

高等学校化学学报 ›› 2024, Vol. 45 ›› Issue (3): 20230527.doi: 10.7503/cjcu20230527
• 综合评述 • 上一篇
赵晓光1, 王云龙1, 尹海波2, 曲亚坤1, 苏海伟2, 房韡1( )
)
收稿日期:2023-12-30
出版日期:2024-03-10
发布日期:2024-02-21
通讯作者:
房韡
E-mail:fangwei.ripp@sinopec.com
作者简介:第一联系人:共同第一作者.
基金资助:
ZHAO Xiaoguang1, WANG Yunlong1, YIN Haibo2, QU Yakun1, SU Haiwei2, FANG Wei1( )
)
Received:2023-12-30
Online:2024-03-10
Published:2024-02-21
Contact:
FANG Wei
E-mail:fangwei.ripp@sinopec.com
Supported by:摘要:
氨是化肥生产和化学工业的重要原料, 也是良好的无碳储能燃料. 相比于工业应用上能耗大、 转化率低的哈勃博施(Haber-Bosch)法, 电催化合成氨的方法能够在温和条件下绿色高效地合成氨. 本文综合评述了以氮气、 硝酸根和一氧化氮作为不同氮源时电催化合成氨的反应机理, 并结合不同氮源的特点分析了各自的研究进展与优势, 分别讨论了氮气难以溶解在水中被吸附和活化、 硝酸盐还原元素价态跨度大难以控制中间体和反应路径及一氧化氮体系复杂、 水溶液中析氢副反应难以控制等问题, 总结了运用不同策略开发高活性、 高稳定性催化剂以提高反应效率和选择性、 优化反应装置以减小传质影响、 选用不同电解质体系改善反应过程等解决思路. 最后, 对不同氮源电催化合成氨的未来发展趋势和应用前景进行了展望.
中图分类号:
TrendMD:
赵晓光, 王云龙, 尹海波, 曲亚坤, 苏海伟, 房韡. 不同氮源用于电催化合成氨的研究进展. 高等学校化学学报, 2024, 45(3): 20230527.
