

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (11): 2370.doi: 10.7503/cjcu20140612
• Physical Chemistry • Previous Articles Next Articles
TANG Haiyan1, ZHAO Maoshuang1, FENG Li1,*( ), CAO Zexing2
), CAO Zexing2
Received:2014-07-02
Online:2014-11-10
Published:2014-10-21
Contact:
FENG Li
E-mail:cumthgfl@163.com
Supported by:CLC Number:
TrendMD:
TANG Haiyan, ZHAO Maoshuang, FENG Li, CAO Zexing. Theoretical Studies on the Taking off of Oxygen-containing Functional Groups in Lignite Model Compounds†[J]. Chem. J. Chinese Universities, 2014, 35(11): 2370.
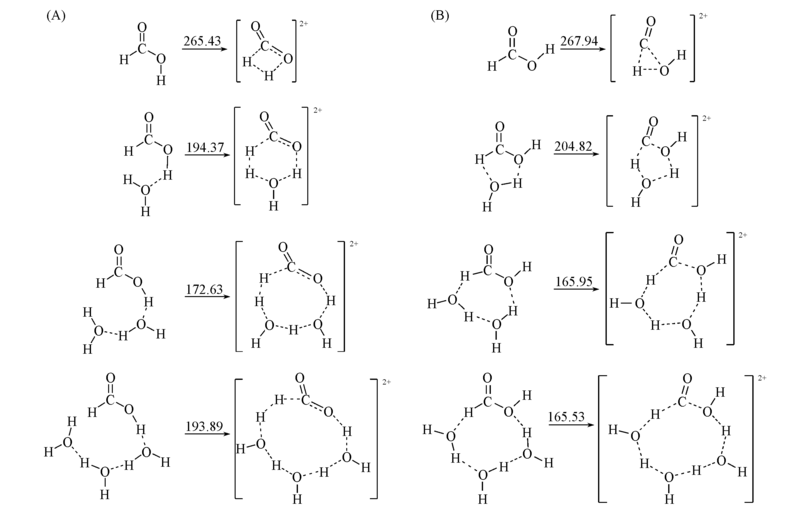
Fig.4 Reaction mechanism of the two reaction paths of formic acid involves the different number of water molecules (A) HCOOH=CO2+H2; (B) HCOOH=H2O+CO2. The reaction activation energies are in kJ/mol.
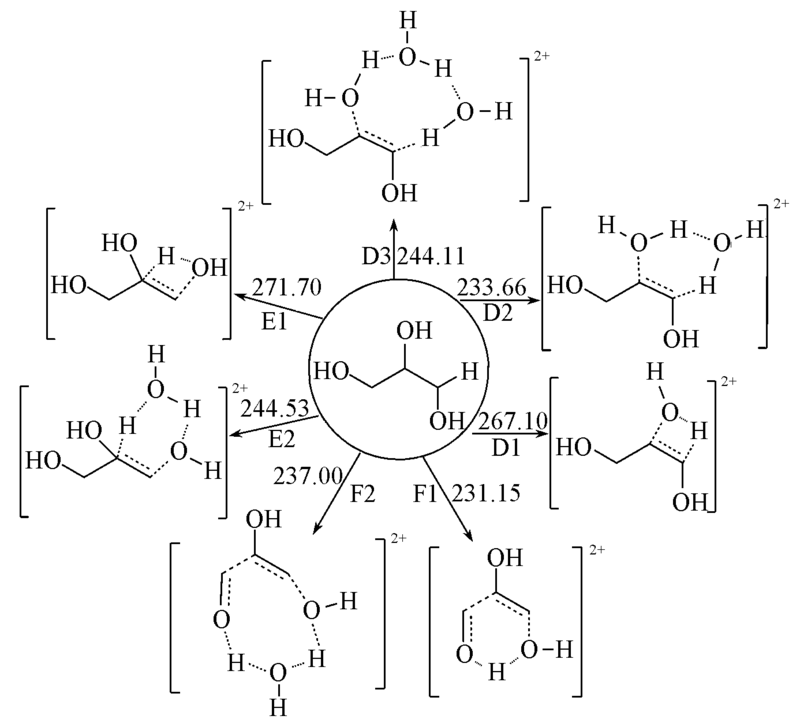
Fig.5 Reaction mechanism of the three reaction paths of glycerol involves the different number of water molecules The reaction activation energies are in kJ/mol.
