

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (8): 1739.doi: 10.7503/cjcu20140260
• Physical Chemistry • Previous Articles Next Articles
DING Kaining*( ), LI Yulu, CHENG Peisi, ZHANG Yongfan
), LI Yulu, CHENG Peisi, ZHANG Yongfan
Received:2014-03-25
Online:2014-08-10
Published:2014-06-16
Contact:
DING Kaining
E-mail:dknfzu@fzu.edu.cn
Supported by:CLC Number:
TrendMD:
DING Kaining, LI Yulu, CHENG Peisi, ZHANG Yongfan. Theoretical Studies on the Reaction Mechanisms of Methoxy Group and Carbon Monoxide over the Surfaces of Pd(111)†[J]. Chem. J. Chinese Universities, 2014, 35(8): 1739.
| Layer | Δd1-2(%) | Δd2-3(%) | Δd3-4 (%) | Esurf /(J·m-2) |
|---|---|---|---|---|
| 3 | 0.33(0.44[ | -0.39(-0.32[ | 1.26(1.40[ | |
| 4 | 0.34 | -0.27 | 1.28 | |
| 5 | 0.31 | -0.31 | 1.30 | |
| 6 | 0.33 | -0.29 | 1.34 | |
| 7 | 0.41 | 0.30 | -0.33 | 1.32 |
Table 1 Calculated relaxation degree and surface energy of 3×3 Pd(111) surface with different layers
| Layer | Δd1-2(%) | Δd2-3(%) | Δd3-4 (%) | Esurf /(J·m-2) |
|---|---|---|---|---|
| 3 | 0.33(0.44[ | -0.39(-0.32[ | 1.26(1.40[ | |
| 4 | 0.34 | -0.27 | 1.28 | |
| 5 | 0.31 | -0.31 | 1.30 | |
| 6 | 0.33 | -0.29 | 1.34 | |
| 7 | 0.41 | 0.30 | -0.33 | 1.32 |
| Layer | Eads(CO)/(kJ·mol-1) | Eads(H3CO)/( kJ·mol-1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Top | Bridge | fcc | hcp | Top | Bridge | fcc | hcp | ||
| 3 | -159.41 | -144.87 | -204.18 | -201.67 | -180.33 | -211.71 | -218.82 | -197.48 | |
| 4 | -151.46 | -186.61 | -199.99 | -196.23 | -189.95 | -200.41 | -212.97 | -196.72 | |
| 5 | -161.08 | -173.22 | -187.02 | -144.78 | -196.14 | -210.04 | -215.06 | -196.42 | |
| 6 | -158.16 | -176.98 | -186.61 | -187.86 | -192.05 | -203.76 | -214.22 | -212.55 | |
| 7 | -155.64 | -187.02 | -196.23 | -192.88 | -195.68 | -205.43 | -216.31 | -210.87 | |
Table 2 Adsorption energy of CO and CH3O over 3×3 Pd(111) surface with different layers
| Layer | Eads(CO)/(kJ·mol-1) | Eads(H3CO)/( kJ·mol-1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Top | Bridge | fcc | hcp | Top | Bridge | fcc | hcp | ||
| 3 | -159.41 | -144.87 | -204.18 | -201.67 | -180.33 | -211.71 | -218.82 | -197.48 | |
| 4 | -151.46 | -186.61 | -199.99 | -196.23 | -189.95 | -200.41 | -212.97 | -196.72 | |
| 5 | -161.08 | -173.22 | -187.02 | -144.78 | -196.14 | -210.04 | -215.06 | -196.42 | |
| 6 | -158.16 | -176.98 | -186.61 | -187.86 | -192.05 | -203.76 | -214.22 | -212.55 | |
| 7 | -155.64 | -187.02 | -196.23 | -192.88 | -195.68 | -205.43 | -216.31 | -210.87 | |
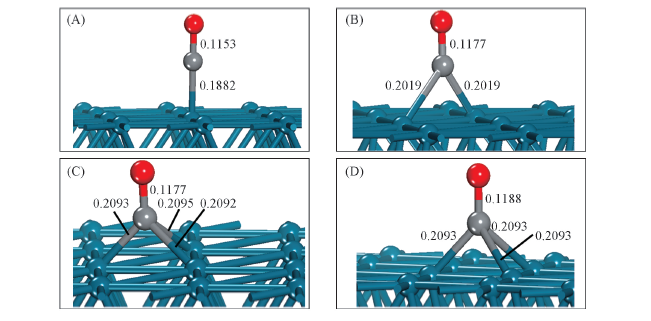
Fig.2 Lateral views for CO adsorbed on Pd(111) surface with four balanced geometrical configurations (A) Top site; (B) bridge site; (C) fcc site; (D) hcp site.
