

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (1): 154.doi: 10.7503/cjcu20130707
• Physical Chemistry • Previous Articles Next Articles
LIU Peng, LI Shushi, WANG Changsheng*( )
)
Received:2013-07-25
Online:2014-01-10
Published:2013-12-11
Contact:
WANG Changsheng
E-mail:chwangcs@lnnu.edu.cn
Supported by:CLC Number:
TrendMD:
LIU Peng, LI Shushi, WANG Changsheng. Effects of Substituents on the Binding Energy in Hydrogen-bonded Complexes Containing Adenine and Thymine†[J]. Chem. J. Chinese Universities, 2014, 35(1): 154.
| R | ρc(C—H…O=C) | ρc(N…H—N) | ρc(N—H…O=C) | ∑ρc | Eb |
|---|---|---|---|---|---|
| NHCH3 | 0.0046 | 0.0409 | 0.0269 | 0.0724 | -58.55 |
| NH2 | 0.0047 | 0.0413 | 0.0267 | 0.0726 | -58.97 |
| Et | 0.0044 | 0.0405 | 0.0277 | 0.0726 | -59.30 |
| CH3 | 0.0044 | 0.0407 | 0.0277 | 0.0728 | -59.39 |
| OCH3 | 0.0048 | 0.0412 | 0.0270 | 0.0730 | -59.53 |
| H | 0.0044 | 0.0409 | 0.0279 | 0.0732 | -59.74 |
| COMe | 0.0049 | 0.0419 | 0.0263 | 0.0731 | -60.25 |
| BH2 | 0.0044 | 0.0414 | 0.0274 | 0.0732 | -60.84 |
| Cl | 0.0049 | 0.0428 | 0.0264 | 0.0741 | -61.43 |
| CHO | 0.0046 | 0.0427 | 0.0264 | 0.0737 | -61.49 |
| Br | 0.0050 | 0.0430 | 0.0263 | 0.0743 | -61.49 |
| CCl3 | 0.0051 | 0.0436 | 0.0258 | 0.0745 | -62.22 |
| COOH | 0.0054 | 0.0449 | 0.0241 | 0.0744 | -62.23 |
| CF3 | 0.0048 | 0.0432 | 0.0260 | 0.0740 | -62.39 |
| SO3H | 0.0058 | 0.0471 | 0.0233 | 0.0762 | -63.17 |
| NO2 | 0.0054 | 0.0443 | 0.0253 | 0.0750 | -63.52 |
| CN | 0.0048 | 0.0443 | 0.0266 | 0.0757 | -63.58 |
Table 1 Electron densities(a.u.) at the hydrogen bond critical points and the binding energies(kJ/mol)
| R | ρc(C—H…O=C) | ρc(N…H—N) | ρc(N—H…O=C) | ∑ρc | Eb |
|---|---|---|---|---|---|
| NHCH3 | 0.0046 | 0.0409 | 0.0269 | 0.0724 | -58.55 |
| NH2 | 0.0047 | 0.0413 | 0.0267 | 0.0726 | -58.97 |
| Et | 0.0044 | 0.0405 | 0.0277 | 0.0726 | -59.30 |
| CH3 | 0.0044 | 0.0407 | 0.0277 | 0.0728 | -59.39 |
| OCH3 | 0.0048 | 0.0412 | 0.0270 | 0.0730 | -59.53 |
| H | 0.0044 | 0.0409 | 0.0279 | 0.0732 | -59.74 |
| COMe | 0.0049 | 0.0419 | 0.0263 | 0.0731 | -60.25 |
| BH2 | 0.0044 | 0.0414 | 0.0274 | 0.0732 | -60.84 |
| Cl | 0.0049 | 0.0428 | 0.0264 | 0.0741 | -61.43 |
| CHO | 0.0046 | 0.0427 | 0.0264 | 0.0737 | -61.49 |
| Br | 0.0050 | 0.0430 | 0.0263 | 0.0743 | -61.49 |
| CCl3 | 0.0051 | 0.0436 | 0.0258 | 0.0745 | -62.22 |
| COOH | 0.0054 | 0.0449 | 0.0241 | 0.0744 | -62.23 |
| CF3 | 0.0048 | 0.0432 | 0.0260 | 0.0740 | -62.39 |
| SO3H | 0.0058 | 0.0471 | 0.0233 | 0.0762 | -63.17 |
| NO2 | 0.0054 | 0.0443 | 0.0253 | 0.0750 | -63.52 |
| CN | 0.0048 | 0.0443 | 0.0266 | 0.0757 | -63.58 |
| R | Eij | Eb | ΔEij | ΔEb | |||
|---|---|---|---|---|---|---|---|
| NHCH3 | 1.00 | 92.84 | 41.67 | 135.52 | -58.55 | -3.51 | -1.19 |
| NH2 | 1.05 | 94.35 | 41.25 | 136.65 | -58.97 | -2.38 | -0.77 |
