

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (10): 2146.doi: 10.7503/cjcu20140393
• Physical Chemistry • Previous Articles Next Articles
SU Tingting1, LIU Junbo1,*( ), TANG Shanshan1, JIN Ruifa2
), TANG Shanshan1, JIN Ruifa2
Received:2014-04-24
Online:2014-10-10
Published:2014-07-28
Contact:
LIU Junbo
E-mail:liujb@mail.ccut.edu.cn
Supported by:CLC Number:
TrendMD:
SU Tingting, LIU Junbo, TANG Shanshan, JIN Ruifa. Theoretical Design and Experimental Performance Research on Barbital Imprinting Polymer†[J]. Chem. J. Chinese Universities, 2014, 35(10): 2146.
| Species | 6-31G(d,p) | 6-31++G(d,p) | 6-311G(d) | Expt.[ |
|---|---|---|---|---|
| C1—N2/nm | 0.1386 | 0.1385 | 0.1385 | 0.1376 |
| N2—C3/nm | 0.1385 | 0.1385 | 0.1384 | 0.1376 |
| C3—C4/nm | 0.1522 | 0.1523 | 0.1521 | 0.1514 |
| C1—O9/nm | 0.1206 | 0.1208 | 0.1199 | 0.1213 |
| C3—O11/nm | 0.1209 | 0.1211 | 0.1203 | 0.1213 |
| N2—H8/nm | 0.1013 | 0.1014 | 0.1012 | 0.0939 |
| N6—H7/nm | 0.1013 | 0.1014 | 0.1012 | 0.0939 |
| C4—C12/nm | 0.1544 | 0.1546 | 0.1544 | 0.1541 |
| C12—C22/nm | 0.1526 | 0.1527 | 0.1526 | 0.1514 |
| C1—N2—C3/(°) | 127.7 | 127.4 | 127.8 | 126.5 |
| N2—C3—C4/(°) | 117.4 | 117.7 | 117.4 | 118.3 |
| C3—C4—C5/(°) | 115.1 | 114.7 | 115.2 | 115.8 |
| C4—C5—N6/(°) | 117.4 | 117.7 | 117.4 | 118.3 |
| C5—N6—C1/(°) | 127.7 | 127.4 | 127.8 | 126.4 |
| N6—C1—N2/(°) | 114.4 | 114.7 | 114.3 | 114.6 |
| C12—C4—C5/(°) | 107.8 | 107.9 | 107.8 | 109.6 |
| C4—C12—C22/(°) | 114.3 | 113.3 | 113.3 | 114.8 |
Table 1 Structural parameters calculated by the M062X with different basis sets [6-31G(d,p), 6-31++G(d,p) and 6-311G(d)] and experimental values of BAR
| Species | 6-31G(d,p) | 6-31++G(d,p) | 6-311G(d) | Expt.[ |
|---|---|---|---|---|
| C1—N2/nm | 0.1386 | 0.1385 | 0.1385 | 0.1376 |
| N2—C3/nm | 0.1385 | 0.1385 | 0.1384 | 0.1376 |
| C3—C4/nm | 0.1522 | 0.1523 | 0.1521 | 0.1514 |
| C1—O9/nm | 0.1206 | 0.1208 | 0.1199 | 0.1213 |
| C3—O11/nm | 0.1209 | 0.1211 | 0.1203 | 0.1213 |
| N2—H8/nm | 0.1013 | 0.1014 | 0.1012 | 0.0939 |
| N6—H7/nm | 0.1013 | 0.1014 | 0.1012 | 0.0939 |
| C4—C12/nm | 0.1544 | 0.1546 | 0.1544 | 0.1541 |
| C12—C22/nm | 0.1526 | 0.1527 | 0.1526 | 0.1514 |
| C1—N2—C3/(°) | 127.7 | 127.4 | 127.8 | 126.5 |
| N2—C3—C4/(°) | 117.4 | 117.7 | 117.4 | 118.3 |
| C3—C4—C5/(°) | 115.1 | 114.7 | 115.2 | 115.8 |
| C4—C5—N6/(°) | 117.4 | 117.7 | 117.4 | 118.3 |
| C5—N6—C1/(°) | 127.7 | 127.4 | 127.8 | 126.4 |
| N6—C1—N2/(°) | 114.4 | 114.7 | 114.3 | 114.6 |