| Species | Eads/(kJ·mol-1) | Species | Eads/(kJ·mol-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nt | Nb | Nf | Nh | Nt | Nb | Nf | Nh | ||
| 1×1 | -181.17 | -171.96 | -74.89 | -84.94 | Calcd.[ | -112.13 | -141.84 | -167.36 | |
| 2×2 | -178.66 | -177.82 | -79.08 | -86.19 | Calcd.[ | -131.38 | -174.47 | -194.14 | -191.21 |
| 3×3 | -159.41 | -144.87 | -204.18 | -201.67 | 4×4 | -181.60 | -158.16 | -184.51 | -182.00 |
| Calcd.[ | -161.92 | -181.59 | -185.35 | -186.19 | |||||
Table 3 Calculated adsorption energy of CO on 1×1, 2×2, 3×3, 4×4 Pd(111) surface
| Species | Eads/(kJ·mol-1) | Species | Eads/(kJ·mol-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nt | Nb | Nf | Nh | Nt | Nb | Nf | Nh | ||
| 1×1 | -181.17 | -171.96 | -74.89 | -84.94 | Calcd.[ | -112.13 | -141.84 | -167.36 | |
| 2×2 | -178.66 | -177.82 | -79.08 | -86.19 | Calcd.[ | -131.38 | -174.47 | -194.14 | -191.21 |
| 3×3 | -159.41 | -144.87 | -204.18 | -201.67 | 4×4 | -181.60 | -158.16 | -184.51 | -182.00 |
| Calcd.[ | -161.92 | -181.59 | -185.35 | -186.19 | |||||
| Parameter | Nt | Nb | Nf | Nh | Free CO |
|---|---|---|---|---|---|
| dC—O/nm(This work) | 0.1153 | 0.1177 | 0.1187 | 0.1188 | 0.1141 |
| dC—O/nm(Calcd.[ | 0.115 | 0.116 | 0.117 | 0.117 | 0.1128 |
| dC—O/nm(Calcd.[ | 0.1152 | 0.1171 | 0.1183 | ||
| dC—O/nm(Calcd.[ | 0.1152 | 0.1178 | 0.1189 | 0.1188 | 0.1146 |
| dC—O/nm(Expt.[ | 0.1131 | ||||
| dPd—C/nm(This work) | 0.1882 | 0.2018 | 0.209 | 0.209 | |
| dPd—C/nm(Calcd.[ | 0.168 | 0.190 | 0.200 | 0.200 | |
| dPd—C/nm(Calcd.[ | 0.1884 | 0.205 | 0.210 | ||
| νC—O/cm-1(This work) | 2031 | 1850 | 1770 | 1769 | 2120 |
| νC—O/cm-1(Calcd.[ | 1987 | 1876 | 1803 | ||
| νC—O/cm-1(Calcd.[ | 2014 | 1848 | 1779 | 1781 | 2087 |
| νC—O/cm-1(Expt.[ | 2138 |
Table 4 Geometry parameters and stretching vibration frequency of C—O for CO adsorbed on Pd(111) surface with four balance configurations
| Parameter | Nt | Nb | Nf | Nh | Free CO |
|---|---|---|---|---|---|
| dC—O/nm(This work) | 0.1153 | 0.1177 | 0.1187 | 0.1188 | 0.1141 |
| dC—O/nm(Calcd.[ | 0.115 | 0.116 | 0.117 | 0.117 | 0.1128 |
| dC—O/nm(Calcd.[ | 0.1152 | 0.1171 | 0.1183 | ||
| dC—O/nm(Calcd.[ | 0.1152 | 0.1178 | 0.1189 | 0.1188 | 0.1146 |
| dC—O/nm(Expt.[ | 0.1131 | ||||
| dPd—C/nm(This work) | 0.1882 | 0.2018 | 0.209 | 0.209 | |
| dPd—C/nm(Calcd.[ | 0.168 | 0.190 | 0.200 | 0.200 | |
| dPd—C/nm(Calcd.[ | 0.1884 | 0.205 | 0.210 | ||
| νC—O/cm-1(This work) | 2031 | 1850 | 1770 | 1769 | 2120 |
| νC—O/cm-1(Calcd.[ | 1987 | 1876 | 1803 | ||
| νC—O/cm-1(Calcd.[ | 2014 | 1848 | 1779 | 1781 | 2087 |
| νC—O/cm-1(Expt.[ | 2138 |
| Species | Charge/e(Δe) | |||
|---|---|---|---|---|
| C | O | CO | Pd | |
| Free | 0.101(0) | -0.101(0) | 0.000(0) | -0.261(0) |
| Nh | 0.332(0.231) | -0.167(-0.066) | 0.165(0.165) | -0.453(-0.192) |