| Et | 0.75 | 91.63 | 44.56 | 136.94 | -59.30 | -2.09 | -0.43 |
| CH3 | 0.75 | 92.34 | 44.69 | 137.78 | -59.39 | -1.26 | -0.35 |
| OCH3 | 1.09 | 94.47 | 42.09 | 137.65 | -59.53 | -1.38 | -0.21 |
| H | 0.71 | 93.30 | 45.02 | 139.03 | -59.74 | 0 | 0 |
| COMe | 1.13 | 97.78 | 40.12 | 139.03 | -60.25 | 0 | 0.51 |
| BH2 | 0.75 | 95.86 | 43.35 | 139.95 | -60.84 | 0.92 | 1.10 |
| Cl | 1.13 | 100.67 | 40.58 | 142.38 | -61.43 | 3.35 | 1.69 |
| CHO | 0.79 | 101.13 | 40.25 | 142.17 | -61.49 | 3.14 | 1.75 |
| Br | 1.17 | 101.50 | 40.21 | 142.88 | -61.49 | 3.85 | 1.75 |
| CCl3 | 1.21 | 104.31 | 38.49 | 144.01 | -62.22 | 4.98 | 2.48 |
| COOH | 1.30 | 110.12 | 30.71 | 142.13 | -62.23 | 3.10 | 2.49 |
| CF3 | 1.09 | 102.63 | 39.46 | 143.18 | -62.39 | 4.14 | 2.65 |
| SO3H | 1.46 | 118.62 | 29.37 | 149.45 | -63.17 | 10.42 | 3.43 |
| NO2 | 1.30 | 107.49 | 37.61 | 146.40 | -63.52 | 7.36 | 3.78 |
| CN | 1.05 | 107.57 | 40.58 | 149.20 | -63.58 | 10.17 | 3.84 |
Table 2 Second-order stabilization energies(Eij, kJ/mol) of the 17 hydrogen-bonded complexes obtained at the B3LYP/6-311G(3df,2p) level and the binding energies(Eb, kJ/mol) obtained at the CP-corrected MP2/6-311++G(3df,2p) level
| R | Eij | Eb | ΔEij | ΔEb | |||
|---|---|---|---|---|---|---|---|
| NHCH3 | 1.00 | 92.84 | 41.67 | 135.52 | -58.55 | -3.51 | -1.19 |
| NH2 | 1.05 | 94.35 | 41.25 | 136.65 | -58.97 | -2.38 | -0.77 |
| Et | 0.75 | 91.63 | 44.56 | 136.94 | -59.30 | -2.09 | -0.43 |
| CH3 | 0.75 | 92.34 | 44.69 | 137.78 | -59.39 | -1.26 | -0.35 |
| OCH3 | 1.09 | 94.47 | 42.09 | 137.65 | -59.53 | -1.38 | -0.21 |
| H | 0.71 | 93.30 | 45.02 | 139.03 | -59.74 | 0 | 0 |
| COMe | 1.13 | 97.78 | 40.12 | 139.03 | -60.25 | 0 | 0.51 |
| BH2 | 0.75 | 95.86 | 43.35 | 139.95 | -60.84 | 0.92 | 1.10 |
| Cl | 1.13 | 100.67 | 40.58 | 142.38 | -61.43 | 3.35 | 1.69 |
| CHO | 0.79 | 101.13 | 40.25 | 142.17 | -61.49 | 3.14 | 1.75 |
| Br | 1.17 | 101.50 | 40.21 | 142.88 | -61.49 | 3.85 | 1.75 |
| CCl3 | 1.21 | 104.31 | 38.49 | 144.01 | -62.22 | 4.98 | 2.48 |
| COOH | 1.30 | 110.12 | 30.71 | 142.13 | -62.23 | 3.10 | 2.49 |
| CF3 | 1.09 | 102.63 | 39.46 | 143.18 | -62.39 | 4.14 | 2.65 |
| SO3H | 1.46 | 118.62 | 29.37 | 149.45 | -63.17 | 10.42 | 3.43 |
| NO2 | 1.30 | 107.49 | 37.61 | 146.40 | -63.52 | 7.36 | 3.78 |
| CN | 1.05 | 107.57 | 40.58 | 149.20 | -63.58 | 10.17 | 3.84 |
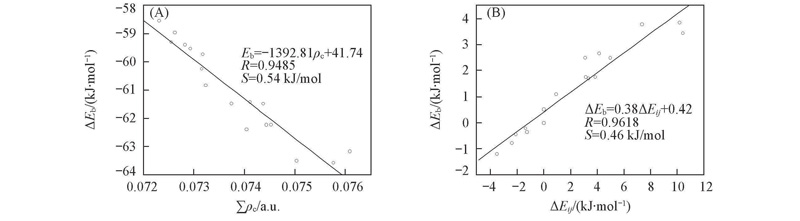
Fig.2 Correlation between the electron densities at the hydrogen bond critical points(∑ρc) and the binding energies(A) and correlation between the second-order stabilization energies ΔEij and the binding energies relative to R=H(B)
| [1] | Turner T.C., McLennan A. G., Bates A. D., White M. R. H.; Translated by Liu J. Y., Li W. J., Wang X. L., Instant Notes in Molecular Biology, 2nd Ed., Science Press, Beijing, 2001, 287—298 |
