

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (5): 962.doi: 10.7503/cjcu20141054
• Physical Chemistry • Previous Articles Next Articles
JIN Junling1,2,*( ), DING Xiang1, OU Lihui1, ZHANG Xiangyang1, SHEN Youming1, GENG Yun2, SU Zhongmin2
), DING Xiang1, OU Lihui1, ZHANG Xiangyang1, SHEN Youming1, GENG Yun2, SU Zhongmin2
Received:2014-11-28
Online:2015-05-10
Published:2015-04-17
Contact:
JIN Junling
E-mail:jinjl174@nenu.edu.cn
Supported by:CLC Number:
TrendMD:
JIN Junling, DING Xiang, OU Lihui, ZHANG Xiangyang, SHEN Youming, GENG Yun, SU Zhongmin. Density Functional Theory Studies on the Photophysical Properties of N,N-Chelate Boron Complexes†[J]. Chem. J. Chinese Universities, 2015, 36(5): 962.
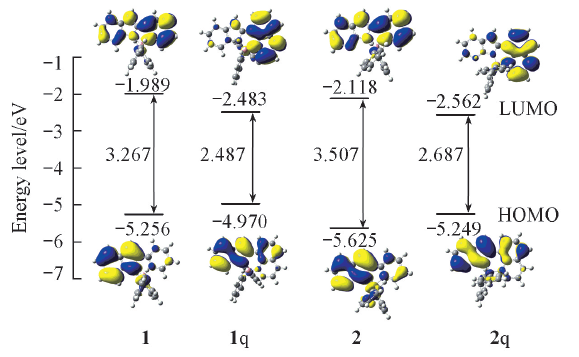
Fig.2 Illustration of the highest occupied molecular orbital(HOMO) and lowest unoccupied molecular orbital(LUMO) plots, the orbi-tal energy levels, and the HOMO-LUMO energy gaps for complexes 1, 1q, 2 and 2q at their optimized S0 geometries in vacuum at B3LYP/6-31G(d) levelThe molecular orbital plots were obtained with an isosurface of 0.02 a.u.
| Complex | Transition | λabs/nm | Ex/eV | f | Composition | λexp./nm |
|---|---|---|---|---|---|---|
| 1 | S0→S1 | 353.9 | 3.503 | 0.367 | HOMO→LUMO(98%) | 378 |
| 1q | S0→S1 | 431.8 | 2.872 | 0.207 | HOMO→LUMO(98%) | 450 |
| 2 | S0→S1 | 343.5 | 3.610 | 0.515 | HOMO→LUMO(99%) | 374 |
| 2q | S0→S1 | 419.8 | 2.954 | 0.237 | HOMO→LUMO(99%) | 446 |
Table 1 Calculated absorption wavelengths(λabs), excitation energies(Ex), oscillator strengths f, and dominant excitation character of complexes 1, 2, 1q, and 2q together with experimental results(λexp.)*
| Complex | Transition | λabs/nm | Ex/eV | f | Composition | λexp./nm |
|---|---|---|---|---|---|---|
| 1 | S0→S1 | 353.9 | 3.503 | 0.367 | HOMO→LUMO(98%) | 378 |
| 1q | S0→S1 | 431.8 | 2.872 | 0.207 | HOMO→LUMO(98%) | 450 |
| 2 | S0→S1 | 343.5 | 3.610 | 0.515 | HOMO→LUMO(99%) | 374 |
| 2q | S0→S1 | 419.8 | 2.954 | 0.237 | HOMO→LUMO(99%) | 446 |
