

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (2): 269.doi: 10.7503/cjcu20150465
• Organic Chemistry • Previous Articles Next Articles
Received:2015-06-12
Online:2016-02-10
Published:2016-01-14
Contact:
TONG Mengliang
E-mail:13973327103@163.com
Supported by:CLC Number:
TrendMD:
YAN Dong, TONG Mengliang. Metal-free Thiolation of C(sp3)—S Bond Adjacent to an Oxygen Atom†[J]. Chem. J. Chinese Universities, 2016, 37(2): 269.
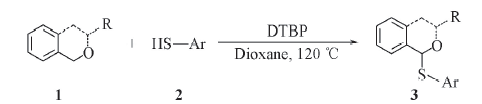
Scheme 1 Thioetherification of Isochromans 3a: R=Me, Ar=Ph; 3b: R=Et, Ar=Ph; 3c: R=i-Pr, Ar=Ph; 3d: R=Ph, Ar=Ph; 3e: R=PhCO, Ar=Ph; 3f: R= Me, Ar=4-Me-Ph; 3g: R=Me, Ar=4-OMe-Ph; 3h: R=Me, Ar=4-Cl-Ph; 3i: R= Me, Ar=4-Me-Ph; 3j: R=i-Pr, Ar=Ph; 3k: R=i-Pr, Ar=4-Cl-Ph; 3l: R=—CH2CH2—, Ar=Ph; 3m: R=—CH2CH2—, Ar=4-Cl-Ph
| Compd. | Appearance | m.p./℃ | HR-MS, m/z([M]+) | IR(KBr), |
|---|---|---|---|---|
| 3a | Yellow oil | — | 230.0758 | 2956, 2854, 1645, 1479, 1387, 1022, 745, 698 |
| 3b | Yellow solid | 67—68 | 244.0913 | 2916, 2848, 1490, 1390, 1014, 810, 723 |
| 3c | Brown solid | 70—72 | 258.1071 | 2935, 2860, 1661, 1507, 1379, 1009, 793 |
| 3d | Pale yellow oil | — | 292.0918 | 3011, 1597, 1495, 1212, 1019, 798, 694 |
| 3e | Brown solid | 87—89 | 320.0866 | 2930, 1634, 1594, 1392, 1226, 1033, 783 |
| 3f | Yellow oil | — | 244.0913 | 3013, 2918, 2843, 1595, 1492, 1238, 1016, 785 |
| 3g | Brown solid | 72—74 | 260.0867 | 2923, 2841, 1593, 1498, 1236, 1012, 831 |
| 3h | Yellow solid | 77—78 | 264.0370 | 2917, 2851, 1478, 1389, 1261, 1101, 1008, 812 |
| 3i | Yellow oil | — | 272.1223 | 2941, 2876, 1497, 1354, 1242, 1104, 1011, 806 |
| 3j | Pale yellow oil | — | 288.1177 | 2923, 2824, 1596, 1369, 1198, 1016, 822, 697 |
| 3k | Yellow oil | — | 292.0678 | 2921, 2837, 1588, 1469, 1382, 1258, 1127, 996, 793 |
| 3l | Yellow solid | 84—86 | 242.0759 | 3012, 2923, 2844, 1596, 1389, 1244, 1012, 803, 698 |
| 3m | Yellow solid | 81—82 | 276.0368 | 3009, 2915, 2832, 1594, 1471, 1254, 1123, 1014, 798 |
Table 1 Appearance, melting points, HR-MS and IR data of compounds 3a—3m
| Compd. | Appearance | m.p./℃ | HR-MS, m/z([M]+) | IR(KBr), |
|---|---|---|---|---|
| 3a | Yellow oil | — | 230.0758 | 2956, 2854, 1645, 1479, 1387, 1022, 745, 698 |
| 3b | Yellow solid | 67—68 | 244.0913 | 2916, 2848, 1490, 1390, 1014, 810, 723 |