| C12—C4—C5/(°) | 107.8 | 107.9 | 107.8 | 109.6 |
| C4—C12—C22/(°) | 114.3 | 113.3 | 113.3 | 114.8 |
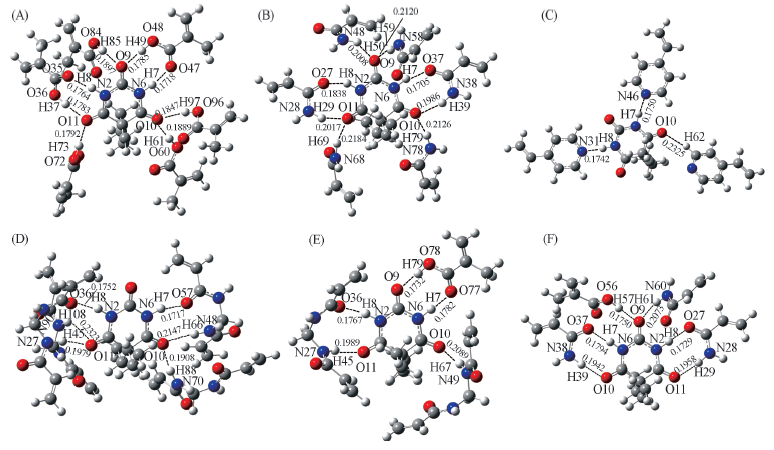
Fig.4 Models of complexes formed from template molecule and functional monomers (A) BAR-MAA; (B) BAR-AM; (C) BAR-4-Vpy; (D) BAR-MBAD; (E) BAR-MAA-MBAD; (F) BAR-MAA-AM.
| Complex | Action site | R/nm | ρ(r) | ▽2ρ(r) | Complex | Action site | R/nm | ρ(r) | ▽2ρ(r) |
|---|---|---|---|---|---|---|---|---|---|
| BAR-MAA(1∶6) | O35…H8—N2 | 0.1764 | 0.0371 | 0.1186 | BAR-4-Vpy(1∶3) | C56—H62…O10 | 0.2325 | 0.0125 | 0.0381 |
| O36—H37…O11 | 0.1783 | 0.0340 | 0.1111 | BAR-MBAD | N27—H45…O11 | 0.1979 | 0.0224 | 0.0723 | |
| O47…H7—N6 | 0.1718 | 0.0416 | 0.1311 | (1∶4) | O36…H8—N2 | 0.1752 | 0.0354 | 0.1270 | |
| O48—H49…O9 | 0.1785 | 0.0339 | 0.1113 | N48—H66…O10 | 0.2147 | 0.0159 | 0.0474 | ||
| O60—H61…O10 | 0.1889 | 0.0258 | 0.0906 | O57…H7—N6 | 0.1717 | 0.0353 | 0.1386 | ||
| O72—H73…O11 | 0.1792 | 0.0309 | 0.1151 | N70—H88…O10 | 0.1908 | 0.0266 | 0.0868 | ||
| O84—H85…O9 | 0.1897 | 0.0257 | 0.0825 | N90—H108…O11 | 0.2323 | 0.0126 | 0.0450 | ||
| O96—H97…O10 | 0.1847 | 0.0293 | 0.0972 | BAR-MAA-MBAD | O36…H8—N2 | 0.1767 | 0.0350 | 0.1185 | |
| BAR-AM(1∶6) | O27…H8—N2 | 0.1838 | 0.0322 | 0.0962 | (1∶1∶2) | N27—H45…O11 | 0.1989 | 0.0219 | 0.0710 |
| N28—H29…O11 | 0.2017 | 0.0212 | 0.0656 | N49—H67…O10 | 0.2089 | 0.0178 | 0.0599 | ||
| O37…H7—N6 | 0.1705 | 0.0340 | 0.1328 | O78—H79…O9 | 0.1732 | 0.0385 | 0.1253 | ||
| N38—H39…O10 | 0.1986 | 0.0233 | 0.0688 | O77…H7—N6 | 0.1782 | 0.0356 | 0.1156 | ||
| N49—H50…O9 | 0.2008 | 0.0215 | 0.0713 | BAR-MAA-AM | O27…H8—N2 | 0.1729 | 0.0411 | 0.1263 | |
| N58—H59…O9 | 0.2120 | 0.0174 | 0.0528 | (1∶1∶3) | O28—H29…O11 | 0.1958 | 0.0245 | 0.0733 | |
| N68—H69…O11 | 0.2184 | 0.0155 | 0.0519 | O37…H7—N6 | 0.1794 | 0.0350 | 0.1088 | ||
| N78—H79…O10 | 0.2126 | 0.0174 | 0.0519 | O38—H39…O10 | 0.1942 | 0.0252 | 0.0765 | ||
| BAR-4-Vpy(1∶3) | N31…H8—N2 | 0.1742 | 0.0485 | 0.1081 | O56—H57…O9 | 0.1750 | 0.0363 | 0.1217 | |
| N46…H7—N6 | 0.1750 | 0.0476 | 0.1069 | O60—H61…O9 | 0.2073 | 0.0186 | 0.0577 |
Table 2 Relevant parameters of the molecular imprinting interaction systems of template molecule and functional monomers
| Complex | Action site | R/nm | ρ(r) | ▽2ρ(r) | Complex | Action site | R/nm | ρ(r) | ▽2ρ(r) |
|---|---|---|---|---|---|---|---|---|---|
| BAR-MAA(1∶6) | O35…H8—N2 | 0.1764 | 0.0371 | 0.1186 | BAR-4-Vpy(1∶3) | C56—H62…O10 | 0.2325 | 0.0125 | 0.0381 |