| Complex | Transition | λem/nm | Em/eV | f | Composition | SS/nm | kr/s-1 | knr/s-1 | Φexp. | λexp./nm |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | S1→S0 | 410.2 | 3.022 | 0.128 | LUMO→HOMO(98%) | 56.3 | 5.10×107 | 1.02×107 | 0.29b | 516 |
| 1q | S1→S0 | 538.5 | 2.302 | 0.060 | LUMO→HOMO(99%) | 96.7 | 1.40×107 | 2.46×1010 | N/Ac | 578 |
| 2 | S1→S0 | 400.1 | 3.099 | 0.185 | LUMO→HOMO(99%) | 56.6 | 7.70×107 | 0.99×107 | 0.61b | 476 |
| 2q | S1→S0 | 521.9 | 2.376 | 0.080 | LUMO→HOMO(99%) | 102.1 | 2.00×107 | 2.62×1010 | N/Ac | 546 |
Table 2 Calculated emission wavelengths(λem), emission energies(Em), oscillator strengths f, dominant excitation character, Stokes shift(SS), radiative rate constants(kr) and nonradiative rate constants(knr) of complexes 1, 2, 1q, and 2q together with the reported experimental results of the emission wavelength(λexp.) and quantum yield(Φexp.)a
| Complex | Transition | λem/nm | Em/eV | f | Composition | SS/nm | kr/s-1 | knr/s-1 | Φexp. | λexp./nm |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | S1→S0 | 410.2 | 3.022 | 0.128 | LUMO→HOMO(98%) | 56.3 | 5.10×107 | 1.02×107 | 0.29b | 516 |
| 1q | S1→S0 | 538.5 | 2.302 | 0.060 | LUMO→HOMO(99%) | 96.7 | 1.40×107 | 2.46×1010 | N/Ac | 578 |
| 2 | S1→S0 | 400.1 | 3.099 | 0.185 | LUMO→HOMO(99%) | 56.6 | 7.70×107 | 0.99×107 | 0.61b | 476 |
| 2q | S1→S0 | 521.9 | 2.376 | 0.080 | LUMO→HOMO(99%) | 102.1 | 2.00×107 | 2.62×1010 | N/Ac | 546 |
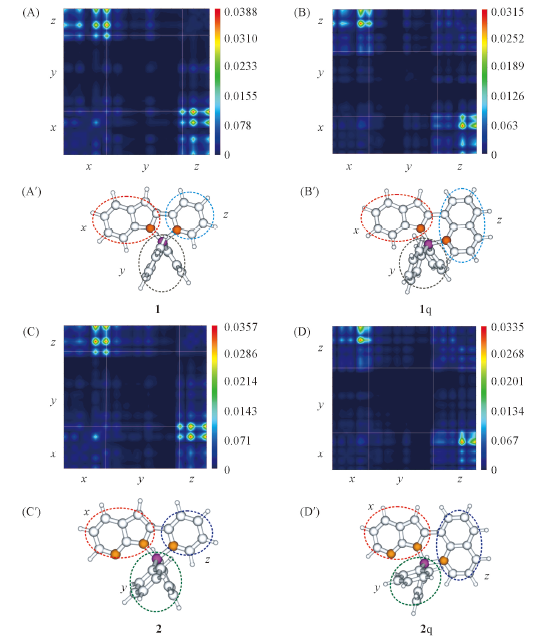
Fig.3 Transition density matrix of the hot excitation(S0 state) for complexes 1(A, A'), 1q(B, B'), 2(C, C') and 2q(D, D') calculated by B3LYP/6-31G(2d,p) in vacuumThe unit of color scale is a.u.2, 1 a.u.=1.6×10-19 C.
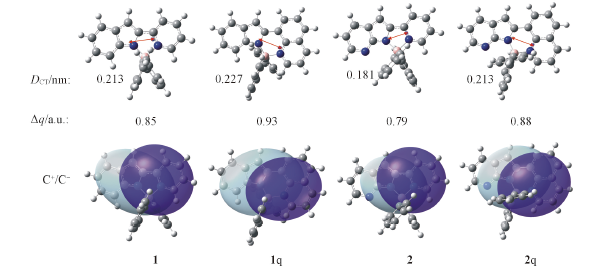
Fig.4 Calculated results for the centroids of charge(C+/C-) and the charge-transfer distance(DCT) between the barycenters of density depletion(gray) and density increment(purple) zone, and the transferred charge(Δq) computed for the first electronic transitionThe calculation level is B3LYP/6-31G(2d,p) level.
