

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (2): 274.doi: 10.7503/cjcu20150695
• Organic Chemistry • Previous Articles Next Articles
PAN Qi1, LI Daixi1,*( ), GUO Baisong2, YANG Chunsheng2, YANG Zhi2
), GUO Baisong2, YANG Chunsheng2, YANG Zhi2
Received:2015-09-08
Online:2016-02-10
Published:2016-01-13
Contact:
LI Daixi
E-mail:dxli75@126.com
Supported by:CLC Number:
TrendMD:
PAN Qi, LI Daixi, GUO Baisong, YANG Chunsheng, YANG Zhi. Active Structure Protection of Monoclonal Antibody Fusion Protein Etanercept†[J]. Chem. J. Chinese Universities, 2016, 37(2): 274.
| Model | Etanercept/trehalose/mannitol | Number of water | Trehalose content(%) | Mannitol content(%) |
|---|---|---|---|---|
| Control | 1/0/0 | 37481 | 0 | 0 |
| THL-50 | 1/50/0 | 51156 | 1.73 | 0 |
| THL-100 | 1/100/0 | 50383 | 3.45 | 0 |
| THL-150 | 1/150/0 | 49604 | 5.16 | 0 |
| THL-200 | 1/200/0 | 48829 | 6.86 | 0 |
| THL-250 | 1/250/0 | 48041 | 8.54 | 0 |
| THL-300 | 1/300/0 | 47244 | 10.23 | 0 |
| THL-MAN | 1/120/112 | 49068 | 4.12 | 2.06 |
Table 1 Models for simulation and the detail components
| Model | Etanercept/trehalose/mannitol | Number of water | Trehalose content(%) | Mannitol content(%) |
|---|---|---|---|---|
| Control | 1/0/0 | 37481 | 0 | 0 |
| THL-50 | 1/50/0 | 51156 | 1.73 | 0 |
| THL-100 | 1/100/0 | 50383 | 3.45 | 0 |
| THL-150 | 1/150/0 | 49604 | 5.16 | 0 |
| THL-200 | 1/200/0 | 48829 | 6.86 | 0 |
| THL-250 | 1/250/0 | 48041 | 8.54 | 0 |
| THL-300 | 1/300/0 | 47244 | 10.23 | 0 |
| THL-MAN | 1/120/112 | 49068 | 4.12 | 2.06 |
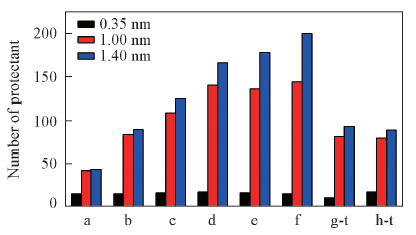
Fig.2 Numbers of protection in different thickness of protective layer around atanercept in each system a-f respectively represents the etanercept solvation systems with 50, 100, 150, 200, 250 and 300 molecular numbers of trehalose. g-t and g-m respectively represents trehalose and mannitol in complex protection system.
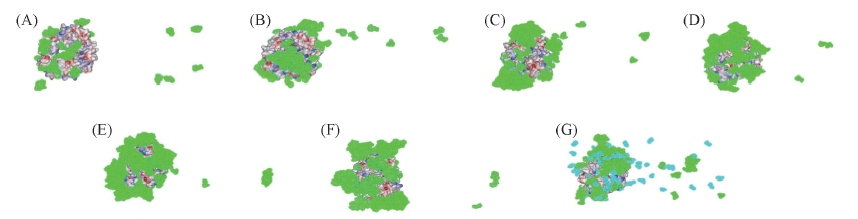
Fig.3 Regional selective adsorption of protectant onto the surface of etanercept in different systems (A)—(F) represent the etanercept solvation systems with 50, 100, 150, 200, 250 and 300 molecular numbers of trehalose. (G) is trehalose and mannitol complex protection system. Closed electrostatic potential surface: etanercept; green: trehalose; cyan: mannitol. In order to show clearly, the molecular of water in systems is removed.
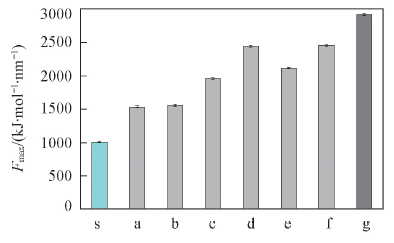
Fig.5 Maximum pull force for dissociating etanercept dimer in different system s. THL-0; a. THL-50; b. THL-100; c. THL-150; d. THL-200; e. THL-250; f. THL-300; g. THL-MAN.