ZHAO Xiaoguang, WANG Yunlong, YIN Haibo, QU Yakun, SU Haiwei, FANG Wei. Research Progress of Electrocatalytic Ammonia Synthesis from Different Nitrogen Sources. Chem. J. Chinese Universities, 2024, 45(3): 20230527.
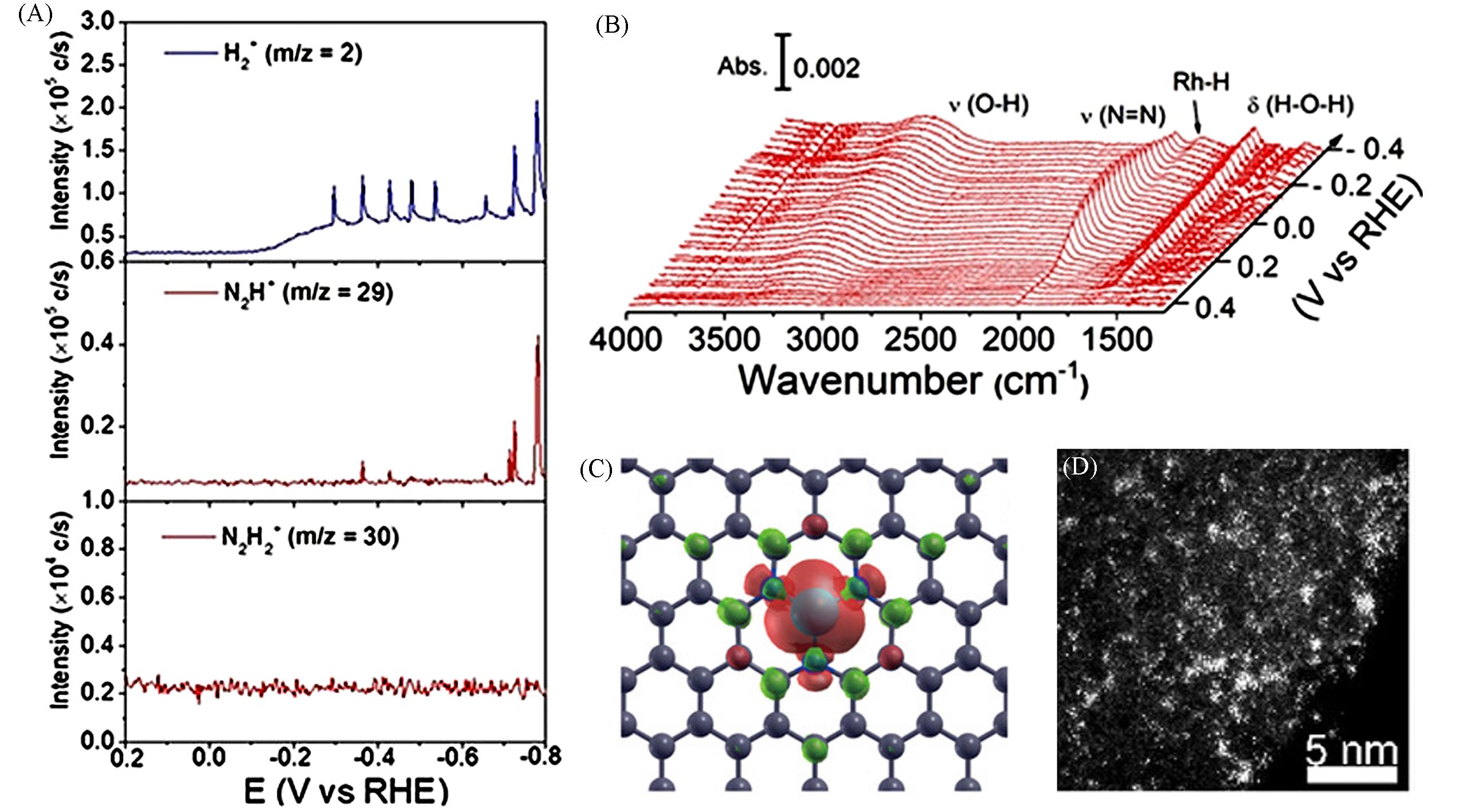
Fig.3 DEMS of intermediates on Rh/C during a scan in a N2⁃saturated 1 mol/L KOH solution(A) and FTIR spectra during the first segment from 0.4 V to -0.4 V on a Rh⁃film electrode in a N2⁃saturated 0.1 mol/L KOH solution(B)[43], optimized structure of FeN3⁃graphene(C)[45], high⁃angle annular dark⁃field scanning transmission electron microscopy(HAADF⁃STEM) image of Mo0/GDY sample(D)[50](B) The reference spectrum was taken at 0.4 V. (A, B) Copyright 2020, Wiley-VCH; (C) Copyright 2016, American Chemical Society; (D) Copyright 2019, American Chemical Society.
