

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (7): 1425.doi: 10.7503/cjcu20190127
• Physical Chemistry • Previous Articles Next Articles
LI Qing, YI Pinggui*( ), TAO Hongwen, LI Yangyang, ZHANG Zhiyu, PENG Wenyu, LI Yuru
), TAO Hongwen, LI Yangyang, ZHANG Zhiyu, PENG Wenyu, LI Yuru
Received:2019-02-28
Online:2019-07-10
Published:2019-07-12
Contact:
YI Pinggui
E-mail:pgyi@hnust.edu.cn
Supported by:CLC Number:
TrendMD:
LI Qing, YI Pinggui, TAO Hongwen, LI Yangyang, ZHANG Zhiyu, PENG Wenyu, LI Yuru. Solvent and Substituent Effects on Spectral Characteristics and Excited-state Intramolecular Proton Transfer of 2-(2-Aminophenyl) Benzothiazole†[J]. Chem. J. Chinese Universities, 2019, 40(7): 1425.
| Compound | Solvent | 1H NMR(500 MHz), δ |
|---|---|---|
| 1(CH3) | DMSO-d6 | 8.75(d, —NH—, 1H), 8.10(d, 1H), 8.03(d, 1H),7.73(m, 1H), 7.53(t, 1H), 7.44(t, 1H), 7.39(t, 1H), 6.84(d, 1H), 6.72(t, 1H), 2.99(d, 3H) |
| 2(H) | DMSO-d6 | 8.09(t, 1H), 8.01(t, 1H), 7.64(t, 1H), 7.52(dd, 1H), 7.42(dd, 1H), 7.34(s, —NH2, 2H), 7.23(m, 1H), 6.89(t, 1H), 6.65(dd, 1H) |
| 3(COCH2Cl) | CDCl3 | 13.24(s, —NH—, 1H), 8.81(d, 1H), 8.05(d, 1H), 7.93(d, 1H), 7.88(d, 1H), 7.48(m, 2H), 7.44(t, 1H), 7.23(t, 1H), 4.31(s, 2H) |
| 4(COCH2Br) | CDCl3 | 13.06(s, —NH—, 1H), 8.75(d, 1H), 8.12(d, 1H), 7.94(d, 1H), 7.89(dd, 1H), 7.53(m, 2H), 7.45(t, 1H), 7.24(m, 1H), 4.15(m, 2H) |
| 5(COCH3) | CDCl3 | 12.38(d, —NH—, 1H), 8.78(d, 1H), 8.00(t, 1H), 7.92(d, 1H), 7.85(d, 1H), 7.53(t, 1H), 7.48(m, 1H), 7.43(d, 1H), 7.16(t, 1H), 2.33(m, 3H) |
| 6(COC2H5) | CDCl3 | 12.41(s, —NH—, 1H), 8.82(d, 1H), 8.00(d, 1H), 7.91(d, 1H), 7.84(d, 1H), 7.52(t, 1H), 7.47(t, 1H), 7.43(t, 1H), 2.59(m, 2H), 1.38(m, 3H) |
Table 1 1H NMR data of compounds 1―6
| Compound | Solvent | 1H NMR(500 MHz), δ |
|---|---|---|
| 1(CH3) | DMSO-d6 | 8.75(d, —NH—, 1H), 8.10(d, 1H), 8.03(d, 1H),7.73(m, 1H), 7.53(t, 1H), 7.44(t, 1H), 7.39(t, 1H), 6.84(d, 1H), 6.72(t, 1H), 2.99(d, 3H) |
| 2(H) | DMSO-d6 | 8.09(t, 1H), 8.01(t, 1H), 7.64(t, 1H), 7.52(dd, 1H), 7.42(dd, 1H), 7.34(s, —NH2, 2H), 7.23(m, 1H), 6.89(t, 1H), 6.65(dd, 1H) |
| 3(COCH2Cl) | CDCl3 | 13.24(s, —NH—, 1H), 8.81(d, 1H), 8.05(d, 1H), 7.93(d, 1H), 7.88(d, 1H), 7.48(m, 2H), 7.44(t, 1H), 7.23(t, 1H), 4.31(s, 2H) |
