

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (8): 1570.doi: 10.7503/cjcu20150135
• Physical Chemistry • Previous Articles Next Articles
SUN Xiaoli1, HUO Ruiping1, BU Yuxiang2, LI Jilai1,*( )
)
Received:2015-02-06
Online:2015-08-10
Published:2015-07-17
Contact:
LI Jilai
E-mail:jilai@jlu.edu.cn
Supported by:CLC Number:
TrendMD:
SUN Xiaoli, HUO Ruiping, BU Yuxiang, LI Jilai. Benchmark Studies of Density Functional Theory on the Hydrogen Adsorption†[J]. Chem. J. Chinese Universities, 2015, 36(8): 1570.
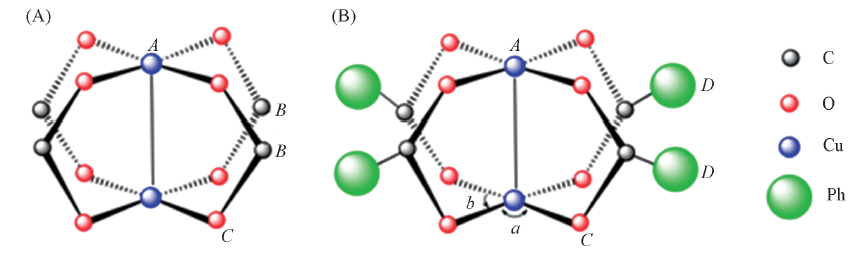
Fig.1 Structures of Model S[Cu2(HCOO)4](A) and model M[Cu2(PhCOO)4](B)A, B, C, D are the adsorption site. ∠a and ∠b are the two BTC angles of crystal(∠a>∠b).
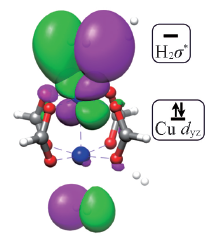
Fig.4 Frontier molecular orbital for the interaction between Cu3(BTC)2 and H2 on the open coordinated copper ionGreen and magenta lobs represent the shape of frontier molecular orbitals.
| Structure | EBE/(kJ·mol-1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B3LYP-D3a | M06 | wB97XD | PW91 | PBE0 | X3LYP | TPSS | B3LYP | BLYP | DFT-D3b | |
| 2A | -32.2 | -35.5 | -28.8 | -24.8 | -23.1 | -19.3 | -16.3 | -15.3 | -9.2 | -16.9 |
| 2C | -8.6 | -6.3 | -5.1 | -7.4 | -3.8 | -3.6 | -2.6 | -1.6 | -0.5 | -7.0 |
| 2A2C | -34.2 | -33.6 | -28.2 | -25.6 | -20.8 | -16.9 | -12.1 | -10.7 | -3.1 | -23.5 |
| 4C | -17.4 | -9.1 | -10.6 | -14.9 | -8.5 | -8.5 | -5.6 | -4.5 | -2.1 | -12.9 |
| 2A4C-1 | -44.0 | -38.3 | -35.0 | -34.2 | -26.3 | -22.1 | -15.9 | -13.7 | -4.5 | -30.3 |
| 2A4C-2 | -48.9 | -46.3 | -41.6 | -38.9 | -30.8 | -25.6 | -21.4 | -17.4 | -8.1 | -31.6 |
| 2A2B2C | -42.9 | -38.6 | -34.0 | -32.8 | -24.6 | -20.1 | -14.2 | -11.6 | -2.3 | -31.3 |
| M-2A | -28.0 | -24.7 | -22.5 | -15.7 | -15.2 | -11.6 | -7.8 | -7.7 | -1.0 | -20.3 |
| M-2D | -6.9 | -3.5 | -6.8 | -5.2 | -1.1 | 1.0 | 0.8 | 3.8 | 6.2 | -10.7 |
| M-2C | -11.5 | -8.4 | -7.8 | -6.8 | -2.6 | -1.8 | -0.4 | 1.2 | 3.4 | -12.8 |
| M-4D | -25.2 | -24.4 | -25.9 | -22.6 | -15.3 | -10.9 | -12.7 | -6.1 | -1.6 | -19.1 |
Table 1 Binding energy of H2 to Cu3(BTC)2 at DFT/B1//B3LYP/B1 level of theory
| Structure | EBE/(kJ·mol-1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B3LYP-D3a | M06 | wB97XD | PW91 | PBE0 | X3LYP | TPSS | B3LYP | BLYP | DFT-D3b | |
| 2A | -32.2 | -35.5 | -28.8 | -24.8 | -23.1 | -19.3 | -16.3 | -15.3 | -9.2 | -16.9 |
| 2C | -8.6 | -6.3 | -5.1 | -7.4 | -3.8 | -3.6 | -2.6 | -1.6 | -0.5 | -7.0 |
| 2A2C | -34.2 | -33.6 | -28.2 | -25.6 | -20.8 | -16.9 | -12.1 | -10.7 | -3.1 | -23.5 |
| 4C | -17.4 | -9.1 | -10.6 | -14.9 | -8.5 | -8.5 | -5.6 | -4.5 | -2.1 | -12.9 |
| 2A4C-1 | -44.0 | -38.3 | -35.0 | -34.2 | -26.3 | -22.1 | -15.9 | -13.7 | -4.5 | -30.3 |
| 2A4C-2 | -48.9 | -46.3 | -41.6 | -38.9 | -30.8 | -25.6 | -21.4 | -17.4 | -8.1 | -31.6 |
| 2A2B2C | -42.9 | -38.6 | -34.0 | -32.8 | -24.6 | -20.1 | -14.2 | -11.6 | -2.3 | -31.3 |