| System | Error/(kJ·mol-1) | System | Error/(kJ·mol-1) | ||
|---|---|---|---|---|---|
| Control | 314.46 | 6.32 | THL-200 | 333.20 | 8.48 |
| THL-50 | 487.63 | 8.63 | THL-250 | 721.78 | 10.46 |
| THL-100 | 684.51 | 8.20 | THL-300 | 888.96 | 8.91 |
| THL-150 | 721.60 | 9.77 | THL-MAN | 518.97 | 9.08 |
Table 2 Average PMF free energies and errors in etanercept dissociation in different systems
| System | Error/(kJ·mol-1) | System | Error/(kJ·mol-1) | ||
|---|---|---|---|---|---|
| Control | 314.46 | 6.32 | THL-200 | 333.20 | 8.48 |
| THL-50 | 487.63 | 8.63 | THL-250 | 721.78 | 10.46 |
| THL-100 | 684.51 | 8.20 | THL-300 | 888.96 | 8.91 |
| THL-150 | 721.60 | 9.77 | THL-MAN | 518.97 | 9.08 |
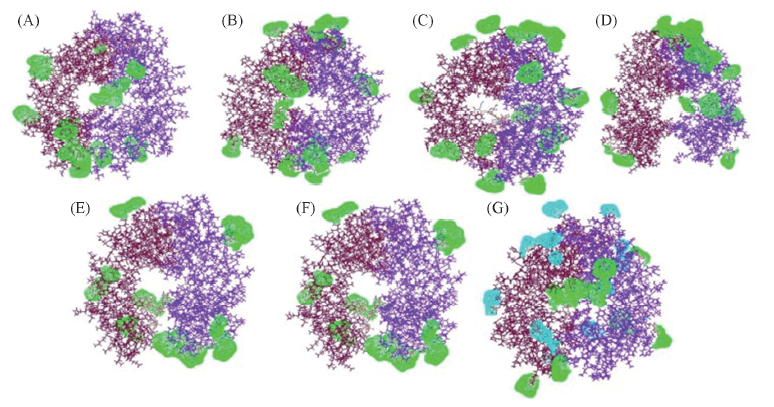
Fig.7 Adsorption of protectant within the range of 0.35 nm from etanercept dimer surface (A)—(F) represent the etanercept solvation systems with 50, 100, 150, 200, 250 and 300 molecular numbers of trehalose. (G) is trehalose and mannitol complex protection system. Dimer with scarlet and indigo: etanercept; green: trehalose; cyan: mannitol. In order to show clearly, the molecular of water in systems is removed.
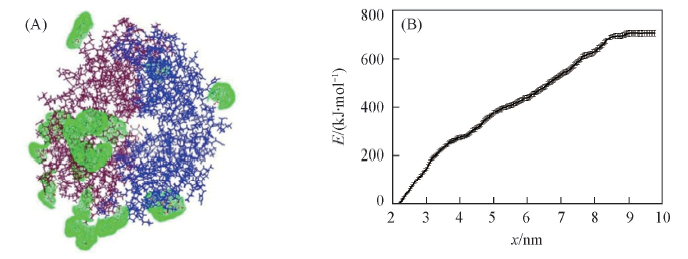
Fig.8 Effect of trehalose adsorptive location on the structural stability of etanercept dimer (A) Adsorption of trehalose within the range of 0.35 nm from the etanercept dimer surface;(B) PMF free energy of etanercept dissociation in new system.
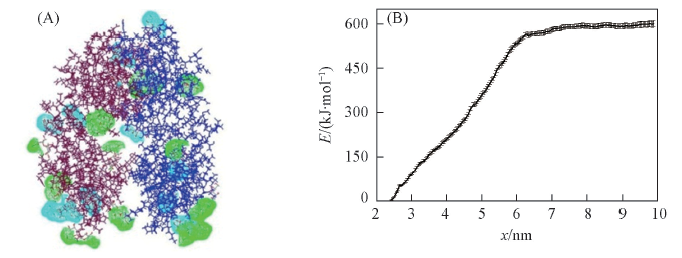
Fig.9 Effect of adsorptive location on the structural stability of etanercept dimer (A) Adsorption of protectant within the range of 0.35 nm from the etanercept dimer surface;(B) the PMF free energy of etanercept dissociation in new complex system.