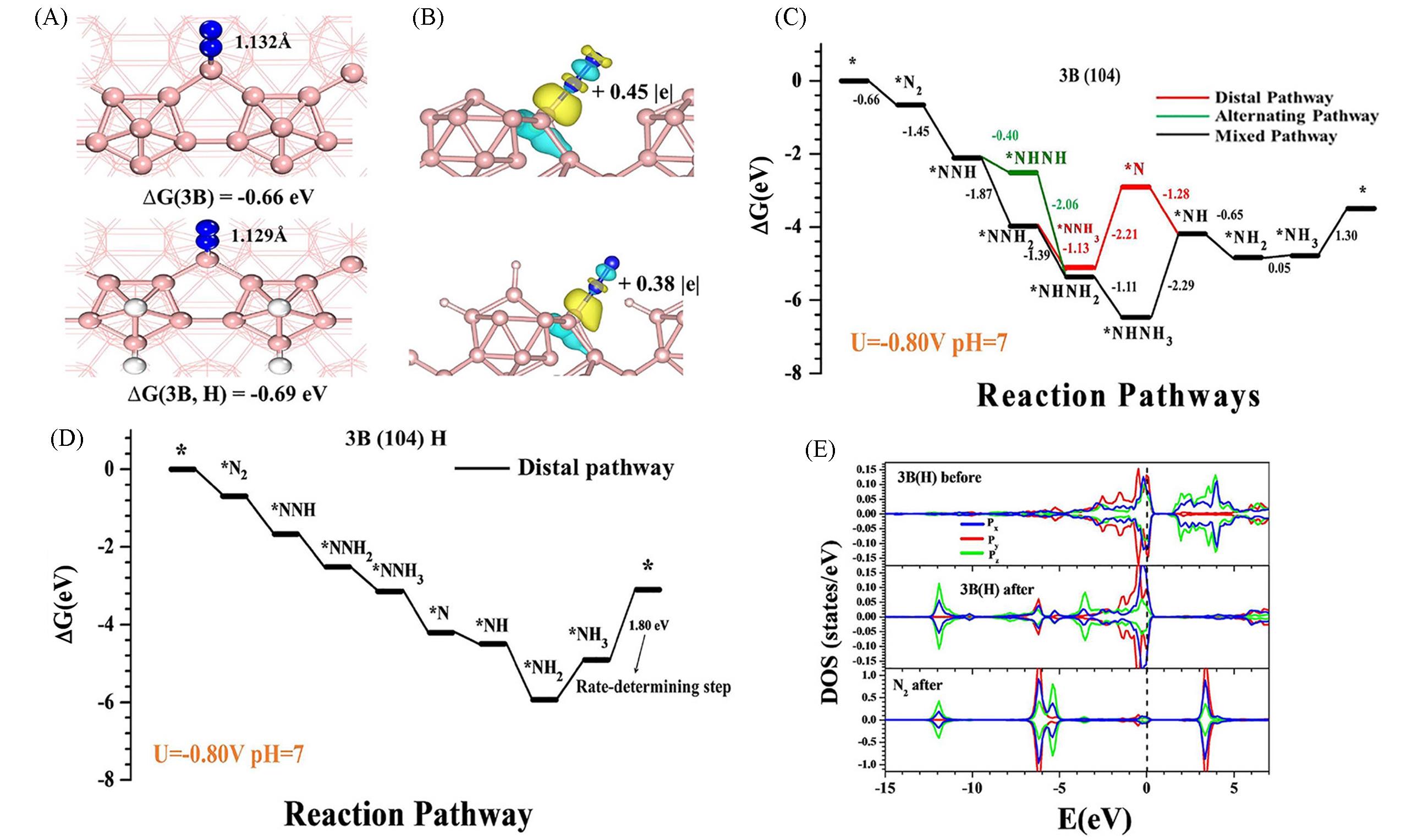
Fig.4 Optimal structure of N2 adsorption with end⁃on coordination on 3B and 3B(H) sites(A), the electron density difference after N2 adsorption on 3B and 3B(H) sites(B), the corresponding free⁃energy diagram of NRR on the B(104) surface at U=-0.80 V(C, D), the density of state(DOS) of 3B(H) site before and after N2 adsorption(E)[56]
| Species | Electrocatalyst | Electrolyte | Potential/V (vs. RHE) | NH3 yield rate | NH3 Faradic efficiency(%) | Ref. |
|---|---|---|---|---|---|---|
| Noble metal catalyst | Ru/C | 2 mol/L KOH | -0.24 | 0.21 μg·h‒1·cm‒2 | 0.28 | [ |
| Ru@ZrO2/NC | 0.1 mol/L HCl | -0.21 | 3.665 mg·h‒1·mg | 21 | [ | |
| THH Au NR | 0.1 mol/L KOH | -0.2 | 1.648 µg·h‒1·cm‒2 | 4.02 | [ | |
| Rh NNs | 0.1 mol/L KOH | -0.2 | 23.88 mg·h‒1·mg‒1 | 0.217 | [ | |
| Pd/C | 0.1 mol/L PBS | 0.1 | 4.5 μg·mg‒1·h‒1 | 8.2 | [ | |
| Non⁃noble metal catalyst | Fe/Fe3O4 | 0.1 mol/L PBS | -0.3 | 0.19 μg·h‒1·cm‒2 | 8.29 | [ |
| Fe⁃CeO2 | 0.5 mol/L LiClO4 | -0.5 | 26.2 μg·mg‒1·h‒1 | 14.7 | [ | |
| Mo⁃D⁃R⁃5h | 0.01 mol/L H2SO4 | -0.49 | 30.9 pmol·s‒1·cm‒2 | 0.71(-0.29 V) | [ | |