| 4(COCH2Br) | CDCl3 | 13.06(s, —NH—, 1H), 8.75(d, 1H), 8.12(d, 1H), 7.94(d, 1H), 7.89(dd, 1H), 7.53(m, 2H), 7.45(t, 1H), 7.24(m, 1H), 4.15(m, 2H) |
| 5(COCH3) | CDCl3 | 12.38(d, —NH—, 1H), 8.78(d, 1H), 8.00(t, 1H), 7.92(d, 1H), 7.85(d, 1H), 7.53(t, 1H), 7.48(m, 1H), 7.43(d, 1H), 7.16(t, 1H), 2.33(m, 3H) |
| 6(COC2H5) | CDCl3 | 12.41(s, —NH—, 1H), 8.82(d, 1H), 8.00(d, 1H), 7.91(d, 1H), 7.84(d, 1H), 7.52(t, 1H), 7.47(t, 1H), 7.43(t, 1H), 2.59(m, 2H), 1.38(m, 3H) |
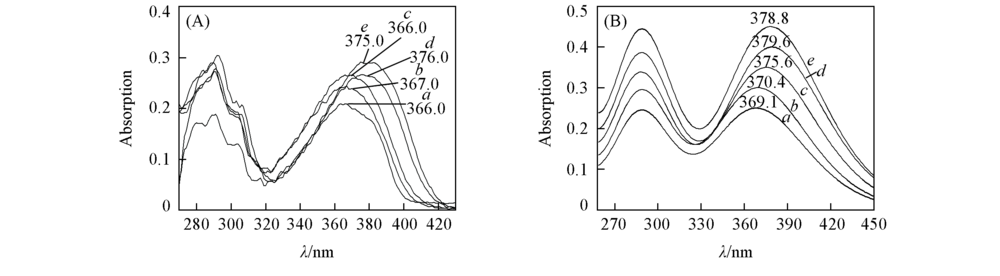
Fig.1 Experimental(A) and calculated(B) absorption spectra of APBT in different solventsa. CYH; b. DCM; c. ACN; d. DMF; e. DMSO. Calculated at TD B3LYP/TZVP theoretical level.
| Solvent | EK-E/(kJ·mol-1) | Ea/(kJ·mol-1) | EH-bond/(kJ·mol-1) | EHOMO/eV | ELUMO/eV | ΔEL-H/eV | |
|---|---|---|---|---|---|---|---|
| CYH | 85.8 | 80.6 | -6.7 | — | -5.668 | -1.769 | 3.899 |
| DCM | 84.5 | 80.6 | -15.7 | -2.4 | -5.726 | -1.839 | 3.887 |
| ACN | 84.2 | 80.6 | -18.5 | -8.7 | -5.621 | -1.841 | 3.780 |
| DMF | 84.2 | 80.6 | -18.6 | -14.8 | -5.580 | -1.824 | 3.756 |
| DMSO | 84.1 | 80.6 | -18.8 | -17.5 | -5.584 | -1.803 | 3.781 |
Table 2 Relative energies(EK-E) of keto form, active energies(Ea), solvation energies(Esolv), hydrogen bond energies(EH-bond), frontier molecular orbital energy levels(EHOMO, ELUMO) and their gaps(ΔEL-H) of ground state APBT in different solvents*
| Solvent | EK-E/(kJ·mol-1) | Ea/(kJ·mol-1) | EH-bond/(kJ·mol-1) | EHOMO/eV | ELUMO/eV | ΔEL-H/eV | |
|---|---|---|---|---|---|---|---|
| CYH | 85.8 | 80.6 | -6.7 | — | -5.668 | -1.769 | 3.899 |
| DCM | 84.5 | 80.6 | -15.7 | -2.4 | -5.726 | -1.839 | 3.887 |
| ACN | 84.2 | 80.6 | -18.5 | -8.7 | -5.621 | -1.841 | 3.780 |
| DMF | 84.2 | 80.6 | -18.6 | -14.8 | -5.580 | -1.824 | 3.756 |
| DMSO | 84.1 | 80.6 | -18.8 | -17.5 | -5.584 | -1.803 | 3.781 |
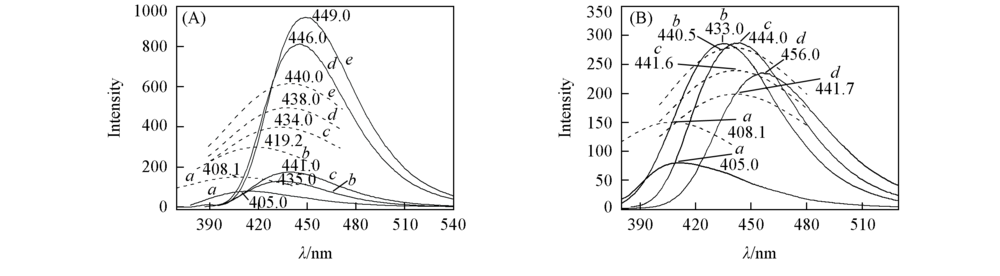
Fig.2 Experimental and calculated fluorescence spectra of APBT in different solvents(A) Aprotic solvent: a. CYH; b. Diox; c. ACN; d. DMF; e. DMSO. (B) protic solvent: a. CYH; b. EtOH; c. MeOH; d. H2O. The solid line represents experimental spectra; the dotted line represents calculated spectra at TD B3LYP/TZVP theoretical level.