| (刘进元, 李文君, 王薛林[译]. 分子生物学, 第2版, 北京, 科学出版社, 2001, 287—298) | |
| [2] | Watson J.D., Beck T. A., Bale S. P., Gane A., Levin M., Laszk R. M., Translated by Feng X. L., Gou X. S., Hu J. F., Hu S. H., Hu S. N., Li J. X., Li L., Liu B., Liu G. Z., Liu M. X., Liu R., Lou X. M., Shi L., Song G., Song Q. F., Song S. H., Su Y. Y., Wang H., Wang C. P., Wang D. M., Wang X., Wu D. Y., Wu L., Yan C. X., Yang M. H., Zhang M., Zhang Q. R., Zhang X., Zhao H., Zhu J. G., Molecular Biology of the Gene, 6th Ed., Science Press, Beijing, 2009, 198—203 |
| (冯小黎, 郭需石, 胡建飞, 胡少晖, 胡松年, 李京湘, 李雷, 刘斌, 刘国振, 刘明旭, 刘韧, 娄晓敏, 时亮, 宋光, 宋其峰, 宋述慧, 苏夜阳, 汪浩, 王彩平, 王敦梅, 王霞, 吴东颖, 吴琳, 闫春霞, 杨焕明, 张明, 张清润, 张欣, 赵辉, 朱金桂[译]. 基因的分子生物学, 第6版, 北京, 科学出版社, 2009, 198—203) | |
| [3] | You Q.D., Medicinal Chemistry, 2th Ed., Chemical Industry Press, Beijing, 2008, 368—374 |
| (尤启冬.药物化学, 第2版, 北京, 化学工业出版社, 2008, 368—374) | |
| [4] | Mohajeri A., Nobandegani F. F., J. Phys. Chem. A, 2008, 112, 281—295 |
| [5] | Kawahara S., Kobori A., Sekine M., Taira K., Uchimaru T., J. Phys. Chem. A, 2001, 105, 10596—10601 |
| [6] | Kawahara S., Uchimaru T., Taira K., Sekine M., J. Phys. Chem. A, 2001, 105, 3894—3898 |
| [7] | Kawahara S., Uchimaru T., Tairi K., Sekine M., J. Phys. Chem. A, 2002, 106, 3207—3212 |
| [8] | Dong H., Hua W. J., Li S. H., J. Phys. Chem. A, 2007, 111, 2941—2945 |
| [9] | Hobza P., Sponer J., Cubero E., Orozco M., Luque F. J., J. Phys. Chem. B, 2000, 104, 6286—6292 |
| [10] | Asensio A., Kobko N., Dannenberg J. J., J. Phys. Chem. A, 2003, 107, 6441—6443 |
| [11] | Garza J., Ramirez J., Vargas R., J. Phys. Chem. A, 2005, 109, 643—651 |
| [12] | Li Y., Wang C. S., Sci. China Chem., 2011, 54, 1759—1769 |
| [13] | Gadre S. R., Pundlik S. S., J. Phys. Chem. B, 1997, 101, 3298—3303 |
| [14] | Liu D. J., Wang C. S., Acta Phys. Chim. Sin., 2012, 28(12), 2809—2816 |
| (刘冬佳, 王长生.物理化学学报, 2012,28(12), 2809—2816) | |
| [15] | Greve C., Preketes N. K., Fidder H., Costard R., Koeppe B., Heisler I. A., Mukamel S., Temps F., Nibbering E. T. J., Elsaes-ser T., J. Phys. Chem. A, 2013, 117, 594—606 |
| [16] | Samanta P. K., Manna A. K., Pati S. K., J. Phys. Chem. B, 2012, 116, 7618—7626 |
| [17] | Hua S. G., Xu L. N., Li W., Li S. H., J. Phys. Chem. B, 2011, 115, 11462—11469 |
| [18] | Shen H. J., Sun H., Li G. H., PLoS Comput. Biol., 2012, 8(12), e1002851-1—e1002851-18 |
| [19] | Wang J. N., Zhu W. L., Li G. H., Hansmann U. H. E., J. Chem. Phys., 2011, 135, 084115-1—084115-5 |
| [20] | Wu J., Zhen X., Shen H. J., Li G. H., Ren P. Y., J. Chem. Phys., 2011, 135, 155104-1—155104-8 |
| [21] | Li Y., Wang C. S., J. Comput. Chem., 2011, 32(13), 2765—2773 |
| [22] | Li Y., Jiang X. N., Wang C. S., J. Comput. Chem., 2011, 32(5), 953—966 |
| [23] | Xu B. S., Shen H. J., Zhu X., Li G. H., J. Comput. Chem., 2011, 32, 3188—3193 |