| 3c | Brown solid | 70—72 | 258.1071 | 2935, 2860, 1661, 1507, 1379, 1009, 793 |
| 3d | Pale yellow oil | — | 292.0918 | 3011, 1597, 1495, 1212, 1019, 798, 694 |
| 3e | Brown solid | 87—89 | 320.0866 | 2930, 1634, 1594, 1392, 1226, 1033, 783 |
| 3f | Yellow oil | — | 244.0913 | 3013, 2918, 2843, 1595, 1492, 1238, 1016, 785 |
| 3g | Brown solid | 72—74 | 260.0867 | 2923, 2841, 1593, 1498, 1236, 1012, 831 |
| 3h | Yellow solid | 77—78 | 264.0370 | 2917, 2851, 1478, 1389, 1261, 1101, 1008, 812 |
| 3i | Yellow oil | — | 272.1223 | 2941, 2876, 1497, 1354, 1242, 1104, 1011, 806 |
| 3j | Pale yellow oil | — | 288.1177 | 2923, 2824, 1596, 1369, 1198, 1016, 822, 697 |
| 3k | Yellow oil | — | 292.0678 | 2921, 2837, 1588, 1469, 1382, 1258, 1127, 996, 793 |
| 3l | Yellow solid | 84—86 | 242.0759 | 3012, 2923, 2844, 1596, 1389, 1244, 1012, 803, 698 |
| 3m | Yellow solid | 81—82 | 276.0368 | 3009, 2915, 2832, 1594, 1471, 1254, 1123, 1014, 798 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
|---|---|---|
| 3a | 7.17—7.26(m, 10H), 5.67(s, 1H), 3.47(s, 3H) | 139.5, 138.0, 134.3, 133.2, 129.5, 128.1, 127.9, 126.4, 90.2, 56.8 |
| 3b | 7.31(dd, J=4.0, 2.9 Hz, 5H), 7.23(d, J=8.2 Hz, 2H), 7.06(m, 3H), 5.68(s, 1H), 3.42(m, 2H), 1.20(t, J=7.5 Hz, 3H) | 139.4, 138.1, 135.1, 133.2, 129.5, 128.1, 128.1, 127.2, 91.3, 58.2, 21.3 |
| 3c | 7.32—7.30(m, 5H), 7.23(d, J=8.2 Hz, 2H), 7.03(t, J=8.2 Hz, 3H), 5.97(s, 1H), 3.53(m, 1H), 1.29(s, 3H), 1.27(s, 3H) | 138.6, 133.7, 131.4, 129.7, 129.3, 129.0, 128.2, 126.3, 85.0, 73.5, 23.9, 23.8 |
| 3d | 7.34—7.28(m, 5H), 7.24—7.20(m, 5H), 7.11—7.08(t, J=8.2 Hz, 2H), 6.93—6.85(m, 3H), 6.07(s, 1H) | 146.5, 139.0, 134.5, 132.5, 132.3, 130.8, 130.7, 129.4, 129.3, 128.5, 127.6, 125.4, 119.8, 89.2 |
| 3e | 7.97(d, J=6.1 Hz, 2H), 7.47(t, J=7.4 Hz, 1H), 7.40(t, J=7.4 Hz, 2H), 7.35—7.15(m, 10H), 7.09(t, J=7.4 Hz, 1H) | 164.2, 138.9, 133.8, 133.3, 131.4, 131.3, 130.5, 129.8, 129.7, 129.7, 129.6, 129.4, 129.3, 128.8, 128.7, 128.6, 128.4, 125.9, 125.8, 85.3 |
| 3f | 7.48(d, J=7.8 Hz, 2H), 7.40—7.30(m, 1H), 7.26—7.00(m, 5H), 6.45(s, 1H), 4.55—4.52(m, 1H), 3.98(dd, J=11.4, 6.3 Hz, 1H), 3.25—3.05(m, 1H), 2.65—2.63(m, 1H), 2.28(s, 3H) | 137.4, 134.0, 133.9, 132.2, 132.1, 129.8, 128.9, 127.7, 127.2, 126.1, 86.4, 77.4, 77.1, 76.9, 58.2, 27.9, 21.2 |