| [1] | Feng L., Liu X. C., Song L. L., Wang X. H., Zhang Y., Cui T. W., Tang H. Y., Powder Technology,2013, 247, 19—23 |
| [2] | Wang Y. G., Zhou J. L., Lin X. C., Coal Science and Technology,2013, 41(9), 182—184 |
| (王永刚, 周剑林, 林雄超. 煤炭科学技术,2013, 41(9), 182—184) | |
| [3] | Zhao N., Lv Y. Z., Li G. J., Chem. Res. Chinese Universities,2013, 29(6), 1180—1184 |
| [4] | Solomon P. R., Coal Structure: Advances in Chemistry Series, 1981, 192, 184—195 |
| [5] | Li A. R., Wu D. H., Wang Q. C., Zhang K., Coal Conversion,2013, 36(1), 9—13 |
| (李爱蓉, 吴道洪, 王其成, 张锴. 煤炭转化,2013, 36(1), 9—13) | |
| [6] | Zhu X. D., Zhu Z. B., Journal of East China University of Science and Tecnology,2000, 26(1), 14—17 |
| (朱学栋, 朱子彬. 华东理工大学学报,2000, 26(1), 14—17) | |
| [7] | Wang S. R., Liang T., Ru B., Guo X. J., Chem. Res. Chinese Universities,2013, 29(4), 782—787 |
| [8] | Bradley L. C., Miller S. F., Miller B. G., Tillman D. A., Energy & Fuels, 2011, 25, 1989—1995 |
| [9] | Qian C. M., Zhou M., Wei J. H., Ye P. H., Yang X., International Journal of Mining Science and Technology,2014, 24, 137—141 |
| [10] | Kuznetsov P. N., Kamenskii E. S., Kolesnikova S. M., Kuznetsova L. I., Solid Fuel Chemistry,2014, 48, 51—57 |
| [11] | Liu L. J., Fei J. X., Cui M. Q., Hu Y. F., Wang J., Fuel Processing Technology,2014, 121, 56—62 |
| [12] | Abbasi A. E., Yozgatligil A., Fuel,2014, 115, 841—849 |
| [13] | Zhao G. W., Journal of Thermal Science, 2014, 23, 275—278 |
| [14] | Wang N., Yu J. L., Tahmasebi A., Han Y.N., Lucas J., Wall T., Jiang Y., Energy & Fuels,2014, 28, 254—263 |
| [15] | Xu Y., Zhang Y. F., Wang Y., Zhang G. J., Chen L., Journal of Analytical and Applied Pyrolysis,2013, 104, 625—631 |
| [16] | Yang X. J., Zhang C., Tan P., Yang T., Fang Q. Y., Chen G., Energy & Fuels,2014, 28, 264—274 |
| [17] | Zhao L. J., Ling L. X., Zhang R. G., Liu X. F., Wang B. J., Journal of Chemical Industry and Engineering,2008, 59(8), 2095—2102 |
| (赵俐娟, 凌丽霞, 章日光, 柳学芳, 王宝俊. 化工学报,2008, 59(8), 2095—2102) | |
| [18] | Ling L. X., Zhao L. J., Zhang R. G., Wang B. J., Journal of Chemical Industry and Engineering,2009, 60(5), 1224—1230 |
| (凌丽霞, 赵俐娟, 章日光, 王宝俊. 化工学报,2009, 60(5), 1224—1230) | |
| [19] | Wang B. J., Ling L. X., Zhang R. G., Xie K. C., Journal of China Coal Society,2009, 34(9), 1239—1248 |
| (王宝俊, 凌丽霞, 章日光, 谢克昌. 煤炭学报,2009, 34(9), 1239—1248) | |
| [20] | Zeng F. G., Jia J. B., Acta Physico-Chimica Sinica,2009, 25(6), 1117—1124 |
| (曾凡桂, 贾建波. 物理化学学报,2009, 25(6), 1117—1124) | |
| [21] | Zhou S. Q., Gu Y. X., Gu X., Chinese Journal of Inorganic Chemistry,2011, 27(6), 1202—1206 |
| (周素芹, 谷亚昕, 固旭. 无机化学学报,2011, 27(6), 1202—1206) | |
| [22] | Wang X. H., Feng L., Cao Z. X., Liu X. C., Tang H. Y., Zhang M., Acta Chimica Sinica,2013, 71(7), 1047—1052 |
| (王新华, 冯莉, 曹泽星, 刘祥春, 汤海燕, 张曼. 化学学报,2013, 71(7), 1047—1052) | |
| [23] | Jüntgen H., Fuel, 1984, 63, 731—737 |
| [24] | Mae K., Maki T., Miura K., Journal of Chemical Engineering of Japan,2002, 35, 778—785 |
| [25] | Xu W. C., Tomita., Fuel,1987, 66, 632—636 |
| [26] | Berkovitz N., Hertog W., Fuel,1962, 41, 507—520 |