| Solvent | Δ | AN1—C2—C3—C4/(°) | f | ||
|---|---|---|---|---|---|
| Enol* | Keto* | Enol* | Keto* | ||
| CYH | 25.6 | 0 | 0 | 0.399 | 0.292 |
| Diox | 39.2 | 0.1 | 0.4 | 0.398 | 0.313 |
| ACN | 36.8 | 0 | 0.1 | 0.601 | 0.429 |
| DMF | 41.2 | -2.6 | -13.7 | 0.588 | 0.398 |
| DMSO | 42.4 | -3.0 | 12.9 | 0.578 | 0.400 |
| EtOH | -14.8 | -21.0 | 98.1 | 0.496 | 0.001 |
| MeOH | -14.3 | -22.7 | 97.8 | 0.490 | 0.001 |
| H2O | -11.8 | -21.9 | -97.7 | 0.510 | 0.002 |
Table 3 Relative energies(ΔEK*-E*), dihedral angles(A) and oscillator strengths(f) of APBT in different solvents*
| Solvent | Δ | AN1—C2—C3—C4/(°) | f | ||
|---|---|---|---|---|---|
| Enol* | Keto* | Enol* | Keto* | ||
| CYH | 25.6 | 0 | 0 | 0.399 | 0.292 |
| Diox | 39.2 | 0.1 | 0.4 | 0.398 | 0.313 |
| ACN | 36.8 | 0 | 0.1 | 0.601 | 0.429 |
| DMF | 41.2 | -2.6 | -13.7 | 0.588 | 0.398 |
| DMSO | 42.4 | -3.0 | 12.9 | 0.578 | 0.400 |
| EtOH | -14.8 | -21.0 | 98.1 | 0.496 | 0.001 |
| MeOH | -14.3 | -22.7 | 97.8 | 0.490 | 0.001 |
| H2O | -11.8 | -21.9 | -97.7 | 0.510 | 0.002 |
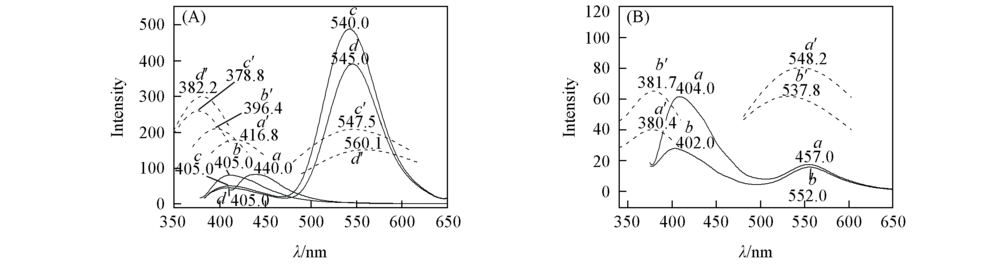
Fig.3 Experimental(a—d) and calculated(a’—d’) fluorescence spectra of APBT and its derivatives(A) a. 1(CH3); b. 2(H); c. 3(COCH2Cl); d. 4(COCH2Br). (B) a. 5(COCH3); b. 6(COC2H5). The solid line represents experimental spectra, dotted line represents calculated spectra at TD PBE1PBE/TZVP theoretical level in cyclohexane.