| 3g | 7.31—7.25(m, 7H), 6.84(d, J=8.2 Hz, 2H), 5.68(s, 1H), 3.82(s, 3H), 3.42(s, 3H) | 141.3, 139.0, 134.3, 133.2, 129.4, 128.2, 127.6, 125.2, 90.2, 58.2, 56.8 |
| 3h | 7.30—7.29(d, J=8.3 Hz, 4H), 7.25—7.22(m, 5H), 5.80(s, 1H), 3.82(s, 3H), 3.38(s, 3H) | 138.3, 136.0, 133.5, 132.8, 132.0, 129.3, 128.2, 127.6, 126.9, 89.2, 55.9 |
| 3i | 7.31—7.22(m, 7H), 7.08(d, J=8.2 Hz, 2H), 5.68(s, 1H), 3.51(m, 2H), 1.27(s, 3H), 1.26(s, 6H) | 139.6, 138.9, 133.3, 131.2, 130.8, 129.1, 128.7, 126.4, 87.9, 78.2, 23.7, 21.2 |
| 3j | 7.32—7.28(m, 6H), 7.24(t, J=7.5 Hz, 1H), 6.98(d, J=8.2 Hz, 2H), 5.68(s, 1H), 3.68(s, 1H), 3.47(m, 1H), 1.24(s, 3H), 1.22(s, 6H) | 142.8, 138.9, 133.3, 131.2, 130.8, 128.7, 126.4, 115.6, 87.9, 75.2, 56.0, 22.7, 21.2 |
| 3k | 7.32—7.28(m, 6H), 7.28—7.21(m, 3H), 5.68(s, 1H), 3.47(m, 1H), 1.24(s, 3H), 1.22(s, 6H) | 138.9, 135.5, 133.6, 132.2, 129.9, 128.9, 128.2, 126.4, 87.9, 75.2, 21.2 |
| 3l | 7.43—7.31(m, 5H), 7.24—7.19(m, 4H), 6.49(s, 1H), 4.55—4.53(m, 2H), 3.20—3.09(m, 1H), 2.72(dd, J=16.5, 2.4 Hz, 1H) | 136.6, 132.9, 132.5, 132.3, 132.2, 128.1, 127.9, 126.9, 126.1, 125.2, 85.1, 57.4, 25.9 |
| 3m | 7.62(d, J=7.8 Hz, 2H), 7.41—7.32(m, 1H), 7.26—7.00(m, 5H), 6.45(s, 1H), 4.55(m, 1H), 3.98(dd, J=11.4, 6.3 Hz, 1H), 3.25—3.05(m, 1H), 2.65(dd, J=16.5, 2.9 Hz, 1H), 2.28(s, 3H) | 138.4, 136.0, 133.5, 132.7, 132.0, 129.3, 128.2, 127.6, 126.9, 126.1, 125.2, 85.1, 57.5, 25.8 |
Table 2 1H NMR, 13C NMR data of compounds 3a—3m
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
|---|---|---|
| 3a | 7.17—7.26(m, 10H), 5.67(s, 1H), 3.47(s, 3H) | 139.5, 138.0, 134.3, 133.2, 129.5, 128.1, 127.9, 126.4, 90.2, 56.8 |
| 3b | 7.31(dd, J=4.0, 2.9 Hz, 5H), 7.23(d, J=8.2 Hz, 2H), 7.06(m, 3H), 5.68(s, 1H), 3.42(m, 2H), 1.20(t, J=7.5 Hz, 3H) | 139.4, 138.1, 135.1, 133.2, 129.5, 128.1, 128.1, 127.2, 91.3, 58.2, 21.3 |
| 3c | 7.32—7.30(m, 5H), 7.23(d, J=8.2 Hz, 2H), 7.03(t, J=8.2 Hz, 3H), 5.97(s, 1H), 3.53(m, 1H), 1.29(s, 3H), 1.27(s, 3H) | 138.6, 133.7, 131.4, 129.7, 129.3, 129.0, 128.2, 126.3, 85.0, 73.5, 23.9, 23.8 |
| 3d | 7.34—7.28(m, 5H), 7.24—7.20(m, 5H), 7.11—7.08(t, J=8.2 Hz, 2H), 6.93—6.85(m, 3H), 6.07(s, 1H) | 146.5, 139.0, 134.5, 132.5, 132.3, 130.8, 130.7, 129.4, 129.3, 128.5, 127.6, 125.4, 119.8, 89.2 |