| [1] | Lorbach, A. , Hubner, A. , Wagner, M. , Dalton Trans., 2012, 41( 20), 6048- 6063 |
| [2] | Li, D. , Wang, K. , Huang, S. , Qu, S. , Liu, X. , Zhu, Q. , Zhang, H. , Wang, Y. , J. Mater. Chem., 2011, 21( 39), 15298- 15304 |
| [3] | Mizuno, Y. , Yisilamu, Y. , Yamaguchi, T. , Tomura, M. , Funaki, T. , Sugihara, H. , Ono, K. , Chem. Eur. J., 2014, 20( 41), 13286- 13295 |
| [4] | Kojima, T. , Kumaki, D. , Nishida J., I. , Tokito, S. , Yamashita, Y. , J. Mater. Chem., 2011, 21( 18), 6607- 6613 |
| [5] | Awuah S., G. , You, Y. , RSC Advances, 2012, 2( 30), 11169- 11183 |
| [6] | Boens, N. , Leen, V. , Dehaen, W. , Chem. Soc. Rev., 2012, 41( 3), 1130- 1172 |
| [7] | Ulrich, G. , Ziessel, R. , Harriman, A. , Angew. Chem. Int. Ed., 2008, 47( 7), 1184- 1201 |
| [8] | Yoshii, R. , Tanaka, K. , Chujo, Y. , Macromolecules, 2014, 47( 7), 2268- 2278 |
| [9] | Massue, J. , Frath, D. , Retailleau, P. , Ulrich, G. , Ziessel, R. , Chem. Eur. J., 2013, 19( 17), 5375- 5386 |
| [10] | Chambon, S. , D'Aleo, A. , Baffert, C. , Wantz, G. , Fages, F. , Chem. Comm., 2013, 49( 34), 3555- 3557 |
| [11] | Wakamiya, A. , Taniguchi, T. , Yamaguchi, S. , Angew. Chem. Int. Ed., 2006, 45( 19), 3170- 3173 |
| [12] | Nagura, K. , Saito, S. , Frö, hlich R. , Glorius, F. , Yamaguchi, S. , Angew. Chem., 2012, 124( 31), 7882- 7886 |
| [13] | Brö, ring M. , Krü, ger R. , Link, S. , Kleeberg, C. , Kö, hler S. , Xie, X. , Ventura, B. , Flamigni, L. , Chem. Eur. J., 2008, 14( 10), 2976- 2983 |
| [14] | Hao, Q. , Yu, S. , Li, S. , Chen, J. , Zeng, Y. , Yu, T. , Yang, G. , Li, Y. , J. Org. Chem., 2013, 79( 1), 459- 464 |
| [15] | Mao, M. , Wang J., B. , Xiao Z., F. , Dai S., Y. , Song Q., H. , Dyes Pigments, 2012, 94( 2), 224- 232 |
| [16] | Suresh, D. , Gomes C. S., B. , Gomes P., T. , Di Paolo R., E. , Macanita A., L. , Calhorda M., J. , Charas, A. , Morgado, J. , Teresa Duarte, M. , Dalton Trans., 2012, 41( 28), 8502- 8505 |
| [17] | Calhorda M., J. , Suresh, D. , Gomes P., T. , Di Paolo R., E. , Macanita A., L. , Dalton Trans., 2012, 41( 42), 13210- 13217 |
| [18] | Liu, H. , Lu, H. , Xu, J. , Liu, Z. , Li, Z. , Mack, J. , Shen, Z. , Chem. Comm., 2014, 50( 9), 1074- 1076 |
| [19] | Liu Q., D. , Mudadu M., S. , Thummel, R. , Tao, Y. , Wang, S. , Adv. Funct. Mater., 2005, 15( 1), 143- 154 |
| [20] | Le Guennic, B. , Chibani, S. , Charaf-Eddin, A. , Massue, J. , Ziessel, R. , Ulrich, G. , Jacquemin, D. , Phys. Chem. Chem. Phys., 2013, 15( 20), 7534- 7540 |
| [21] | 李倩, 耿允, 段雨爱, 王光宇, 苏忠民. 高等学校化学学报, 2014, 35( 7), 1471- 1479 |
| Li, Q. , Geng, Y. , Duan Y., A. , Wang G., Y. , Su Z., M. , Chem. J. Chinese Universities, 2014, 35( 7), 1471- 1479 | |
| [22] | 苏欣, 张吉, 吴勇, 耿允, 苏忠民. 高等学校化学学报, 2013, 34( 8), 1945- 1952 |
| Su, X. , Zhang, J. , Wu, Y. , Geng, Y. , Su Z., M. , Chem. J. Chinese Universities , 2013, 34( 8), 1945- 1952 | |
| [23] | Teng Y., L. , Kan Y., H. , Su Z., M. , Liao, Y. , Yang S., Y. , Wang R., S. , Theo. Chem. Acc., 2007, 117( 1), 1- 5 |
| [24] | Yang, G. , Su, T. , Shi, S. , Su, Z. , Zhang, H. , Wang, Y. , J. Phys. Chem. A, 2007, 111( 14), 2739- 2744 |
| [25] | Lu T. , |
| [26] | Lu, T. , Chen, F. , J. Comput. Chem., 2012, 33( 5), 580- 592 |