| O36—H37…O11 | 0.1783 | 0.0340 | 0.1111 | BAR-MBAD | N27—H45…O11 | 0.1979 | 0.0224 | 0.0723 | |
| O47…H7—N6 | 0.1718 | 0.0416 | 0.1311 | (1∶4) | O36…H8—N2 | 0.1752 | 0.0354 | 0.1270 | |
| O48—H49…O9 | 0.1785 | 0.0339 | 0.1113 | N48—H66…O10 | 0.2147 | 0.0159 | 0.0474 | ||
| O60—H61…O10 | 0.1889 | 0.0258 | 0.0906 | O57…H7—N6 | 0.1717 | 0.0353 | 0.1386 | ||
| O72—H73…O11 | 0.1792 | 0.0309 | 0.1151 | N70—H88…O10 | 0.1908 | 0.0266 | 0.0868 | ||
| O84—H85…O9 | 0.1897 | 0.0257 | 0.0825 | N90—H108…O11 | 0.2323 | 0.0126 | 0.0450 | ||
| O96—H97…O10 | 0.1847 | 0.0293 | 0.0972 | BAR-MAA-MBAD | O36…H8—N2 | 0.1767 | 0.0350 | 0.1185 | |
| BAR-AM(1∶6) | O27…H8—N2 | 0.1838 | 0.0322 | 0.0962 | (1∶1∶2) | N27—H45…O11 | 0.1989 | 0.0219 | 0.0710 |
| N28—H29…O11 | 0.2017 | 0.0212 | 0.0656 | N49—H67…O10 | 0.2089 | 0.0178 | 0.0599 | ||
| O37…H7—N6 | 0.1705 | 0.0340 | 0.1328 | O78—H79…O9 | 0.1732 | 0.0385 | 0.1253 | ||
| N38—H39…O10 | 0.1986 | 0.0233 | 0.0688 | O77…H7—N6 | 0.1782 | 0.0356 | 0.1156 | ||
| N49—H50…O9 | 0.2008 | 0.0215 | 0.0713 | BAR-MAA-AM | O27…H8—N2 | 0.1729 | 0.0411 | 0.1263 | |
| N58—H59…O9 | 0.2120 | 0.0174 | 0.0528 | (1∶1∶3) | O28—H29…O11 | 0.1958 | 0.0245 | 0.0733 | |
| N68—H69…O11 | 0.2184 | 0.0155 | 0.0519 | O37…H7—N6 | 0.1794 | 0.0350 | 0.1088 | ||
| N78—H79…O10 | 0.2126 | 0.0174 | 0.0519 | O38—H39…O10 | 0.1942 | 0.0252 | 0.0765 | ||
| BAR-4-Vpy(1∶3) | N31…H8—N2 | 0.1742 | 0.0485 | 0.1081 | O56—H57…O9 | 0.1750 | 0.0363 | 0.1217 | |
| N46…H7—N6 | 0.1750 | 0.0476 | 0.1069 | O60—H61…O9 | 0.2073 | 0.0186 | 0.0577 |
| Complex | ΔEBSSE/(kJ·mol-1) | Complex | ΔEBSSE/(kJ·mol-1) |
|---|---|---|---|
| BAR-MAA | -114.51 | BAR-MBAD-MAA | -46.19 |
| BAR-AM | -63.53 | BAR-MBAD | -22.05 |
| BAR-MAA-AM | -53.49 | BAR-4-Vpy | -14.44 |
Table 3 Binding energies of complexes calculated at the M062X/6-31G(d,p) level
| Complex | ΔEBSSE/(kJ·mol-1) | Complex | ΔEBSSE/(kJ·mol-1) |
|---|---|---|---|
| BAR-MAA | -114.51 | BAR-MBAD-MAA | -46.19 |
| BAR-AM | -63.53 | BAR-MBAD | -22.05 |
| BAR-MAA-AM | -53.49 | BAR-4-Vpy | -14.44 |
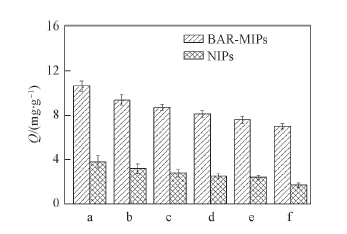
Fig.5 Adsorption capacities of MIPs and NIPs with different functional monomers a.BAR-MAA; b. BAR-AM; c. BAR-MBAD;d. BAR-MAA-AM; e. BAR-MAA-MBAD; f. BAR-4-Vpy.
| Substrate | Q/(mg·g-1) | α | ||
|---|---|---|---|---|
| BAR-MIPs | NIPs | BAR-MIPs | NIPs | |
| BAR | 10.5 | 3.8 | ||
| DMBA | 5.8 | 3.2 | 1.81 | 1.18 |
| TMB | 6.8 | 3.1 | 1.54 | 1.22 |