| Nf | 0.341(0.240) | -0.123(-0.022) | 0.218(0.218) | -0.550(-0.289) |
| Nb | 0.362(0.261) | -0.206(-0.105) | 0.156(0.156) | -0.414(-0.153) |
| Nt | 0.414(0.313) | -0.267(-0.166) | 0.147(0.147) | -0.407(-0.146) |
Table 5 Mulliken charge distribution for the adsorption system of CO/Pd(111)
| Species | Charge/e(Δe) | |||
|---|---|---|---|---|
| C | O | CO | Pd | |
| Free | 0.101(0) | -0.101(0) | 0.000(0) | -0.261(0) |
| Nh | 0.332(0.231) | -0.167(-0.066) | 0.165(0.165) | -0.453(-0.192) |
| Nf | 0.341(0.240) | -0.123(-0.022) | 0.218(0.218) | -0.550(-0.289) |
| Nb | 0.362(0.261) | -0.206(-0.105) | 0.156(0.156) | -0.414(-0.153) |
| Nt | 0.414(0.313) | -0.267(-0.166) | 0.147(0.147) | -0.407(-0.146) |
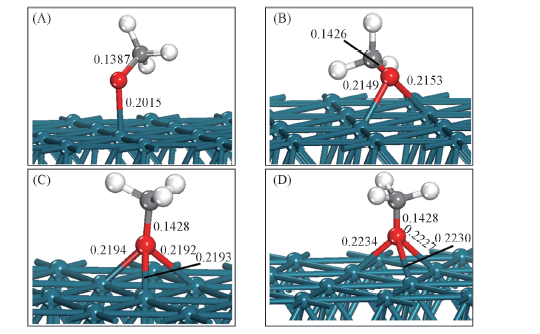
Fig.3 Lateral views for CH3O adsorbed on Pd(111) surface with balanced geometrical configurations (A) Top site; (B) bridge site; (C) fcc site; (D) hcp site.
| Parameter | dC—O/ nm | dC—H/ nm | ∠H—C—H/ (°) | ∠O—C—H/ (°) | dPd—O/ nm | νs(C—O)/ cm-1 | νs(C—H)/ cm-1 | νas(C—H)/ cm-1 |
|---|---|---|---|---|---|---|---|---|
| Lt | 0.1387 | 0.1113 | 106.4 | 113.5 | 0.2015 | 1007 | 2577 | 2787 |
| Lb | 0.1426 | 0.1108 | 108.8 | 109.3 | 0.2151 | 989 | 2857 | 2975 |
| Lf | 0.1428 | 0.1097 | 109.3 | 109.6 | 0.2193 | 986 | 2969 | 3046 |
| Lh | 0.1425 | 0.1098 | 108.7 | 109.9 | 0.2230 | 995 | 2966 | 3040 |
| This work | 0.1356 | 0.1107 | 105.0 | 114.4 | 1101 | 2837 | 2912 | |
| Calcd.[ | 0.1405 | 0.1112 | 107.6 | 111.3 |
Table 6 Geometry parameters and stretching vibration frequency for CH3O adsorbed on Pd(111) surface with four balance configurations
| Parameter | dC—O/ nm | dC—H/ nm | ∠H—C—H/ (°) | ∠O—C—H/ (°) | dPd—O/ nm | νs(C—O)/ cm-1 | νs(C—H)/ cm-1 | νas(C—H)/ cm-1 |
|---|---|---|---|---|---|---|---|---|
| Lt | 0.1387 | 0.1113 | 106.4 | 113.5 | 0.2015 | 1007 | 2577 | 2787 |
| Lb | 0.1426 | 0.1108 | 108.8 | 109.3 | 0.2151 | 989 | 2857 | 2975 |
| Lf | 0.1428 | 0.1097 | 109.3 | 109.6 | 0.2193 | 986 | 2969 | 3046 |
| Lh | 0.1425 | 0.1098 | 108.7 | 109.9 | 0.2230 | 995 | 2966 | 3040 |
| This work | 0.1356 | 0.1107 | 105.0 | 114.4 | 1101 | 2837 | 2912 | |
| Calcd.[ | 0.1405 | 0.1112 | 107.6 | 111.3 |
| Species | Charge/e(Δe) | |||
|---|---|---|---|---|
| C | O | CH3O | Pd | |
| Free | 0.159(0) | -0.336(0) | 0.000(0) | -0.261(0) |
| Lt | 0.050(-0.109) | -0.462(-0.126) | -0.156(-0.156) | -0.041(0.220) |
| Lb | 0.055(-0.104) | -0.518(-0.182) | -0.199(-0.199) | 0.045(0.306) |
| Lf | 0.077(-0.082) | -0.556(-0.220) | -0.223(-0.223) | 0.080(0.341) |
| Lh | 0.079(-0.080) | -0.554(-0.218) | -0.204(-0.204) | 0.078(0.339) |
Table 7 Mulliken charge distribution for the adsorption system of CH3O/Pd(111) (translated from Pd to CH3O radical /e-)
| Species | Charge/e(Δe) | |||
|---|---|---|---|---|