| 3e | 7.97(d, J=6.1 Hz, 2H), 7.47(t, J=7.4 Hz, 1H), 7.40(t, J=7.4 Hz, 2H), 7.35—7.15(m, 10H), 7.09(t, J=7.4 Hz, 1H) | 164.2, 138.9, 133.8, 133.3, 131.4, 131.3, 130.5, 129.8, 129.7, 129.7, 129.6, 129.4, 129.3, 128.8, 128.7, 128.6, 128.4, 125.9, 125.8, 85.3 |
| 3f | 7.48(d, J=7.8 Hz, 2H), 7.40—7.30(m, 1H), 7.26—7.00(m, 5H), 6.45(s, 1H), 4.55—4.52(m, 1H), 3.98(dd, J=11.4, 6.3 Hz, 1H), 3.25—3.05(m, 1H), 2.65—2.63(m, 1H), 2.28(s, 3H) | 137.4, 134.0, 133.9, 132.2, 132.1, 129.8, 128.9, 127.7, 127.2, 126.1, 86.4, 77.4, 77.1, 76.9, 58.2, 27.9, 21.2 |
| 3g | 7.31—7.25(m, 7H), 6.84(d, J=8.2 Hz, 2H), 5.68(s, 1H), 3.82(s, 3H), 3.42(s, 3H) | 141.3, 139.0, 134.3, 133.2, 129.4, 128.2, 127.6, 125.2, 90.2, 58.2, 56.8 |
| 3h | 7.30—7.29(d, J=8.3 Hz, 4H), 7.25—7.22(m, 5H), 5.80(s, 1H), 3.82(s, 3H), 3.38(s, 3H) | 138.3, 136.0, 133.5, 132.8, 132.0, 129.3, 128.2, 127.6, 126.9, 89.2, 55.9 |
| 3i | 7.31—7.22(m, 7H), 7.08(d, J=8.2 Hz, 2H), 5.68(s, 1H), 3.51(m, 2H), 1.27(s, 3H), 1.26(s, 6H) | 139.6, 138.9, 133.3, 131.2, 130.8, 129.1, 128.7, 126.4, 87.9, 78.2, 23.7, 21.2 |
| 3j | 7.32—7.28(m, 6H), 7.24(t, J=7.5 Hz, 1H), 6.98(d, J=8.2 Hz, 2H), 5.68(s, 1H), 3.68(s, 1H), 3.47(m, 1H), 1.24(s, 3H), 1.22(s, 6H) | 142.8, 138.9, 133.3, 131.2, 130.8, 128.7, 126.4, 115.6, 87.9, 75.2, 56.0, 22.7, 21.2 |
| 3k | 7.32—7.28(m, 6H), 7.28—7.21(m, 3H), 5.68(s, 1H), 3.47(m, 1H), 1.24(s, 3H), 1.22(s, 6H) | 138.9, 135.5, 133.6, 132.2, 129.9, 128.9, 128.2, 126.4, 87.9, 75.2, 21.2 |
| 3l | 7.43—7.31(m, 5H), 7.24—7.19(m, 4H), 6.49(s, 1H), 4.55—4.53(m, 2H), 3.20—3.09(m, 1H), 2.72(dd, J=16.5, 2.4 Hz, 1H) | 136.6, 132.9, 132.5, 132.3, 132.2, 128.1, 127.9, 126.9, 126.1, 125.2, 85.1, 57.4, 25.9 |
| 3m | 7.62(d, J=7.8 Hz, 2H), 7.41—7.32(m, 1H), 7.26—7.00(m, 5H), 6.45(s, 1H), 4.55(m, 1H), 3.98(dd, J=11.4, 6.3 Hz, 1H), 3.25—3.05(m, 1H), 2.65(dd, J=16.5, 2.9 Hz, 1H), 2.28(s, 3H) | 138.4, 136.0, 133.5, 132.7, 132.0, 129.3, 128.2, 127.6, 126.9, 126.1, 125.2, 85.1, 57.5, 25.8 |
| Entry | Oxidant | n(Oxidant)/mmol | T/℃ | Solvent | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | DTBP | 4.0 | 120 | Dioxane | 39 |
| 2 | K2S2O8 | 4.0 | 120 | Dioxane | |
| 3 | TBHP | 4.0 | 120 | Dioxane | 20 |
| 4 | DDQ | 4.0 | 120 | Dioxane | 12 |
| 5 | BPO | 4.0 | 120 | Dioxane | Trace |
| 6 | DTBP | 3.0 | 120 | Dioxane | 44 |
| 7 | DTBP | 2.0 | 120 | Dioxane | 53 |