| PBS | 8.3 | 3.3 | 1.26 | 1.15 |
Table 4 Selective factor α of BAR-MIPs and NIPs
| Substrate | Q/(mg·g-1) | α | ||
|---|---|---|---|---|
| BAR-MIPs | NIPs | BAR-MIPs | NIPs | |
| BAR | 10.5 | 3.8 | ||
| DMBA | 5.8 | 3.2 | 1.81 | 1.18 |
| TMB | 6.8 | 3.1 | 1.54 | 1.22 |
| PBS | 8.3 | 3.3 | 1.26 | 1.15 |
| T/K | Time of used | Mean(%) | RSD(%) | |||
|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | |||
| 293 | 95.23 | 94.28 | 92.57 | 90.74 | 93.20 | 2.12 |
| 303 | 93.72 | 91.65 | 89.23 | 87.91 | 90.62 | 2.57 |
| 313 | 91.86 | 89.64 | 86.75 | 84.87 | 88.28 | 3.08 |
Table 5 Adsorption rate(%) of BAR-MIPs
| T/K | Time of used | Mean(%) | RSD(%) | |||
|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | |||
| 293 | 95.23 | 94.28 | 92.57 | 90.74 | 93.20 | 2.12 |
| 303 | 93.72 | 91.65 | 89.23 | 87.91 | 90.62 | 2.57 |
| 313 | 91.86 | 89.64 | 86.75 | 84.87 | 88.28 | 3.08 |
| [1] | Lian Z. R., Wang J. T., Mar. Pollut. Bull., 2012, 64, 2656—2662 |
| [2] | Zhang Y. Q., Shan X., Gao X. Q., Sep. Purif. Technol., 2011, 76(3), 337—344 |
| [3] | Liang R. N., Kou L. J., Chen Z. P., Qin W., Sensor Actuat. B-Chem., 2013, 188, 972—977 |
| [4] | Marina N., Sandra B., Dragana M. P., Davorka S., Forensic Sci. Int., 2013, 231(1/3), 317—324 |
| [5] | Li W. J., Hu Y. Y., Han F., Xu H. Q., Song W., Lv Y. N., Zheng P., Chem. J. Chinese Universities,2013, 34(5), 1219—1225 |
| (李文静, 胡艳云, 韩芳, 徐慧群, 宋伟, 吕亚宁, 郑平. 高等学校化学学报, 2013, 34(5), 1219—1225) | |
| [6] | Sun J. N., Liu J. B., Tang S. S., Jin R. F., Chinese J. Struct. Chem., 2013, 32(8), 1204—1210 |
| [7] | Liu J. B., Tang S. S., Sun J. N., Jin R. F., Chem. J. Chinese Universities,2013, 34(11), 2566—2573 |
| (刘俊渤, 唐珊珊, 孙佳妮, 靳瑞发. 高等学校化学学报, 2013, 34(11), 2566—2573) | |
| [8] | Wang Z.L., Studies on Immunological Rapid Determination of Phenobarbital Residues in Animal Food, Northwest A & F University, Xi’an, 2006 |
| (王自良. 苯巴比妥残留免疫学快速检测技术研究, 西安, 西北农林科技大学, 2006) | |
| [9] | Zhang H. T., Wang Z. L., Wang Y. R., Journal of Shanxi Agricultural Sciences,2008, 36(4), 29—31 |
| (张海棠, 王自良, 王艳荣. 山西农业科学, 2008, 36(4), 29—31) | |
| [10] | Deng Q. L., Yan C., Zhang Z. C., Gao R. Y., Wang Q. S., Chinese J. Analys. Lab., 2003, 22, 113—115 |
| (邓启良, 阎超, 张智超, 高如瑜, 王琴孙. 分析试验室, 2003, 22, 113—115) | |
| [11] | Beltran A., Borrull F., Cormack P. A. G., Marcé R. M., J. Chromatogr. A,2011, 1218(29), 4612—4618 |
| [12] | Dong C. K., Li X., Guo Z. C., Qi J. Y., Anal. Chim. Acta,2009, 647(1), 117—124 |