| Compound | Tautomer | Δ | RN5—H6/nm | RN1—H6/nm | ∠N1—H6—N5/(°) | QN5/e | QH6/e |
|---|---|---|---|---|---|---|---|
| 1(CH3) | Enol* | 28.9 | 0.104 | 0.175 | 143.4 | -0.616 | 0.437 |
| Keto* | 0.164 | 0.108 | 139.5 | -0.672 | 0.468 | ||
| 2(H) | Enol* | 24.0 | 0.104 | 0.179 | 138.7 | -0.764 | 0.436 |
| Keto* | 0.168 | 0.106 | 137.3 | -0.309 | 0.202 | ||
| 3(COCH2Cl) | Enol* | -11.2 | 0.105 | 0.171 | 144.8 | -0.600 | 0.453 |
| Keto* | 0.198 | 0.103 | 124.3 | -0.673 | 0.494 | ||
| 4(COCH2Br) | Enol* | -8.0 | 0.105 | 0.170 | 145.5 | -0.633 | 0.442 |
| Keto* | 0.201 | 0.102 | 123.0 | -0.732 | 0.477 | ||
| 5(COCH3) | Enol* | 7.0 | 0.105 | 0.171 | 146.0 | -0.606 | 0.445 |
| Keto* | 0.183 | 0.104 | 132.3 | -0.691 | 0.482 | ||
| 6(COC2H5) | Enol* | 10.5 | 0.105 | 0.171 | 146.0 | -0.604 | 0.445 |
| Keto* | 0.181 | 0.104 | 133.6 | -0.689 | 0.480 |
Table 4 Relative energies(EK*-E*) between keto and enol, selected bond lengths(R), bond angles and NBO charges(Q) of excited state APBT and its derivatives*
| Compound | Tautomer | Δ | RN5—H6/nm | RN1—H6/nm | ∠N1—H6—N5/(°) | QN5/e | QH6/e |
|---|---|---|---|---|---|---|---|
| 1(CH3) | Enol* | 28.9 | 0.104 | 0.175 | 143.4 | -0.616 | 0.437 |
| Keto* | 0.164 | 0.108 | 139.5 | -0.672 | 0.468 | ||
| 2(H) | Enol* | 24.0 | 0.104 | 0.179 | 138.7 | -0.764 | 0.436 |
| Keto* | 0.168 | 0.106 | 137.3 | -0.309 | 0.202 | ||
| 3(COCH2Cl) | Enol* | -11.2 | 0.105 | 0.171 | 144.8 | -0.600 | 0.453 |
| Keto* | 0.198 | 0.103 | 124.3 | -0.673 | 0.494 | ||
| 4(COCH2Br) | Enol* | -8.0 | 0.105 | 0.170 | 145.5 | -0.633 | 0.442 |
| Keto* | 0.201 | 0.102 | 123.0 | -0.732 | 0.477 | ||
| 5(COCH3) | Enol* | 7.0 | 0.105 | 0.171 | 146.0 | -0.606 | 0.445 |
| Keto* | 0.183 | 0.104 | 132.3 | -0.691 | 0.482 | ||
| 6(COC2H5) | Enol* | 10.5 | 0.105 | 0.171 | 146.0 | -0.604 | 0.445 |
| Keto* | 0.181 | 0.104 | 133.6 | -0.689 | 0.480 |
| [1] | Formosinho S. J., Arnaut L. G., J. Photoch. Photobio. A, 1993, 75(1), 21—48 |
| [2] | Kungwan N., Plasser F., Aquino A.J., Phys. Chem. Chem. Phys., 2012, 14(25), 9016—9025 |
| [3] | Fujisawa T., Kuramochi H., Hosoi H., J. Am. Chem. Soc., 2016, 138(12), 3942—3945 |
| [4] | Kang B., Ko K. C., Park S. Y., Phys. Chem. Chem. Phys., 2011, 13(13), 6332—6339 |
| [5] | Luque A.M., Mulder W. H., Calvente J. J., Anal. Chem., 2012, 84(13), 5778—5786 |
| [6] | Bacchi A., Carcelli M., Compari C., Fisicaro E., Pala N., Rispoli G., Rogolino D., Sanchez T.W., Sechi M., Sinisi V., Neamati N., J. Med. Chem., 2011, 54(24), 8407—8420 |
| [7] | Yi P. G., Yang X. C., Yu X. Y., Chem. J. Chinese Universities, 2012, 33(12), 2657—2662 |
| (易平贵, 阳习春, 于贤勇. 高等学校化学学报, 2012, 33(12), 2657—2662) | |
| [8] | Mancini D. T., Sen K.,Barbatti M., Phys. Chem. Chem. Phys., 2016, 16(16), 3444—3449 |
| [9] | Balamurali M. M., Dogra S. K., Chem. Phys., 2004, 305, 95—103 |
| [10] | Rini M., Dreyer J., Nibbering E. T., J. Chem. Phys. Left., 2003, 374, 13—19 |
| [11] | Dr M.M. H., Wu Y., Dr C. J. F., Chem-Eur. J., 2004, 10(12), 3015—3025 |
| [12] | Chou P. T., Martinez M. L.,Clements J. H., Chem. Phys. Lett., 1993, 204(5/6), 395—399 |