| [27] | Eskay T. P., Britt P. F., Energy & Fuels,1996, 10, 1257—1261 |
| [28] | Ibarra J. V., Moliner R., Gavilin M. P., Fuel,1991, 70, 408—413 |
| [29] | Mae K., Maki T., Okutsu H., Minra K., Fuel,2000, 79, 417—425 |
| [30] | Li C. Z., Yu J. L., Chang L. P., Advances in the Science of Victorian Brown Coal, Chemical Idustry Press, Beijing, 2009, 184—187 |
| (李春柱, 余江龙, 常丽萍. 维多利亚褐煤科学进展, 北京: 化学工业出版社, 2009, 184—187) | |
| [31] | Zhang D. J., Xian X. F., Chem. J. Chinese Universities,1990, 11(7), 912—914 |
| (张代钧, 鲜学福. 高等学校化学学报, 1990, 11(7), 912—914) | |
| [32] | Liu S. Y., Zhang Z. Q., Wang H. F., Journal of Molecular Modeling,2012, 18, 359—365 |
| [33] | Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas Ö., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision A.02, Gau-ssian Inc., Wallingford CT, 2009 |
| [34] | Ruelle P., Kesselring U. W., Nam-Tran H., J. Am. Chem. Soc., 1986, 108, 371—375 |
| [35] | Gao C. G., Long Z. W., Tan X. F., Long B., Long C. Y., Qin S. J., Zhang W. J., Acta Chimica Sinica,2013, 71(5), 849—856 |
| (高成贵, 隆正文, 谭兴凤, 龙波, 龙超云, 秦水介, 张为俊. 化学学报,2013, 71(5), 849—856) | |
| [36] | Murakami K., Shirato H., Ozaki J., Fuel Processing Technology,1996, 46, 183—194 |
| [37] | Tang Q., Yu F. W., Lü H. Y., Ji B. J., Chemical Industry and Engineering Progress,2010, 29(1), 48—51 |
| (唐强, 于凤文, 吕红云, 计建炳. 化工进展,2010, 29(1), 48—51) |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [3] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [4] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [5] | SUN Cuihong, LYU Liqiang, LIU Ying, WANG Yan, YANG Jing, ZHANG Shaowen. Mechanism and Kinetics on the Reaction of Isopropyl Nitrate with Cl, OH and NO3 Radicals [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210591. |
| [6] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [7] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [8] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [9] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [10] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| [11] | HUANG Luoyi, WENG Yueyue, HUANG Xuhui, WANG Chaojie. Theoretical Study on the Structures and Properties of Flavonoids in Plantain [J]. Chem. J. Chinese Universities, 2021, 42(9): 2752. |
| [12] | MENG Fanwei, GAO Qi, YE Qing, LI Chenxi. Potassium Poisoning Mechanism of Cu-SAPO-18 Catalyst for Selective Catalytic Reduction of NOx by Ammonia [J]. Chem. J. Chinese Universities, 2021, 42(9): 2832. |
| [13] | MA Lijuan, GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun. Theoretical Investigation of Hydrogen Storage Properties of Sc, Ti, V-decorated and B/N-doped Monovacancy Graphene [J]. Chem. J. Chinese Universities, 2021, 42(9): 2842. |
| [14] | LIU Changhui, LIANG Guojun, LI Yanlu, CHENG Xiufeng, ZHAO Xian. Density Functional Theory Study of NH3 Adsorption on Boron Nanotubes [J]. Chem. J. Chinese Universities, 2021, 42(7): 2263. |
| [15] | LIU Yang, LI Qingbo, SUN Jie, ZHAO Xian. Direct Synthesis of Graphene on AlN Substrates via Ga Remote Catalyzation [J]. Chem. J. Chinese Universities, 2021, 42(7): 2271. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||