| [13] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Montgomery J. A., Vreven T., Kudin J. K. N., Burant J. C., Milliam J. M., Iyengar S. S., Tomasi J., Barone V., Mennucci B., Cossi M., Scalmani G., Rega N., Petersson G. A., Nakatsuji H., Hada M., Ehara M., Toyota K., Fakuda R., Hasegawa J., Ishida M., Akajima T., Honda Y., Kitao O., Nakai H., Klene M., Li X., Knox J. E., Hratchian H. P., Cross J. B., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Ayala P. Y., Morokuma K., Voth G. A., Salvador P., Dannenberg J. J., Zakrzewski V. G., Dapprich S., Daniels A. D., Strain M. C., Farkas O., Malick D. K., Rabuck A. D., Raghavachari K., Foresman J. B., Ortiz J. V., Cui Q., Baboul A. G., Clifford S., Cioslowski J., Stefanov B. B., Liu G., Liashenko A., Piskorz P., Komaromi I., Martin R. L., Fox D. J., Keith T., Al-Laham M. A., Peng C. Y., Nanayakkara A., Challacombe M., Gill P. M. W., Johnson B., Chen W., Wong M. W., Gonzalez C., Pople J. A.,Gaussian 09, Revision A.2, Gaussian Inc., Pittsburgh PA, 2009 |
| [14] | Boys F., Bernardi F., Mol. Phys., 1970, 19, 553 |
| [15] | Zhang Y., Xu X., Prog. Chem., 2012, 24(6), 1023—1037 |
| (张颖, 徐昕. 化学进展, 2012, 24(6), 1023—1037) | |
| [16] | Su P. F., Tan K., Wu A. A., Lv X., Zhao Y., Cao Z. X., Wu W., Journal of Xiamen University(Natural Science), 2011, 50(2), 311—318 |
| (苏培峰, 谭凯, 吴安安, 吕鑫, 赵仪, 曹泽星, 吴玮. 厦门大学学报(自然科学版), 2011, 50(2), 311—318) | |
| [17] | Sun T., Wang Y. B., Acta Phys. Chim. Sin., 2011, 27(11), 2553—2558 |
| (孙涛, 王一波. 物理化学学报, 2011, 27(11), 2553—2558) | |
| [18] | Maria das Dores M. C. R. S., Manuel A. V. R. S., Vera L. S. F., Maria V. R., Pilar J., Manuel T., Juan Z. D., Pilar C., Rosa M. C., José E., J. Chem. Thermodyn., 2009, 41, 1400—1407 |
| [19] | Bader R. F. W., J. Phys. Chem. A, 1998, 102, 7314—7323 |
| [20] | Lu T., Chen F. W., J. Comput. Chem., 2012, 33, 580 |
| [21] | Popelier P. L. A., Bader R. F. W., J. Phy. Chem. A,1998, 102, 1873—1878 |
| [22] | Jeffrey G. A., Crystallogr. Rev., 2003, 9(2/3), 135—176 |
| [23] | Steiner T., Crystallogr. Rev., 2003, 9(2/3), 177—228 |
| [1] | WENG Meiqi, SHANG Guiming, WANG Jiatai, LI Shenghua, FAN Zhi, LIN Song, GUO Minjie. Template Simulation of Organophosphorus Nerve Agent Molecularly Imprinted Polymers [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220136. |
| [2] | GAO Zhiwei, LI Junwei, SHI Sai, FU Qiang, JIA Junru, AN Hailong. Analysis of Gating Characteristics of TRPM8 Channel Based on Molecular Dynamics [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220080. |