| [13] | Lim S.J., Seo J., Park S. Y., J. Am. Chem. Soc., 2006, 128(45), 14542—14547 |
| [14] | Feng Y., Bai L., Wang S., Chem. Res. Chinese Universities, 2017, 33(4), 534—539 |
| [15] | Jung H.S., Kim H. J., Vicens J., Tetrahedron Lett., 2009, 50(9), 983—987 |
| [16] | Shawkat M., Aly A. U., Maytham A., J. Phys. Chem. B, 2015, 119(6), 2596—603 |
| [17] | Green O., Gajst O., Simkovitch R., J. Phys. Chem. A, 2017, 121(16), 3079—3087 |
| [18] | Balamurali M. M., Dogra S. K., Chem. Phys., 2004, 305(1), 95—103 |
| [19] | Yi P.G., Peng H. L., Yu X. Y., Li X. F., Wang Z. X., Wang T., Zhou J. M., J. Chem., 2010, 68(9), 875—882 |
| (易平贵, 彭洪亮, 于贤勇, 李筱芳, 汪朝旭, 王涛. 化学学报, 2010, 68(9), 875—882) | |
| [20] | Chen C.L., Tseng H. W., Chen Y. A., Chou P. T., J. Phys. Chem. A, 2016, 120, 1020—1028 |
| [21] | Myung G.C., Min J. C., Hyein R., Jongin H., Chang S. K., Dyes and Pigments, 2017, 143, 123—128 |
| [22] | Hyein R., Myung G.C., Eun J. C., Chang S. K., Dyes and Pigments, 2018, 149, 620—625 |
| [23] | Fadda A.A., Refat H. M., Zaki M. E. A., J. Cheminformatics, 2001, 31(22), 3537—3545 |
| [24] | Mukhina O. A., Kutateladze A. G., J. Am. Chem. Soc., 2016, 47(33), 2110—2113 |
| [25] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Keith T., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision C.01, Gaussian Inc., Wallingford CT, 2011 |
| [26] | Cossi M., Barone V., Mennucci B., Tomasi J., Chem. Phys. Lett., 1998, 286(3/4), 253—260 |
| [27] | Mennucci B., Tomasi J., J. Chem. Phys., 1997, 106(12), 5151—5158 |
| [28] | Xiang J. F., Yi P. G., Ren Z. Y., Yu X. Y.,Chen J., Liu W., Li T. M., Acta Phys.-Chem. Sin., 2016, 32(3), 624—630 |
| (向俊峰, 易平贵, 任志勇, 于贤勇, 陈建, 刘武, 李桃梅. 物理化学学报, 2016, 32(3), 624—630) | |
| [29] | Yi P. G., Liu J., Chen J., Yu X. Y., Li X. F., Zheng B. S., Tao H. W., Hao Y. L., Chem. J. Chinese Universities, 2014, 35(6), 1219—1223 |
| (易平贵, 刘金, 陈建, 于贤勇, 李筱芳, 郑柏树, 陶洪文, 郝艳雷. 高等学校化学学报, 2014, 35(6), 1219—1223) | |
| [30] | Ouyang J. M., Lin W. H.,Guo Z. J., Chinese J. Inorg. Chem., 2000, 16(4), 573—579 |
| (欧阳健明, 林伟汉, 郭志坚. 无机化学学报, 2000, 16(4), 573—579) | |
| [31] | Wang J.F., Feng J. K., Ren A. M., Macromolecules, 2004, 37(9), 3451—3458 |
| [32] | Liu B. Q., Chen Y. T., Chen Y. W., Chung K. Y.,Tsai Y. H., Li Y. J., Chao C. M., Liu K. M., Tseng H. W., Chou P. T., Methods Ethods. Appl. Fluores., 2016, 4(1), 014004 |
| [33] | Yi P. G., Liu Z. J., Wang Z. X., Yu X. Y., Zhou J. M., Hou B., Int. J. Quantum Chem., 2013, 113(9), 1316—1324 |
| [34] | Li X. Y., Chen Y. M., Cui N., Zhang W. Y., Wang Z. M., Chem. J. Chinese Universities, 2017, 38(3), 448—455 |
| (李学颖, 陈延明, 崔娜, 张万宇, 王志明. 高等学校化学学报, 2017, 38(3), 448—455) | |
| [35] | Rodembusch F.S., Leusin F. P., Campo L. F., Stefani V., J. Lumin., 2007, 126(2), 728—734 |
| [1] | ZHAO Dongxia, ZHANG Haixia, FENG Wenjuan, YANG Zhongzhi. Molecular Face Guiding the Proton Transfer Reactions of Hydroxyl Carbene and Its Derivatives [J]. Chem. J. Chinese Universities, 2021, 42(7): 2187. |