| C | O | CH3O | Pd | |
| Free | 0.159(0) | -0.336(0) | 0.000(0) | -0.261(0) |
| Lt | 0.050(-0.109) | -0.462(-0.126) | -0.156(-0.156) | -0.041(0.220) |
| Lb | 0.055(-0.104) | -0.518(-0.182) | -0.199(-0.199) | 0.045(0.306) |
| Lf | 0.077(-0.082) | -0.556(-0.220) | -0.223(-0.223) | 0.080(0.341) |
| Lh | 0.079(-0.080) | -0.554(-0.218) | -0.204(-0.204) | 0.078(0.339) |
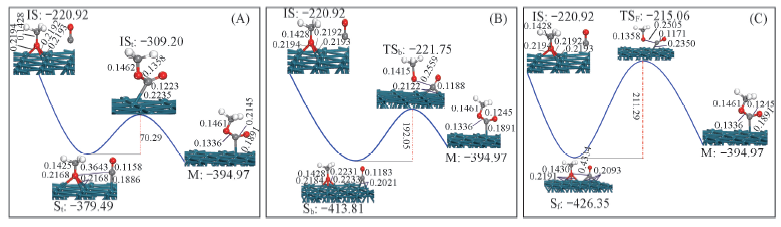
Fig.4 Schematic diagram of adsorbed CH3OOC formed by CH3O adsorbed on fcc site and CO on top site(A), bridge site(B)and fcc site(C) of Pd(111) surface Eabs is in kJ/mol, distances are in nm.
| [1] | Ma X. B., Xu G. H., Chen J. W., Chen H. F., Chinese J. Chem. Eng., 1995, 46(1), 50—56 |
| (马新宾, 许根慧, 陈锦文, 陈洪钫.化工学报, 1995, 46(1), 50—56) | |
| [2] | Lin X., Ji Y., Tan J. Q., Xiao W. D., Chinese J. Catal., 2008, 29(4), 325—329 |
| (林茜, 计扬, 谭俊清. 催化学报, 2008, 29(4), 325—329) | |
| [3] | Song Y., Li Z. H., Gao Z. H., He F., Xu G. H., Liu C. H., Zhu Q. M., Chinese J. Catal., 2000, 21(6), 537—541 |
| (宋瑛, 李振花, 高正虹, 何菲, 许根慧, 刘崇微, 朱起明. 催化学报, 2000, 21(6), 537—541) | |
| [4] | Ji Y., Liu G., Li W., Xiao W., J. Mol. Catal. A: Chem., 2009, 314(1/2), 63—70 |
| [5] | Chen G. K., Yan H. M., Xue B., Nat. Gas Chem. Indus., 1995, 20(4), 5—9 |
| (陈庚申, 严慧敏. 天然气化工, 1995, 20(4), 5—9) | |
| [6] | Xu Z. N., Sun J., Lin C. S., Jiang X. M., Chen Q. S., Peng S. Y., Wang M. S., Guo G. C., ACS Catalysis, 2013, 3(2), 118—122 |
| [7] | Delley B., J. Chem. Phys., 2000, 113(18), 7756—7764 |
| [8] | Perdew J. P., Wang Y., Phys. Rev. B, 1992, 45(23), 13244—13249 |
| [9] | Delley B., Phys. Rev. B, 2002, 66(15), 155125—155131 |
| [10] | Rose M. K., Mitsui T., Dunphy J., Borg A., Ogletree D. F., Surf. Sci., 2002, 512, 48—60 |
| [11] | Gravil P. A., Toulhoat H., Surf. Sci., 1999, 430, 176—191 |
| [12] | Loffreda D., Simon D., Sautet P., Surf. Sci., 1999, 425(1), 68—80 |
| [13] | Radilla J., Boronat M., Corma A., Illas F., Theor. Chem. Acc., 2010, 126(3/4), 223—229 |
| [14] | Ilya V. Y., Riadh S., Konstantin M. N., Notker R., J. Phys. Chem. B, 2003, 107, 255—264 |
| [15] | Zhang J., Wang Z., Wang Z. X., Surf. Interface Anal., 2011, 43(7), 1038—1045 |
| [16] | Herzberg G., Crawford B. L. Jr., J. Phys. Chem., 1946, 50(3), 288 |
| [17] | Lide D.R., CRC Handbook of Chemistry and Physics, CRC Press, Boca Raton, FL, 2000, 9—22 |
| [18] | Jackels C. F., J. Chem. Phys., 1982, 76(1), 505—515 |
| [19] | Yamamoto Y., Catalysis Surveys from Asia, 2010, 14(3/4), 103—110 |
| [20] | Song K., Ji Y., Xiao W. D., Guangdong Chemical Industry, 2007, 34(6), 12—14 |
| (宋轲, 计扬, 肖文德. 广东化工, 2007, 34(6), 12—14) | |
| [21] | Li Z. H., He C. Y., Xiang T. L., Wang B. W., Ma X. B., Xu G. H., Chemical Reaction Engineering and Technology, 2004, 20(3), 280—283(李振花, 何翠英, 项铁丽, 王保伟, 马新宾, 许根慧. 化学反应工程与工艺, 2004, 20(3), 280—283) |