| [24] | Zhang Y. X., Shen H. J., Zhang M. B., Li G. H., J. Phys. Chem. B, 2013, 117, 982—988 |
| [25] | Xu B. S., Schones D. E., Wang Y. M., Liang H. J., Li G. H., PLoS ONE, 2013, 8(1), e52460-1—e52460-10 |
| [26] | Huang C. Y., Li Y., Wang C. S., Sci. China Chem., 2013, 56(2), 238—248 |
| [27] | Lee C., Yang W., Parr R. G., Phys. Rev. B, 1988, 37, 785—789 |
| [28] | Miehlich B., Savin A., Stoll H., Preuss H., Chem. Phys. Lett., 1989, 157, 200—206 |
| [29] | Becke A. D., J. Chem. Phys., 1993, 98, 5648—5652 |
| [30] | Moller C., Plesset M. S., Phys. Rev., 1934, 46, 618—622 |
| [31] | Foster J. P., Weinhold F., J. Am. Chem. Soc., 1980, 102, 7211—7218 |
| [32] | Reed A. E., Weinhold F., J. Chem. Phys., 1983, 78, 4066—4073 |
| [33] | Reed A. E., Weinhold F., J. Chem. Phys., 1985, 83, 1736—1740 |
| [34] | Reed A. E., Weinhold F., Curtiss A., Pochatko D. J., J. Chem. Phys., 1986, 84, 5687—5705 |
| [35] | Reed A. E., Curtiss A., Weinhold F., Chem. Rev., 1988, 88, 899—926 |
| [36] | Biegler K. F., Schonbohm J., Bayles D., J. Comput. Chem., 2001, 22, 545—559 |
| [37] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Montgomery J. A. Jr., Vreven T., Kudin K. N., Burant J. C., Millam J. M., Iyengar S. S., Tomasi J., Barone V., Mennucci B., Cossi M., Scalmani G., Rega N., Petersson G. A., Nakatsuji H., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Klene M., Li X., Knox J. E., Hratchian H. P., Cross J. B., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Ayala P. Y., Morokuma K., Voth G. A., Salvador P., Dannenberg J. J., Zakrzewski V. G., Dapprich S., Daniels A. D., Strain M. C., Farkas O., Malick D. K., Rabuck A. D., Raghavachari K., Foresman J. B., Ortiz J. V., Cui Q., Baboul A. G., Clifford S., Cioslowski J., Stefanov B. B., Liu G., Liashenko A., Piskorz P., Komaromi I., Martin R. L., Fox D. J., Keith T., Al-Laham M. A., Peng C. Y., Nanayakkara A., Challacombe M., Gill P. M. W., Johnson, B., Chen W., Wong M. W., Gonzalez C., Pople J. A.Gaussian 03, Revision B.02, Gaussian Inc., Pittsburgh,PA, 2003 |
| [38] | Angelina E. L., Peruchena N. M., J. Phys. Chem. A, 2011, 115, 4701—4710 |
| [39] | Yang Y., J. Phys. Chem. A, 2011, 115, 9043—9054 |
| [1] | MIN Jing, WANG Liyan. 1H NMR Study on the Conformation of Aromatic Amides Limited by Three-center Hydrogen Bonds [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220084. |
| [2] | ZHANG Yong, XU Jun, BAO Yu, CUI Shuxun. Quantifying the Degree of Weakening Effect of Nonpolar Organic Solvent on the Strength of Intramolecular Hydrogen Bonding by Single-molecule Force Spectroscopy [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210863. |
| [3] | CUI Shaoli, ZHANG Weijia, SHAO Xueguang, CAI Wensheng. Revealing the Effect of Threonine on the Binding Ability of Antifreeze Proteins with Ice Crystals by Free-energy Calculations [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210838. |