| M-2A | -28.0 | -24.7 | -22.5 | -15.7 | -15.2 | -11.6 | -7.8 | -7.7 | -1.0 | -20.3 |
| M-2D | -6.9 | -3.5 | -6.8 | -5.2 | -1.1 | 1.0 | 0.8 | 3.8 | 6.2 | -10.7 |
| M-2C | -11.5 | -8.4 | -7.8 | -6.8 | -2.6 | -1.8 | -0.4 | 1.2 | 3.4 | -12.8 |
| M-4D | -25.2 | -24.4 | -25.9 | -22.6 | -15.3 | -10.9 | -12.7 | -6.1 | -1.6 | -19.1 |
| Model | EAE/(kJ·mol-1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B3LYP-D3a | M06 | wB97XD | PW91 | PBE0 | X3LYP | TPSS | B3LYP | BLYP | DFT-D3b | |
| S | -8.0 | -7.4 | -6.4 | -11.0 | -4.9 | -4.2 | -3.2 | -2.8 | -1.2 | -5.2 |
| M | -7.4 | -6.1 | -6.3 | -4.9 | -3.3 | -2.2 | -1.7 | -0.7 | 1.0 | -6.7 |
| Exp.[ | -4.5—-6.5 | |||||||||
Table 2 Adsorption energy of H2 to Cu3(BTC)2 at DFT/B1//B3LYP/B1 level of theory
| Model | EAE/(kJ·mol-1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B3LYP-D3a | M06 | wB97XD | PW91 | PBE0 | X3LYP | TPSS | B3LYP | BLYP | DFT-D3b | |
| S | -8.0 | -7.4 | -6.4 | -11.0 | -4.9 | -4.2 | -3.2 | -2.8 | -1.2 | -5.2 |
| M | -7.4 | -6.1 | -6.3 | -4.9 | -3.3 | -2.2 | -1.7 | -0.7 | 1.0 | -6.7 |
| Exp.[ | -4.5—-6.5 | |||||||||
| Method | EBE/(kJ·mol-1) | EAE/(kJ·mol-1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2A | 2C | 2A2C | 4C | 2A4B-1 | 2A4B-2 | 2A2B2C | M-2A | M-2D | M-2C | M-4D | Model S | Model M | |
| B3-TZ-D3 | -27.8 | -4.3 | -26.6 | -10.4 | -33.2 | -38.1 | -31.1 | -24.2 | -5.2 | -7.9 | -22.6 | -5.6 | -6.1 |
| B3-QZ-D3 | 26.6 | -3.6 | -24.7 | -8.9 | -30.6 | -35.8 | -28.5 | -6.1 | |||||
Table 3 Binding energy and adsorption energy of H2 to Cu3(BTC)2 at B3LYP-D3/def2-TZVPP and B3LYP-D3/def2-QZVPP level of theory
| Method | EBE/(kJ·mol-1) | EAE/(kJ·mol-1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2A | 2C | 2A2C | 4C | 2A4B-1 | 2A4B-2 | 2A2B2C | M-2A | M-2D | M-2C | M-4D | Model S | Model M | |
| B3-TZ-D3 | -27.8 | -4.3 | -26.6 | -10.4 | -33.2 | -38.1 | -31.1 | -24.2 | -5.2 | -7.9 | -22.6 | -5.6 | -6.1 |
| B3-QZ-D3 | 26.6 | -3.6 | -24.7 | -8.9 | -30.6 | -35.8 | -28.5 | -6.1 | |||||
| Model | EAE/(kJ·mol-1) | |||
|---|---|---|---|---|
| A | B | C | D | |
| S | -13.9 | (-0.2) | -2.4(-1.4) | |
| M | -12.1 | -3.9 | -4.9 | |
Table 4 Adsorption energy of H2 to the possible binding site of Cu3(BTC)2 at B3LYP/TZ-D3 level*
| Model | EAE/(kJ·mol-1) | |||
|---|---|---|---|---|
| A | B | C | D | |
| S | -13.9 | (-0.2) | -2.4(-1.4) | |
| M | -12.1 | -3.9 | -4.9 | |
| [1] | Li J. L., Zhang X., Huang X. R., Phys. Chem. Chem. Phys., 2012, 14, 246—256 |
| [2] | Sun X. L., Huang X. R., Li J. L., Huo R. P., Sun C. C., J. Phys. Chem. A, 2012, 116, 1475—1485 |
| [3] | Huo R. P., Zhang X., Huang X. R., Li J. L., Sun C. C., Acta Chim. Sinica, 2013, 71, 743—748 |
| (霍瑞萍, 张祥, 黄旭日, 李吉来, 孙家锺. 化学学报, 2013, 71, 743—748) | |
| [4] | Li J. L., Mata R. A., Ryde U., J. Chem. Theory. Comput., 2013, 9, 1799—1807 |
