—3488 |
| [8] | Cui G. H., Li J. R., Tian J. L., Bu X. H., Batten S. R., Cryst. Growth Des., 2005, 5, 1775—1780 |
| [9] | Liu Q., Ren Z. G., Deng L., Zhang W. H., Zhao X., Sun Z. R., Lang J. P., Dalton Trans., 2015, 44, 130—137 |
| [10] | Liu D., Ren Z.G., Li H. X., Chen Y., Wang J., Zhang Y., Lang J. P., CrystEngComm, 2010, 12, 1912—1919 |
| [11] | Long L. S., Cryst. Eng. Comm., 2010, 12, 1354—1365 |
| [12] | Gao L. J., Wang L., Wang S. Y., Jing S. B., Chem. J. Chinese Universities, 2016, 37(9), 1589—1595 |
| (高丽娟, 王莉, 王圣燕, 井淑波.高等学校化学学报, 2016,37(9), 1589—1595) | |
| [13] | Hu F. L., Wang S. L., Wu B., Yu H., Wang F., Lang J. P., Cryst. Eng. Comm., 2014, 16, 6354—6363 |
| [14] | Sun Y. G., Wu Y. L., Xiong G., Smet P. F., Ding F., Guo M. Y., Zhu M. C., Gao E. J., Poelman D., Verpoort F., Dalton Trans., 2010, 39, 11383—11395 |
| [15] | Wei Y., Yu Y., Wu K., Cryst. Growth Des., 2008, 8, 2087—2089 |
| [16] | Liu Y. R., Li L., Yang T., Yu X. W., Su C. Y., Cryst. Eng. Comm., 2009, 11, 2712—2718 |
| [17] | Huang F. P., Tian J. L., Li D. D., Chen G. J., Gu W., Yan S. P., Liu X., Liao D. Z., Cheng P., Cryst. Eng. Comm., 2010, 12, 395—400 |
| [18] | Ma X., Li X., Cha Y. E., Jin L. P., Cryst. Growth Des., 2012, 12, 5227—5232 |
| [19] | Wang P., Fan R. Q., Yang Y. L., Liu X. R., Xiao P., Li X. Y., Hasi W., Cao W. W., Cryst. Eng. Comm., 2013, 15, 4489—4506 |
| [20] | Wang S. J., Tian Y. W., Xiong G., You L. X., Ding F., Guo M. Y., Gao E. J., Smet P. F., Poelman D., Xiao L. J., Sun Y. G., Cryst. Eng. Comm., 2012, 14, 8689—8697 |
| [21] | Sun Y. G., Zong W. H., Xiong G., Guo M. Y., Ding F., Wang S. J., You L. X., Ren B. Y., Xu Z. H., Gao E. J., Polyhedron, 2014, 83, 68—76 |
| [22] | Li Z. Y., Dai J. W., Wang N., Qiu H. H., Yue S. T., Liu Y. L., Cryst. Growth Des., 2010, 10, 2746—2751 |
| [23] | Wang H., Li X. F., Song W. D., Ma X. T., Liu J. H., Acta Crystallogr. E, 2010, 66, m151 |
| [24] | Li L., Zhang Y. T., Cheng J. S., Zhao R. F., Chinese J. Struc. Chem., 2011, 30, 563—567 |
| [25] | Wang W., Sun J. Y., Zhang D. J., Song T. Y., Song W., Zhang L. Y., Chen Y. L., Fan Y., Zhang P., Inorg. Chim. Acta, 2012, 384, 105—110 |
| [26] | Guo Z., Li G., Zhou L., Su S., Lei Y., Dang S., Zhang H., Inorg. Chem., 2009, 48, 8069—8071 |
| [27] | Mallick A., Saha S., Pachfule P., Roy S., Banerjee R., J. Mater. Chem., 2010, 20, 9073—9080 |
| [28] | Wu Z. F., Tan B., Feng M. L., Lan A. J., Huang X. Y., J. Mater. Chem. A, 2014, 2, 6426—6431 |
| [29] | Wu Z. F., Tan B., Deng Z. H., Xie Z. L., Fu J. J., Shen N. N., Huang X. Y., Chem. Eur. J., 2016, 22, 1334—1339 |
| [30] | Sun Y. Q., Liu Q., Zhong J. C., Pan Q. F., Chen Y. P., J. Solid State Chem., 2013, 206, 85—90 |
| [31] | Huang Y. L., Gong Y. N., Jiang L., Lu T. B., Chem. Commun., 2013, 49, 1753—1755 |
| [32] | Wei Y., Yu Y., Sa R., Li Q., Wu K., Cryst. Eng. Comm., 2009, 11, 1054—1060 |
| [33] | Long L. S., Wu Y. R., Huang R. B., Zheng L. S., Inorg. Chem., 2004, 43, 3798—3800 |
| [34] | Cruzan J. D., Braly L. B., Liu K., Brown M. G., Loeser J. G., Saykally R. J., Science, 1996, 271, 59—62 |
| [35] | Zhang X.F., Deng Z. P., Huo L. H., Feng Q. M., Gao S.,Eur. J. Inorg. Chem., 2012, 5506—5514 |
| [36] | Fan R. Q., Wang L. Y., Wang P., Chen H., Sun C. F., Yang Y. L., Su Q., J. Solid State Chem., 2012, 196, 332—340 |
| [37] | Shu Q. D., Kong S. N., Wei Y. Z., Shu M. H., Polyhedron, 2016, 118, 96—102 |
| [38] | Zhang R. G., Huang W., Wang B. J., Chinese J. Catal., 2007, 28, 641—645 |
| (章日光, 黄伟, 王宝俊, 催化学报, 2007, 28, 641—645) | |