| [4] | HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614. |
| [5] | GAO Huiling, CAO Zhenzhen, GU Fang, WANG Haijun. Monte Carlo Simulation on Self-healing Behaviour of Hydrogen-bonded Hydrogel [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220482. |
| [6] | PENG Qin, FANG Yeguang, ZHANG Tengshuo, CUI Ganglong, FANG Weihai. Theoretical Study on the Excited State Properties and Photophysical Mechanism of Selenothymine and Adenine Base Pairs in DNA Environment [J]. Chem. J. Chinese Universities, 2021, 42(7): 2136. |
| [7] | WANG Le, QIN Liulei, LIU Yang, REN Li, XU Huiting, LIU Zunqi. Synthesis, Structure and Dielectric Properties of One-dimensional Chain Hydrogen Glycine Supramolecular Compound [(Gly)2+(18-crown-6)2(MnCl4)2‒] [J]. Chem. J. Chinese Universities, 2021, 42(3): 691. |
| [8] | NI Qingsheng, DU Miao, SHAN Guorong, SONG Yihu, WU Ziliang, ZHENG Qiang. Regulation of Rheological Behavior of Polyvinyl Alcohol Aqueous Solution by One-dimensional Particles [J]. Chem. J. Chinese Universities, 2021, 42(12): 3738. |
| [9] | GONG Shanshan, WU Tong, WANG Guange, HUANG Qing, SU Yuefeng, WU Feng. Screening of Deep Eutectic Solvent Based on Efficient Recovery of Spent Lithium⁃ion Battery Cathode Materials [J]. Chem. J. Chinese Universities, 2021, 42(10): 3151. |
| [10] | BAI Lan, ZHAI Lei, WANG Changou, HE Minhui, MO Song, FAN Lin. Thermal Expansion Behavior of Amide-containing Polyimide Films with Ultralow Thermal Expansion Coefficient † [J]. Chem. J. Chinese Universities, 2020, 41(4): 795. |
| [11] | WANG Mengyu, CAO Simin, LI Haoyang, ZHANG Mengjie, LI Dong, ZHAO Zenan, XU Jianhua. Fluorescence Resonance Energy Transfer Between Coenzyme NADH and Tryptophan [J]. Chem. J. Chinese Universities, 2020, 41(11): 2473. |
| [12] | QIN Liulei,LIU Yang,GUAN Xiaoqin,ZHENG Xiaoyuan,ZHANG Ziyu,LIU Zunqi. Synthesis and Switchable Dielectric Properties of an Inorganic-organic Hybrid Complex [H2(DABCO)CuCl4]·H2O † [J]. Chem. J. Chinese Universities, 2020, 41(1): 70. |
| [13] | LI Qing, YI Pinggui, TAO Hongwen, LI Yangyang, ZHANG Zhiyu, PENG Wenyu, LI Yuru. Solvent and Substituent Effects on Spectral Characteristics and Excited-state Intramolecular Proton Transfer of 2-(2-Aminophenyl) Benzothiazole† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1425. |
| [14] | XU Yan,LIU Cui,HAN Chengjuan,PAN Mingyu,SUN Zhaoqi,HAN Bingyu,YANG Zhongzhi. Development of Polarization Force Field for Guanine and Amino Acid Residues Systems† [J]. Chem. J. Chinese Universities, 2019, 40(2): 288. |
| [15] | XU Yu,HUA Er. Hydrogen Bonding Study on Protic Ionic Liquids Composed of N-Alkyl Ethylenediaminum Cations with Trifluoroacetic Anion† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1954. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||