| N@MoS2 | 0.1 mol/L Na2SO4 | -0.3 | 69.82 μg·h‒1·mg‒1 | 9.14 | [ | |
| Mo0/GDY | 0.1 mol/L Na2SO4 | -0.96 | 145.4 μg·h‒1·mg‒1 | 21 | [ | |
| MoO2/C700 | 1 mol/L KOH | -0.7 | 173.7 μg·h‒1·mg‒1 | 27.6 | [ | |
| WO3⁃C3N4⁃R | 0.1 mol/L Li2SO4 | -0.3 | 43.5 μg·h‒1·mg‒1 | 11.2 | [ | |
| Non⁃metallic catalyst | NPC | 0.05 mol/L H2SO4 | -0.9 | 1.40 mmol·g‒1·h‒1 | 1.42 | [ |
| BG | 0.05 mol/L H2SO4 | -0.5 | 9.8 μg·h‒1·cm‒2 | 10.8 | [ | |
| BNS | 0.1 mol/L Na2SO4 | -0.8 | 13.22 μg·h‒1·mg‒1 | 4.4 | [ |
Table 1 Summary of performance of electrocatalytic NH3 synthesis by NRR
| Species | Electrocatalyst | Electrolyte | Potential/V (vs. RHE) | NH3 yield rate | NH3 Faradic efficiency(%) | Ref. |
|---|---|---|---|---|---|---|
| Noble metal catalyst | Ru/C | 2 mol/L KOH | -0.24 | 0.21 μg·h‒1·cm‒2 | 0.28 | [ |
| Ru@ZrO2/NC | 0.1 mol/L HCl | -0.21 | 3.665 mg·h‒1·mg | 21 | [ | |
| THH Au NR | 0.1 mol/L KOH | -0.2 | 1.648 µg·h‒1·cm‒2 | 4.02 | [ | |
| Rh NNs | 0.1 mol/L KOH | -0.2 | 23.88 mg·h‒1·mg‒1 | 0.217 | [ | |
| Pd/C | 0.1 mol/L PBS | 0.1 | 4.5 μg·mg‒1·h‒1 | 8.2 | [ | |
| Non⁃noble metal catalyst | Fe/Fe3O4 | 0.1 mol/L PBS | -0.3 | 0.19 μg·h‒1·cm‒2 | 8.29 | [ |