| [27] | Frisch M., J. , Trucks G., W. , Schlegel H., B. , Scuseria G., E. , Robb M., A. , Cheeseman J., R. , Scalmani, G. , Barone, V. , Mennucci, B. , Petersson G., A. , Nakatsuji, H. , Caricato, M. , Li, X. , Hratchian H., P. , Izmaylov A., F. , Bloino, J. , Zheng, G. , Sonnenberg J., L. , Hada, M. , Ehara, M. , Toyota, K. , Fukuda, R. , Hasegawa, J. , Ishida, M. , Nakajima, T. , Honda, Y. , Kitao, O. , Nakai, H. , Vreven, T. , Montgomery J. A., Jr. , Peralta J., E. , Ogliaro, F. , Bearpark, M. , Heyd J., J. , Brothers, E. , Kudin K., N. , Staroverov V., N. , Kobayashi, R. , Normand, J. , Raghavachari, K. , Rendell, A. , Burant J., C. , Iyengar S., S. , Tomasi, J. , Cossi, M. , Rega, N. , Millam J., M. , Klene, M. , Knox J., E. , Cross J., B. , Bakken, V. , Adamo, C. , Jaramillo, J. , Gomperts, R. , Stratmann R., E. , Yazyev, O. , Austin A., J. , Cammi, R. , Pomelli, C. , Ochterski J., W. , Martin R., L. , Morokuma, K. , Zakrzewski V., G. , Voth G., A. , Salvador, P. , Dannenberg J., J. , Dapprich, S. , Daniels A., D. , Farkas, O. , Foresman J., B. , Ortiz J., V. , Cioslowski, J. , Fox D., J. , Gaussian, 09W , Revision A.02. Gaussian, Inc. , Wallingford CT, 2009 |
| [28] | Li M., C. , Hayashi, M. , Lin S., H. , J. Phys. Chem. A, 2011, 115( 50), 14531- 14538 |
| [29] | Jin J., L. , Li H., B. , Geng, Y. , Wu, Y. , Duan Y., A. , Su Z., M. , ChemPhysChem, 2012, 13( 16), 3714- 3722 |
| [30] | Yin, S. , Peng, Q. , Shuai, Z. , Fang, W. , Wang Y., H. , Luo, Y. , Phys. Rev. B, 2006, 73( 20), 205409/ 1- 205409/5 |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [3] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [4] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [5] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [6] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [7] | MA Lijuan, GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun. Theoretical Investigation of Hydrogen Storage Properties of Sc, Ti, V-decorated and B/N-doped Monovacancy Graphene [J]. Chem. J. Chinese Universities, 2021, 42(9): 2842. |
| [8] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| [9] | HUANG Luoyi, WENG Yueyue, HUANG Xuhui, WANG Chaojie. Theoretical Study on the Structures and Properties of Flavonoids in Plantain [J]. Chem. J. Chinese Universities, 2021, 42(9): 2752. |
| [10] | WANG Jian, ZHANG Hongxing. Theoretical Study on the Structural-photophysical Relationships of Tetra-Pt Phosphorescent Emitters [J]. Chem. J. Chinese Universities, 2021, 42(7): 2245. |
| [11] | HU Wei, LIU Xiaofeng, LI Zhenyu, YANG Jinlong. Surface and Size Effects of Nitrogen-vacancy Centers in Diamond Nanowires [J]. Chem. J. Chinese Universities, 2021, 42(7): 2178. |
| [12] | YANG Yiying, ZHU Rongxiu, ZHANG Dongju, LIU Chengbu. Theoretical Study on Gold-catalyzed Cyclization of Alkynyl Benzodioxin to 8-Hydroxy-isocoumarin [J]. Chem. J. Chinese Universities, 2021, 42(7): 2299. |
| [13] | YING Fuming, JI Chenru, SU Peifeng, WU Wei. λ-DFCAS: A Hybrid Density Functional Complete Active Space Self Consistent Field Method [J]. Chem. J. Chinese Universities, 2021, 42(7): 2218. |
| [14] | LIU Yang, LI Qingbo, SUN Jie, ZHAO Xian. Direct Synthesis of Graphene on AlN Substrates via Ga Remote Catalyzation [J]. Chem. J. Chinese Universities, 2021, 42(7): 2271. |
| [15] | LIU Changhui, LIANG Guojun, LI Yanlu, CHENG Xiufeng, ZHAO Xian. Density Functional Theory Study of NH3 Adsorption on Boron Nanotubes [J]. Chem. J. Chinese Universities, 2021, 42(7): 2263. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||