| [39] | Liu X. F., Guo J. P., Xuan C. S., Zhang R. G., Ling L. X., Wang B. J., Chinese J. Inorg. Chem., 2008, 24, 708—714 |
| (柳学芳, 郭建平, 宣春生, 章日光, 凌丽霞, 王宝俊. 无机化学学报, 2008, 24, 708—714) |
| [1] | MIN Jing, WANG Liyan. 1H NMR Study on the Conformation of Aromatic Amides Limited by Three-center Hydrogen Bonds [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220084. |
| [2] | ZHANG Yong, XU Jun, BAO Yu, CUI Shuxun. Quantifying the Degree of Weakening Effect of Nonpolar Organic Solvent on the Strength of Intramolecular Hydrogen Bonding by Single-molecule Force Spectroscopy [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210863. |
| [3] | CUI Shaoli, ZHANG Weijia, SHAO Xueguang, CAI Wensheng. Revealing the Effect of Threonine on the Binding Ability of Antifreeze Proteins with Ice Crystals by Free-energy Calculations [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210838. |
| [4] | HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614. |
| [5] | GAO Huiling, CAO Zhenzhen, GU Fang, WANG Haijun. Monte Carlo Simulation on Self-healing Behaviour of Hydrogen-bonded Hydrogel [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220482. |
| [6] | WANG Le, QIN Liulei, LIU Yang, REN Li, XU Huiting, LIU Zunqi. Synthesis, Structure and Dielectric Properties of One-dimensional Chain Hydrogen Glycine Supramolecular Compound [(Gly)2+(18-crown-6)2(MnCl4)2‒] [J]. Chem. J. Chinese Universities, 2021, 42(3): 691. |
| [7] | NI Qingsheng, DU Miao, SHAN Guorong, SONG Yihu, WU Ziliang, ZHENG Qiang. Regulation of Rheological Behavior of Polyvinyl Alcohol Aqueous Solution by One-dimensional Particles [J]. Chem. J. Chinese Universities, 2021, 42(12): 3738. |
| [8] | GONG Shanshan, WU Tong, WANG Guange, HUANG Qing, SU Yuefeng, WU Feng. Screening of Deep Eutectic Solvent Based on Efficient Recovery of Spent Lithium⁃ion Battery Cathode Materials [J]. Chem. J. Chinese Universities, 2021, 42(10): 3151. |
| [9] | BAI Lan, ZHAI Lei, WANG Changou, HE Minhui, MO Song, FAN Lin. Thermal Expansion Behavior of Amide-containing Polyimide Films with Ultralow Thermal Expansion Coefficient † [J]. Chem. J. Chinese Universities, 2020, 41(4): 795. |
| [10] | QIN Liulei,LIU Yang,GUAN Xiaoqin,ZHENG Xiaoyuan,ZHANG Ziyu,LIU Zunqi. Synthesis and Switchable Dielectric Properties of an Inorganic-organic Hybrid Complex [H2(DABCO)CuCl4]·H2O † [J]. Chem. J. Chinese Universities, 2020, 41(1): 70. |
| [11] | XU Yan,LIU Cui,HAN Chengjuan,PAN Mingyu,SUN Zhaoqi,HAN Bingyu,YANG Zhongzhi. Development of Polarization Force Field for Guanine and Amino Acid Residues Systems† [J]. Chem. J. Chinese Universities, 2019, 40(2): 288. |
| [12] | LI Cheng,SONG Jixue,LIU Tingting,LI Yan,LIU Bingnan,WANG Liang,XIAO Shan,LI Lin,GENG Xuhui,WANG Jihui. Detection of Egg White Lysozyme Oligomers Based on Fluorescence Lifetime of ThT† [J]. Chem. J. Chinese Universities, 2019, 40(1): 90. |
| [13] | XU Yu,HUA Er. Hydrogen Bonding Study on Protic Ionic Liquids Composed of N-Alkyl Ethylenediaminum Cations with Trifluoroacetic Anion† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1954. |
| [14] | OUYANG Shunli, ZHANG Mingzhe, ZHANG Yongzhao, HU Qingcheng, WEI Haiyan, WU Nannan, HUANG Baokun. Raman Spectroscopic Investigation on the Effect of Hydrogen Bond on Molecular Structure in Ternary Aqueous Solution† [J]. Chem. J. Chinese Universities, 2018, 39(4): 758. |
| [15] | HAN Bingyu, LI Yue, LIU Cui. Investigation on the Hydrogen Bonding Interaction Between Amino Acid Side Chains and Base Pairs Containing Oxidized Guanine† [J]. Chem. J. Chinese Universities, 2017, 38(6): 1068. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||