| Fe⁃CeO2 | 0.5 mol/L LiClO4 | -0.5 | 26.2 μg·mg‒1·h‒1 | 14.7 | [ | |
| Mo⁃D⁃R⁃5h | 0.01 mol/L H2SO4 | -0.49 | 30.9 pmol·s‒1·cm‒2 | 0.71(-0.29 V) | [ | |
| N@MoS2 | 0.1 mol/L Na2SO4 | -0.3 | 69.82 μg·h‒1·mg‒1 | 9.14 | [ | |
| Mo0/GDY | 0.1 mol/L Na2SO4 | -0.96 | 145.4 μg·h‒1·mg‒1 | 21 | [ | |
| MoO2/C700 | 1 mol/L KOH | -0.7 | 173.7 μg·h‒1·mg‒1 | 27.6 | [ | |
| WO3⁃C3N4⁃R | 0.1 mol/L Li2SO4 | -0.3 | 43.5 μg·h‒1·mg‒1 | 11.2 | [ | |
| Non⁃metallic catalyst | NPC | 0.05 mol/L H2SO4 | -0.9 | 1.40 mmol·g‒1·h‒1 | 1.42 | [ |
| BG | 0.05 mol/L H2SO4 | -0.5 | 9.8 μg·h‒1·cm‒2 | 10.8 | [ | |
| BNS | 0.1 mol/L Na2SO4 | -0.8 | 13.22 μg·h‒1·mg‒1 | 4.4 | [ |
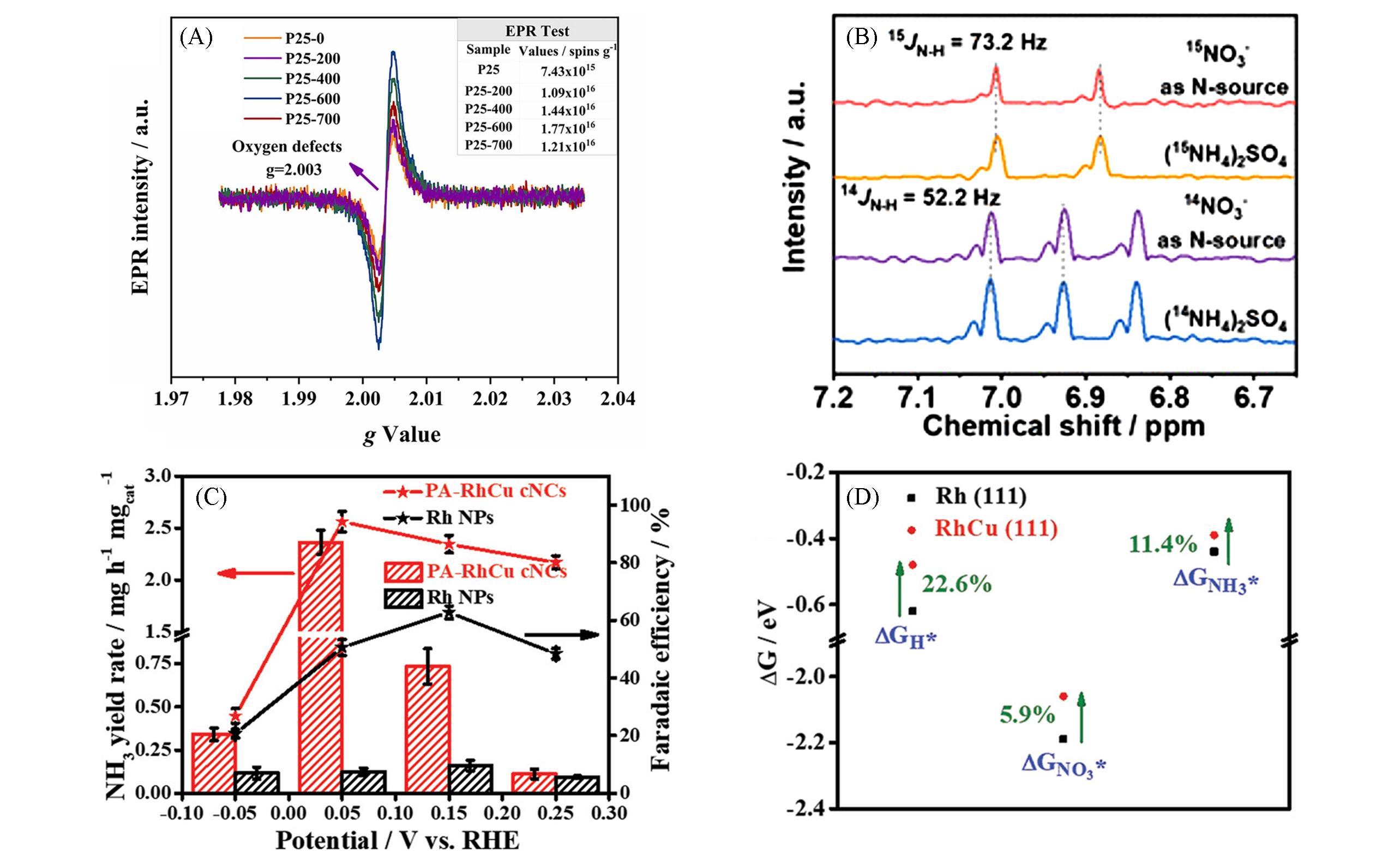
Fig.6 EPR spectra of P25⁃M(M=0, 200, 400 and 600)(A)[68], 1H NMR spectra of the electrolyte using 15NO3- and 14NO3- as the reactant(B)[70], NH3 yield and Faradaic efficiency of NH3 for NO3-⁃RR at PA⁃RhCu cNCs and Rh NPs at different potentials(C) and ΔGH*, ΔGNO3*, and ΔGNH3* on Rh(111) and RhCu(111) surfaces(D)[83](A) Inset table is referred to corresponding spin quantum numbers. Copyright 2022, Elsevier; (B) Copyright 2021, American Chemical Society; (C, D) Copyright 2022, Wiley-VCH.