| [1] | Goldenberg M. M., Clin. Ther., 1999, 21, 75—87 |
| [2] | Mease P. J., Goffe B. S., Metz J., Vanderstoep A., Finck B., Burge D. J., Lancet, 2000, 356, 385—390 |
| [3] | Heijde D. V. D., Silva J. C. D., Dougados M., Geher P., Horst-Bruinsma I. V. D., Juanola X., Olivieri I., Raeman F., Settas L., Sieper J., Szechinski J., Walker D., Boussuge M. P., Wajdula J. S., Paolozzi L., Fatenejad S., Ann. Rheum. Dis., 2006, 65(12), 1572—1577 |
| [4] | Pirjo T., Lindahl P., Honkanen V., Lahdenne P., Kotaniemi K., Ann. Rheum. Dis., 2007, 66(4), 548—550 |
| [5] | Goffe B., Cather J. C., J. Am. Acad. Dermatol., 2003, 49(2S), S105—S111 |
| [6] | Wong M., Ziring D., Korin Y., Desai S., Kim S., Lin J., Gjertson D., Braun J., Reed E., Singh R. R., Clin. Immunol., 2008, 126(2), 121—136 |
| [7] | Feofilova E. P., Usov A. I., Mysyakina I. S., Kochkina G. A., Microbiology, 2014, 83(3), 271—283 |
| [8] | Wu Y. J., Cui Y. L., Zheng Q. C., Zhang H. X., Chem. J. Chinese Universities, 2014, 34(12), 2605—2611 |
| (吴云剑, 崔颖璐, 郑清川, 张红星. 高等学校化学学报, 2014, 34(12), 2605—2611) | |
| [9] | Spoel D. V. D., Lindahl E., Hess B., Groenhof G., Mark A. E., Berendsen H. J. C., J. Comput. Chem., 2005, 26(16), 1701—1718 |
| [10] | Mu G., Wilmanns M., Schulten K., Biophys. J., 2002, 83(6), 3435—3445 |
| [11] | Isralewitz B., Baudry J., Gullingsrud J., Kosztin D., Schulten K., J. Mol. Graph. Model., 2001, 19(1), 13—25 |
| [12] | Shen L. L., Shen J. H., Luo X. M., Cheng F., Xu Y. C., Chen K. X., Arnold E., Ding J. P., Jiang H. L., Biophys. J., 2003, 84(6), 3547—3563 |
| [13] | Jani V., Sonavane U. B., Joshi R., J. Mol. Model., 2014, 20(6), 1—14 |
| [14] | Kim I., Allen T. W., J .Chem. Phys., 2012, 136(16), 164103 |
| [15] | Manning M., Murphy B., Etanercept Formulations Stabilized with Combination of Sugars and Polyols UP 0101640A1, 2013-04-25 |
| [16] | Lindorff L. K., Piana S. K., Maragakis P., Klepeis J. L., Dror R. O., Shaw D. E., Proteins: Struc. Func. & Bioinf., 2010, 78(8), 1950—1958 |
| [17] | Hornak V., Abel R., Okur A., Strockbine B., Roitberg A., Simmerling C., Proteins, 2006, 65(3), 712—725 |
| [18] | Price D. J., J. Chem. Phys., 2004, 121(20), 10096—10103 |
| [19] | Wu Y. J., Zheng Q. C., Xu Y., Chu W. T., Cui Y. L., Wang Y., Zhang H. X., Chem. Res. Chinese Universities, 2014, 30(6), 1011—1017 |
| [20] | Lan H.N., Wang Y. X., Zhang M. Z., Han W. W., Zheng X., Chem. Res. Chinese Universities, 2013, 29(1), 139—143 |
| [21] | Thurtell J. K., Thurtell G. W., J. Chem. Phys., 1988, 88(10), 6641—6646 |
| [22] | Bussi G., Donadio D., Parrinello M., J. Chem. Phys., 2007, 126(1), 14101—14107 |
| [23] | Waichman K., Barmashenko B. D., Rosenwaks S., J. Chem. Phys., 2010, 133(8), 362—364 |
| [24] | Wang Y. H., Li W., Chem. Res. Chinese Universities, 2005, 21(1), 73—77 |
| [25] | Souaile M., Roux B., Comp. Phys. Comm., 2001, 135(1), 40—57 |
| [26] | Roux B., Comp. Phys. Comm., 1995, 19(1—3), 275—282 |
| [27] | Kumar V., Sharma V. K., Kalonia D. S., Int. J. Pharm., 2009, 3661(1/2), 88—98 |
| [1] | BAI Jingqi, BAI Shan, REN Lixia, ZHU Kongying, ZHAO Yunhui, LI Xiaohui, YUAN Xiaoyan. Trehalose-modified Poly(vinyl alcohol) and Their Antifogging/Antifrosting Coatings [J]. Chem. J. Chinese Universities, 2021, 42(8): 2683. |
| [2] | QI Renrui, LI Minghao, CHANG Hao, FU Xueqi, GAO Bo, HAN Weiwei, HAN Lu, LI Wannan. Theoretical Study on the Unbinding Pathway of Xanthine Oxidase Inhibitors Based on Steered Molecular Dynamics Simulation [J]. Chem. J. Chinese Universities, 2021, 42(3): 758. |
| [3] | ZHU Jingxuan,YU Zhengfei,LIU Ye,ZHAN Dongling,HAN Jiarui,TIAN Xiaopian,HAN Weiwei. Exploration of Increasing the Non-specificity Substrates Activity for the Phosphotriesterase-like Lactonase Using Molecular Dynamics Simulations† [J]. Chem. J. Chinese Universities, 2019, 40(1): 138. |
| [4] | ZHOU Chao, SHI Ting, ZHAO Yilei, WANG Xiaolei. Theoretical Analysis of Oxygen Diffusion in the Micro-channels of Quinoline Oxygenase† [J]. Chem. J. Chinese Universities, 2017, 38(10): 1813. |
| [5] | ZHANG Luge, XUE Zexu, ZHANG Chong, YAN Hui. Molecular Dynamics Studies on the Selective Deposition of 3(5)-(9-Anthryl) Pyrazole onto Self-assembled Monolayers† [J]. Chem. J. Chinese Universities, 2016, 37(3): 505. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||