| [22] | Chen Z. X., Neyman K. M., Lim K. H., Rösch N., Langmuir, 2004, 20(19), 8068—8077 |
| [23] | Wang G. C., Zhou Y. H., Nakamura J., J. Chem. Phys., 2005, 122(4), 044707-1—044707-8 |
| [24] | Ren R., Niu C., Bu S., Zhou Y., Lv Y., Wang G., Journal of Natural Gas Chemistry, 2011, 20(1), 90—98 |
| [25] | Gates J., Kesmodel L., J. Catal., 1983, 83(2), 437—445 |
| [26] | Yang H., Whitten J. L., Langmuir, 1995, 11(3), 853—859 |
| [27] | Huberty J., Madix R., Surf. Sci., 1996, 360(1), 144—156 |
| [28] | Guang Z. R., Zheng H. Y., Wang B. J., Li Z., Chem. J. Chinese Universities, 2010, 31(6),1246—1251(章日光, 郑华艳, 王宝俊, 李忠. 高等学校化学学报, 2010, 31(6), 1246—1251) |
| (Ed.: Y, Z) |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | WANG Lijun, LI Xin, HONG Song, ZHAN Xinyu, WANG Di, HAO Leiduan, SUN Zhenyu. Efficient Electrocatalytic CO2 Reduction to CO by Tuning CdO-Carbon Black Interface [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220317. |
| [3] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [4] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [5] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [6] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [7] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [8] | MA Lijuan, GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun. Theoretical Investigation of Hydrogen Storage Properties of Sc, Ti, V-decorated and B/N-doped Monovacancy Graphene [J]. Chem. J. Chinese Universities, 2021, 42(9): 2842. |
| [9] | HUANG Luoyi, WENG Yueyue, HUANG Xuhui, WANG Chaojie. Theoretical Study on the Structures and Properties of Flavonoids in Plantain [J]. Chem. J. Chinese Universities, 2021, 42(9): 2752. |
| [10] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| [11] | ZHENG Ruoxin, ZHANG Igor Ying, XU Xin. Development and Benchmark of Lower Scaling Doubly Hybrid Density Functional XYG3 [J]. Chem. J. Chinese Universities, 2021, 42(7): 2210. |
| [12] | LIU Changhui, LIANG Guojun, LI Yanlu, CHENG Xiufeng, ZHAO Xian. Density Functional Theory Study of NH3 Adsorption on Boron Nanotubes [J]. Chem. J. Chinese Universities, 2021, 42(7): 2263. |
| [13] | LIU Yang, LI Qingbo, SUN Jie, ZHAO Xian. Direct Synthesis of Graphene on AlN Substrates via Ga Remote Catalyzation [J]. Chem. J. Chinese Universities, 2021, 42(7): 2271. |
| [14] | WANG Jian, ZHANG Hongxing. Theoretical Study on the Structural-photophysical Relationships of Tetra-Pt Phosphorescent Emitters [J]. Chem. J. Chinese Universities, 2021, 42(7): 2245. |
| [15] | HU Wei, LIU Xiaofeng, LI Zhenyu, YANG Jinlong. Surface and Size Effects of Nitrogen-vacancy Centers in Diamond Nanowires [J]. Chem. J. Chinese Universities, 2021, 42(7): 2178. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||