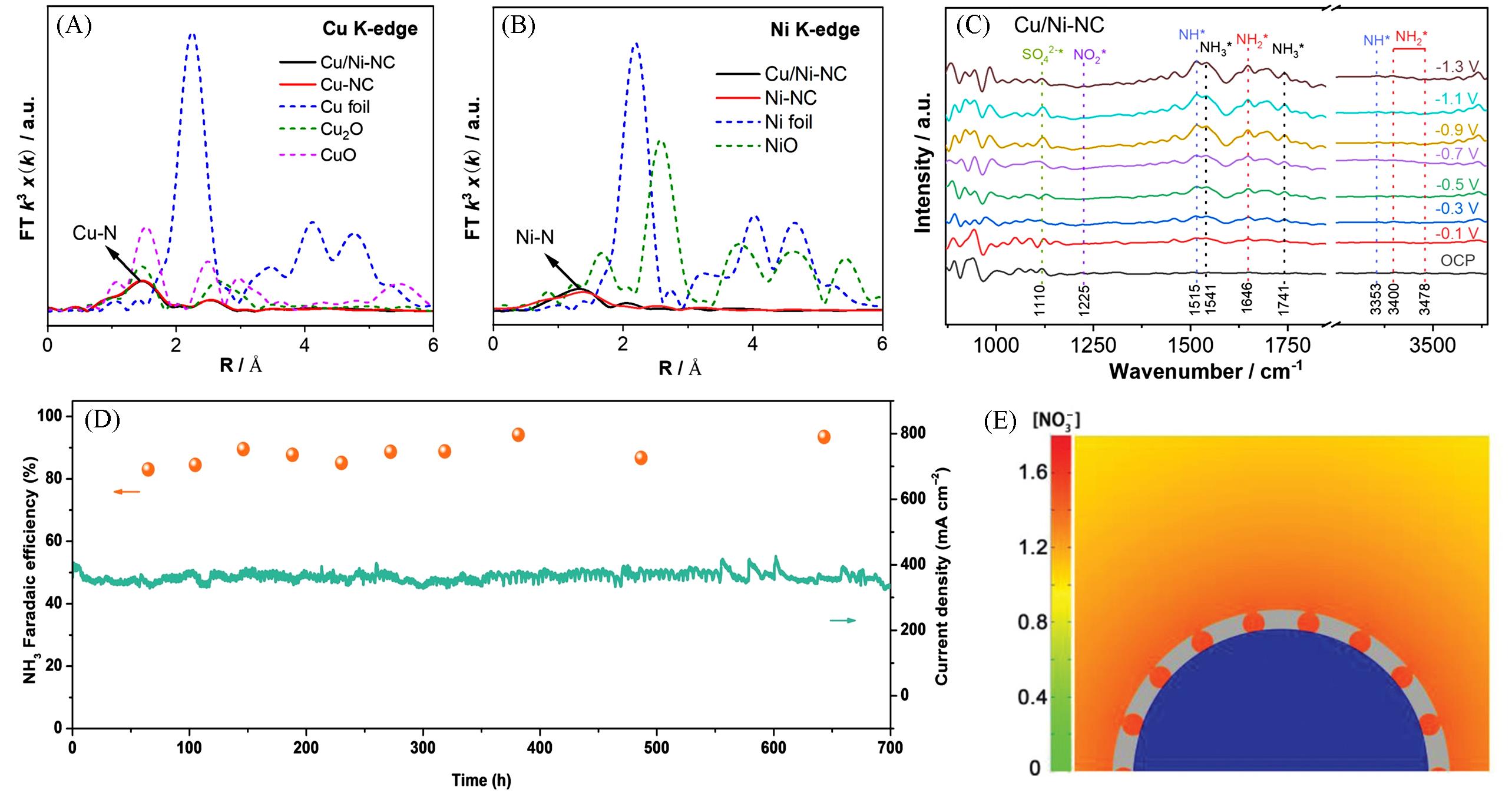
Fig.7 FT magnitude of the experimental EXAFS spectra of Cu/Ni⁃NC and references at Cu K⁃edge(A) and Ni K⁃edge(B), respectively, in situ ATR⁃SEIRAS spectra of Cu/Ni⁃NC from OCP to -1.3 V(vs. RHE) in electrolytes containing 0.1 mol/L Na2SO4 and 100 mg/L NO3⁃N(C)[88], time⁃dependent NH3 Faradaic efficiency and current density over Cu nanosheets during long⁃term stability test in a course of 700 h(D)[96], simulated concentrations and distributions of local NO3- on the surface of Cu encapsulated with porous carbon at the diffusion time of 7 µs(E)[97]Copyright 2023, Wiley-VCH.
| Species | Electrocatalyst | Electrolyte | Potential/V (vs. RHE) | NH3 yield rate | NH3 Faradic efficiency(%) | Ref. |
|---|---|---|---|---|---|---|
| Vacancy engineering | TiO2-x | 0.5 mol/L Na2SO4+50 ppm | -1.36 | 0.045 mmol·h‒1·mg‒1 | 85 | [ |
| P25⁃600 | 0.5 mol/L Na2SO4+100 ppm | -1.0 | 0.052 mmol·h‒1·mg‒1 | 78 | [ | |