| 8 | DTBP | 1.5 | 120 | Dioxane | 80 |
| 9 | DTBP | 1.0 | 120 | Dioxane | 32 |
| 10 | DTBP | 1.5 | 100 | Dioxane | 58 |
| 11 | DTBP | 1.5 | 140 | Dioxane | 67 |
| 12 | DTBP | 1.5 | 120 | Benzene | 69 |
| 13 | DTBP | 1.5 | 120 | PhCl | 56 |
| 14 | DTBP | 1.5 | 120 | Toluene | 13 |
Table 3 Optimization of the reactions between thiophenol and benzyl ethera
| Entry | Oxidant | n(Oxidant)/mmol | T/℃ | Solvent | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | DTBP | 4.0 | 120 | Dioxane | 39 |
| 2 | K2S2O8 | 4.0 | 120 | Dioxane | |
| 3 | TBHP | 4.0 | 120 | Dioxane | 20 |
| 4 | DDQ | 4.0 | 120 | Dioxane | 12 |
| 5 | BPO | 4.0 | 120 | Dioxane | Trace |
| 6 | DTBP | 3.0 | 120 | Dioxane | 44 |
| 7 | DTBP | 2.0 | 120 | Dioxane | 53 |
| 8 | DTBP | 1.5 | 120 | Dioxane | 80 |
| 9 | DTBP | 1.0 | 120 | Dioxane | 32 |
| 10 | DTBP | 1.5 | 100 | Dioxane | 58 |
| 11 | DTBP | 1.5 | 140 | Dioxane | 67 |
| 12 | DTBP | 1.5 | 120 | Benzene | 69 |
| 13 | DTBP | 1.5 | 120 | PhCl | 56 |
| 14 | DTBP | 1.5 | 120 | Toluene | 13 |
| Entry | Compd. | Yield(%)b | Entry | Compd. | Yield(%)b |
|---|---|---|---|---|---|
| 1 |  | 79 | 7 |  | 82 |
| 2 |  | 78 | 8 |  | 74 |
| 3 |  | 82 | 9 |  | 86 |
| 4 |  | 79 | 10 |  | 81 |
| 5 |  | 77 | 11 |  | 76 |
| 6 |  | 80 | 12 |  | 76 |
Table 4 Scope of the reactiona
| Entry | Compd. | Yield(%)b | Entry | Compd. | Yield(%)b |
|---|---|---|---|---|---|
| 1 |  | 79 | 7 |  | 82 |
| 2 |  | 78 | 8 |  | 74 |
| 3 |  | 82 | 9 |  | 86 |
| 4 |  | 79 | 10 |  | 81 |
| 5 |  | 77 | 11 |  | 76 |
| 6 |  | 80 | 12 |  | 76 |
| [1] | Li C. J., Acc. Chem. Res., 2009, 42(2), 335—344 |
| [2] | Yi W. G., Yan D., Wu C., Lan L. X., Chem. J. Chinese Universities, 2014, 35(12), 2563—2566 |
| (易卫国, 鄢东, 吴超, 兰立新. 高等学校化学学报, 2014, 35(12), 2563—2566) | |
| [3] | Zhang Y., Feng B. N., Chin. J. Org. Chem., 2014, 34(12), 2406—2411 |
| (张艳, 冯柏年. 有机化学, 2014, 34(12), 2406—2411) | |
| [4] | Kumar R. A., Saidulu G., Prasad K. R., Kumar G. S., Sridhar B., Reddy K. R., Adv. Synth. Catal., 2012, 354(16), 2985—2991 |
| [5] | Chu L., Qing F. L., Chem. Commun., 2010, 46, 6285—6287 |
| [6] | Wu C., Huang W. Y., He W. M., Xiang J. N., Chem. Lett., 2013, 42, 1233—1234 |
| [7] | Shu X. Z., Xia X. F., Yang Y. F., Ji K. G., Liu X. Y., Liang Y. M., J. Org. Chem., 2009, 74(19), 7464—7469 |