| [3] | HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614. |
| [4] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [5] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [6] | LEI Xiaotong, JIN Yiqing, MENG Xuanyu. Prediction of the Binding Site of PIP2 in the TREK-1 Channel Based on Molecular Modeling [J]. Chem. J. Chinese Universities, 2021, 42(8): 2550. |
| [7] | LI Congcong, LIU Minghao, HAN Jiarui, ZHU Jingxuan, HAN Weiwei, LI Wannan. Theoretical Study of the Catalytic Activity of VmoLac Non-specific Substrates Based on Molecular Dynamics Simulations [J]. Chem. J. Chinese Universities, 2021, 42(8): 2518. |
| [8] | ZENG Yonghui, YAN Tianying. Vibrational Density of States Analysis of Proton Hydration Structure [J]. Chem. J. Chinese Universities, 2021, 42(6): 1855. |
| [9] | LIU Aiqing, XU Wensheng, XU Xiaolei, CHEN Jizhong, AN Lijia. Molecular Dynamics Simulation of Polymer/rod Nanocomposite [J]. Chem. J. Chinese Universities, 2021, 42(3): 875. |
| [10] | QI Renrui, LI Minghao, CHANG Hao, FU Xueqi, GAO Bo, HAN Weiwei, HAN Lu, LI Wannan. Theoretical Study on the Unbinding Pathway of Xanthine Oxidase Inhibitors Based on Steered Molecular Dynamics Simulation [J]. Chem. J. Chinese Universities, 2021, 42(3): 758. |
| [11] | FAN Juanjuan, HAN Yuanyuan, CUI Jie. Monte Carlo Simulation of the Transformation Control of ABC Triblock Copolymer Micelles from Multicompartment Structure to Multicore Structure [J]. Chem. J. Chinese Universities, 2021, 42(3): 857. |
| [12] | SONG Wenyao, ZHOU Zhanglang, YANG Xinli, CHEN Lan, GE Guanglu. Tunable Enantioselective Adsorption of the As⁃synthesized Mesoporous Silica Through Chiral Imprinting [J]. Chem. J. Chinese Universities, 2021, 42(10): 3144. |
| [13] | YE Chenghao, LIANG Heng, LI Enmin, XU Liyan, LI Peng, CHEN Guanghui. High-throughput Virtual Screening of CDK2/Cyclin A2 Target Inhibitors [J]. Chem. J. Chinese Universities, 2021, 42(10): 3135. |
| [14] | WANG Xiaoru,ZHANG Na,XING Jun. Preparation and Application of Melamine Imprinted Material Using Itaconic Acid as Multidentate Functional Monomer [J]. Chem. J. Chinese Universities, 2020, 41(7): 1521. |
| [15] | LI Xiangyuan,YAO Xiaoxia,SHENTU Jiangtao,SUN Xiaohui,LI Juanqin,LIU Mingxia,XU Shimin. Combustion Reaction Mechanism Construction by Two-parameter Rate Constant Method † [J]. Chem. J. Chinese Universities, 2020, 41(3): 512. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||