| [2] | ZENG Yonghui, YAN Tianying. Vibrational Density of States Analysis of Proton Hydration Structure [J]. Chem. J. Chinese Universities, 2021, 42(6): 1855. |
| [3] | TENG Yunyang, QU Zexing, ZHOU Zhongjun, HUANG Xuri. Theoretical Study on Photoinduced Stepwise Dearomatization of Benzenoid Arenes with Different States [J]. Chem. J. Chinese Universities, 2021, 42(3): 752. |
| [4] | WANG Ruxin, ZHAO Zhongjun, HE Feiyao, YUE Hanlu, DENG Fulong, LI Hong, LI Wenwen, DUAN Yixiang. Characteristic Analysis of C1—C3n-Aldehydes and n-Alcohols in Proton Transfer Reaction Time-of-flight Mass Spectrometry [J]. Chem. J. Chinese Universities, 2021, 42(12): 3632. |
| [5] | WEI Xin, DENG Yaoliang, ZHENG Xuming, ZHAO Yanying. Ground Structure and Excited State Proton Transfer Reaction of 2-Aminobenzothiazole [J]. Chem. J. Chinese Universities, 2019, 40(8): 1679. |
| [6] | LI Xun,XUE Yurui,SONG Yu,ZHANG Wenke. Coordinate Interaction Between Monosulfide and Au Surfaces† [J]. Chem. J. Chinese Universities, 2018, 39(12): 2774. |
| [7] | SUN Xiaoli, MENG Lingmin, JIN Mingxing, LI Jilai. Mechanism Research of Reactions of Metal Carbides and Carbenes Cation[M-X] +(M=Au, Ag, Cu; X=C, CH2) with Methane [J]. Chem. J. Chinese Universities, 2017, 38(8): 1406. |
| [8] | WANG Yunming, CHANG Peiyang, GU Fang, WANG Haijun. Intramolecular Reaction in Self-condensing Vinyl Polymerization System with Solvent Effect† [J]. Chem. J. Chinese Universities, 2017, 38(4): 660. |
| [9] | LI Xueying, CHEN Yanming, CUI Na, ZHANG Wanyu, WANG Zhiming. Excited State Intramolecular Proton Transfer of Salicylaldehyde Derivatives and Its Application in Fluorescent Probe Field† [J]. Chem. J. Chinese Universities, 2017, 38(3): 448. |
| [10] | GAO Lijuan, WANG Li, WANG Shengyan, JING Shubo. Influence of Solvent on Structure of Ni(Ⅱ) Metal-organic Frameworks† [J]. Chem. J. Chinese Universities, 2016, 37(9): 1589. |
| [11] | WANG Xiao, YANG Xinguo, SHEN Qili. Synthesis and Gel Properties of a Novel Amide Gelator with Melamine Moieties and Rationalizing Gelation Behavior by Hansen Solubility Parameters† [J]. Chem. J. Chinese Universities, 2016, 37(11): 2068. |
| [12] | CHANG Peiyang, WANG Yunming, GU Fang, WANG Haijun. Cyclization in Hyperbranched Polymerization System of ABg Type† [J]. Chem. J. Chinese Universities, 2015, 36(8): 1627. |
| [13] | XIANG Junfeng, YI Pinggui, YU Xianyong, CHEN Jian, HAO Yanlei, REN Zhiyong. Excited-state Proton Transfer of 2-(2-Hydroxyphenyl)benzothiazole in the Confined Nanocavity† [J]. Chem. J. Chinese Universities, 2015, 36(4): 654. |
| [14] | SHEN Chengyin, WANG Hongmei, HUANG Chaoqun, LU Yan, XIA Lei, CHEN Xiaojing, WANG Hongzhi, CHU Yannan. On-line Detection of Volatile Sulfur Compounds in Breath Gas by Proton Transfer Reaction Mass Spectrometry† [J]. Chem. J. Chinese Universities, 2015, 36(2): 236. |
| [15] | SONG Yanli, LIU Yajun. Spin-orbit Coupling ab initio Investigation on the Photolysis Mechnism of CH2BrI† [J]. Chem. J. Chinese Universities, 2015, 36(11): 2163. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||