| [8] | He T., Yu L., Zhang L., Wang L., Wang M., Org. Lett., 2011, 13(19), 5016—5019 |
| [9] | Chen L., Shi E., Liu Z., Chen S., Wei W., Li H., Xu K., Wan X., Chem. Eur. J., 2011, 17(15), 4085—4089 |
| [10] | Li X., Wang H. Y., Shi Z. J., New J. Chem., 2013, 37, 1704—1706 |
| [11] | Chen D., Pan F. Y., Gao J. R., Yang J. G., Synlett., 2013, 24(16), 2085—2088 |
| [12] | Xiang S. K., Zhang B., Zhang L. H., Cui Y. X., Jiao N., Sci. China Chem., 2012, 55(1), 50—54 |
| [13] | Correia C. A., Li C. J., Heterocycles, 2010, 82(1), 555—562 |
| [14] | Zhang Y. H., Li C. J., J. Am. Chem. Soc., 2006, 128(13), 4242—4243 |
| [15] | Kang X., Yan R. L., Yu G. Q., Pang X. B., Liu X. X., Li X. N., Xiang L. K., Huang G. S., J. Org. Chem., 2014, 79(21), 10605—10610 |
| [16] | Varun B. V., Prabhu K. R., J. Org. Chem., 2014, 79(20), 9655—9668 |
| [17] | Corbet J. P., Mignani G., Chem. Rev., 2006, 106(7), 2651—2710 |
| [18] | Frei R., Waser J., J. Am. Chem. Soc., 2013, 135(26), 9620—9623 |
| [19] | Saravanan P., Anbarasan P., Org. Lett., 2014, 16(3), 848—851 |
| [20] | Zhao J., Xuan L. N., Zhao H. C., Cheng J., Fu X. Y., Li S., Jing F., Liu Y. M., Chen B. Q., Chem. Res. Chinese Universities, 2014, 30(5), 764—769 |
| [21] | Guo S. R., Yuan Y. Q., Xiang J. N., Org. Lett., 2013, 15(18), 4654—4657 |
| [1] | LIN Junxu, XI Zhiwei, LI Zhiping, WANG Yingchun. Palladium Catalyzed Selective Synthesis of Pyrrolofuran Derivatives and Carbamates from Propargylic Alcohols and Tert butyl isonitrile#br# [J]. Chem. J. Chinese Universities, 0, (): 20220473. |
| [2] | GUO Zhiqiang, YANG Boru, XI Chanjuan. Recent Advances in Reductive Functionalization of Carbon Dioxide with Borohydride Reagents [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220199. |
| [3] | ZHANG Zhen, DENG Yu, ZHANG Qinfang, YU Dagang. Visible Light-driven Carboxylation with CO2 [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220255. |
| [4] | YU Jing, WU Chao, LI Chenyang, CHEN Danfeng, DING Liuyue, MA Xiantao. Catalyst-free and Highly Efficient O-Silylation of Alcohols and Phenols [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210588. |
| [5] | HU Chuanchuan, PANG Jingxiang, HE Chuangchuang, LI Wei, SUN Shutao. Sc(OTf)3 Catalyzed 1,6-Conjugate Allylation of δ-CN p-QMs: Synthesis of Allyl Substituted Diarylacetonitrile Compounds [J]. Chem. J. Chinese Universities, 2021, 42(9): 2805. |