| [5] | Shi Q., Bergquist K. E., Huo R. P., Li J. L., Lund M., Vacha R., Sundin A., Butkus E., Orentas E., Warnmark K., J. Am. Chem. Soc., 2013, 135, 15263—15268 |
| [6] | Sun X. L., Li J. L., Huang X. R., Sun C. C., Acta Chim. Sinica, 2013, 71, 749—754 |
| (孙小丽, 李吉来, 黄旭日, 孙家锺. 化学学报, 2013, 71, 749—754) | |
| [7] | Li J. L., Geng C. Y., Bu Y. X., Huang X. R., Sun C. C., J. Comput. Chem., 2009, 30, 1135—1145 |
| [8] | Li J. L., Geng C. Y., Huang X. R., Sun C. C., J. Chem. Theory. Comput., 2006, 2, 1551—1564 |
| [9] | Li J. L., Geng C. Y., Huang X. R., Zhang X., Sun C. C., Organometallics, 2007, 26, 2203—2210 |
| [10] | Geng C. Y., Li J. L., Huang X. R., Liu H. L., Li Z., Sun C. C., J. Comput. Chem., 2008, 29, 686—693 |
| [11] | Geng C.Y., Li J. L., Huang X. R., Sun C. C., Chem. Phys., 2006, 324, 474—482 |
| [12] | Roy D., Marianski M., Maitra N. T., Dannenberg J. J., J. Chem. Phys., 2012, 137, 134109 |
| [13] | Ess D. H., Houk K. N., J. Phys. Chem. A, 2005, 109, 9542—9553 |
| [14] | Sun X. H., Sun X. L., Geng C. Y., Zhao H. T., Li J. L., J. Phys. Chem. A, 2014, 118, 7146—7158 |
| [15] | Andreji M., Mata R. A., J. Chem.Theory. Comput., 2014, 10, 5397—5404 |
| [16] | Zhang X., Schwarz H., Theor. Chem. Acc., 2011, 129, 389—399 |
| [17] | Korth M., Grimme S., J. Chem. Theory. Comput., 2009, 5, 993—1003 |
| [18] | Li J. L., González-Navarrete P., Schlangen M., Schwarz H., Chem. Eur. J., 2015, 21, 7780—7789 |
| [19] | Cohen A. J., Mori-Sánchez P., Yang W., Chem. Rev., 2012, 112, 289—320 |
| [20] | Boguslawski K., Jacob C. R., Reiher M., J. Chem. Theory. Comput., 2011, 7, 2740—2752 |
| [21] | Yu Y. X., ACS Appl. Mater. Interfaces, 2014, 6, 16267—16275 |
| [22] | Yu Y. X., J. Mater. Chem. A, 2014, 2, 8910—8917 |
| [23] | Wang C.J., Tang C. M., Zhang Y. J., Gao F. Z., Chem. J. Chinese Universities, 2014, 35,2131—2137 |
| (王成杰, 唐春梅, 张轶杰, 高凤志. 高等学校化学学报, 2014, 35,2131—2137) | |
| [24] | Grimme S., Chem. Eur. J., 2012, 18, 9955—9964 |
| [25] | Grimme S., J. Comput. Chem., 2006, 27, 1787—1799 |
| [26] | Goerigk L., Grimme S., Phys. Chem. Chem. Phys., 2011, 13, 6670—6688 |
| [27] | Korth M., Grimme S., J. Chem. Theory Comput., 2009, 5, 993—1003 |
| [28] | Wang S. Y., Zhong C. L., Acta Chim. Sinica, 2006, 64, 2375—2378 |
| (王三跃, 仲崇立. 化学学报, 2006, 64, 2375—2378) | |
| [29] | Li J. L., Geng C. Y., Huang X. R., Sun C. C., J. Chem. Theory Comput., 2006, 2, 1551—1564 |
| [30] | Toda J., Fischer M., Jorge M., Gomes J. R. B., Chem. Phys. Lett., 2013, 587, 7—13 |
| [31] | Grajciar L., Nachtigall P., Bludsk O., Rubeš M., J. Chem. Theory Comput., 2015, 11, 230—238 |
| [32] | Terencio T., Di Renzo F., Berthomieu D., Trens P., J. Phys. Chem. C, 2013, 117, 26156—26165 |
| [33] | Brittain D. R. B., Lin C. Y., Gilbert A. T. B., Izgorodina E. I., Gill P. M. W., Coote M. L., Phys. Chem. Chem. Phys., 2009, 11, 1138—1142 |