| [6] | LIU Huazheng, PAN Xiaoguang, LI Hua, WAN Renzhong, LIU Xigong. Na2CO3-catalyzed 1,6-Conjugate Addition of Trimethylsilyl Azide to δ-CF3-δ-Aryl-disubstituted Para-Quinone Methides: Efficient Construction of Diarylmethanes Bearing CF3- and N3-Substituted Quaternary Stereocenters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2772. |
| [7] | GUO Yang, LIN Kai, XIE Kaiqiang, LIU Sheng. Novel Approach to Isatins via Pd-Cu Catalyzed Oxidative Transformation [J]. Chem. J. Chinese Universities, 2021, 42(9): 2798. |
| [8] | LIN Junjie, WANG Shuang, LI Weiqiang, CUI Xin, HUANG Chao. Efficient Synthesis of Pyridine [2,3-d]pyrimidine Derivatives by Catalyst-free Tandem Cyclization Under Microwave Irradiation [J]. Chem. J. Chinese Universities, 2020, 41(12): 2749. |
| [9] | ZHOU Jinlong, LIU Xiaolong, FU Yao. Visible-light-induced Selective Oxidation of Alcohols [J]. Chem. J. Chinese Universities, 2020, 41(11): 2435. |
| [10] | ZHANG Shuxin, FENG Yu, FAN Qinghua. Progress of Transition Metal⁃catalyzed Asymmetric Hydrogenation in China† [J]. Chem. J. Chinese Universities, 2020, 41(10): 2107. |
| [11] | QIN Wenbing, LIN Weifeng, LI Xin, XIONG Wei, LIU Guokai. Difluoromethylation of Dicyanoalkylidenes by Electrophilic S-(Difluoromethyl)sulfonium Salt: Efficient Construction of Difluoromethylated All-carbon-substituted Centers† [J]. Chem. J. Chinese Universities, 2020, 41(10): 2230. |
| [12] | WANG Yang, WANG Sidi, TANG Shaokun. Synthesis and Characterization of Imine-based Covalent Organic Framework(COF-LZU1) in Supercritical Carbon Dioxide [J]. Chem. J. Chinese Universities, 2020, 41(8): 1792. |
| [13] | XU Wenyi,FENG Yisi. Oxidative Trifluoromethylation of CF3SO2Na with Olefins Mediated by Diacetyl† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1567. |
| [14] | HUANG Mingyao, ZHU Shoufei. Recent Advances of Catalytic Asymmetric C—B Bond Forming Reactions† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1426. |
| [15] | SUN Mengying,Lü Jingchun,XU Hong,ZHANG Linping,ZHONG Yi,CHEN Zhize,SUI Xiaofeng,MAO Zhiping. Synthesis and Electrochromic Performance of Phosphazene-viologen Polymer † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1399. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