| [34] | Sun X. L., Geng C. Y., Huo R. P., Ryde U., Bu Y. X., Li J. L., J. Phys. Chem. B, 2014, 118, 1493—1500 |
| [35] | Huo R. P., Zhang X., Huang X. R., Li J. L., Sun C. C., J. Phys. Chem. A, 2011, 115, 3576—3582 |
| [36] | Li N., Huo R. P., Zhang X., Huang X. R., Li J. L., Sun C. C., Chem. Phys. Lett., 2011, 503, 210—214 |
| [37] | Dewar M., Bull. Soc. Chem. Fr., 1951, 18, C79 |
| [38] | Chatt J., Duncanson L.A.,J. Chem. Soc., 1953, 2939 |
| [39] | Chatt J., Duncanson L.A., Venanzi M. L.,J. Chem. Soc., 1955, 4456—4460 |
| [40] | Bernardo C. P., Bauman N., Piecuch P., Silva P., J. Mol. Model., 2013, 19, 5457—5467 |
| [41] | Zhao Y., Truhlar D. G., J. Phys. Chem. A, 2005, 109, 5656—5667 |
| [42] | Hirscher M., Panella B., Schmitz B., Microporous. Mesoporous. Mater., 2010, 129, 335—339 |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | JIANG Hongbin, DAI Wenchen, ZHANG Rao, XU Xiaochen, CHEN Jie, YANG Guang, YANG Fenglin. Research on Co3O4/UiO-66@α-Al2O3 Ceramic Membrane Separation and Catalytic Spraying Industry VOCs Waste Gas [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220025. |
| [3] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [4] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [5] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [6] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [7] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [8] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| [9] | HUANG Luoyi, WENG Yueyue, HUANG Xuhui, WANG Chaojie. Theoretical Study on the Structures and Properties of Flavonoids in Plantain [J]. Chem. J. Chinese Universities, 2021, 42(9): 2752. |
| [10] | MA Lijuan, GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun. Theoretical Investigation of Hydrogen Storage Properties of Sc, Ti, V-decorated and B/N-doped Monovacancy Graphene [J]. Chem. J. Chinese Universities, 2021, 42(9): 2842. |
| [11] | WANG Jian, ZHANG Hongxing. Theoretical Study on the Structural-photophysical Relationships of Tetra-Pt Phosphorescent Emitters [J]. Chem. J. Chinese Universities, 2021, 42(7): 2245. |
| [12] | HU Wei, LIU Xiaofeng, LI Zhenyu, YANG Jinlong. Surface and Size Effects of Nitrogen-vacancy Centers in Diamond Nanowires [J]. Chem. J. Chinese Universities, 2021, 42(7): 2178. |
| [13] | YANG Yiying, ZHU Rongxiu, ZHANG Dongju, LIU Chengbu. Theoretical Study on Gold-catalyzed Cyclization of Alkynyl Benzodioxin to 8-Hydroxy-isocoumarin [J]. Chem. J. Chinese Universities, 2021, 42(7): 2299. |
| [14] | LIU Yang, LI Qingbo, SUN Jie, ZHAO Xian. Direct Synthesis of Graphene on AlN Substrates via Ga Remote Catalyzation [J]. Chem. J. Chinese Universities, 2021, 42(7): 2271. |
| [15] | ZHENG Ruoxin, ZHANG Igor Ying, XU Xin. Development and Benchmark of Lower Scaling Doubly Hybrid Density Functional XYG3 [J]. Chem. J. Chinese Universities, 2021, 42(7): 2210. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||