| Fe2TiO5 | PBS+0.1 mol/L | -1.0 | 0.77 mmol·h‒1·mg‒1 | 87.5 | [ | |
| PCN⁃600 | 0.5 mol/L Na2SO4+100 ppm | -1.6 | 0.03262 mmol·h‒1·g‒1 | 89.96 | [ | |
| Interface engineering | Ag/Cu2O | 0.5 mol/L Na2SO4+100 ppm | -0.8 | 0.225 mmol·h‒1·cm‒2 | 96.45 | [ |
| Co/CoO NSAs | 0.1 mol/L Na2SO4+200 ppm | -1.06 | 194.46 μmol·h‒1·cm‒2 | 93.8 | [ | |
| CuCl_BEF | 0.5 mol/L Na2SO4+100 ppm | -1.0 | 1.82 mg·h‒1·cm‒2 | 98.6 | [ | |
| Alloy engineering | CuCo alloy | 1 mol/L KOH+100 mmol/L | -0.2 | 960 mmol·h‒1·g‒1 | 100±1 | [ |
| RuFe NFs | 0.5 mol/L Na2SO4+0.1 mol/L | -0.65 | 8.68 mg·h‒1·mg‒1 | 74.4(-0.3 V) | [ | |
| PdCu/Cu2O | 0.5 mol/L Na2SO4+100 ppm | -0.8 | 0.19 mmol·h‒1·cm‒2 | 94.32 | [ | |
| PA⁃RhCu cNCs | 0.1 mol/L HClO4+0.05 mol/L KNO3 | 0.05 | 2.4 mg·h‒1·mg‒1 | 93.7 | [ | |
| meso⁃PdN NCs | 0.1 mol/L Na2SO4+5 mmol/L | -0.7 | 3760 μg·h‒1·mg‒1 | 96.1 | [ | |
| Single⁃atom catalyst | Fe SACs | 0.1 mol/L K2SO4+0.5 mol/L | -0.66 | 20 mg·h‒1·mg‒1 | 75 | [ |
| Cu/Ni⁃NC | 0.5 mol/L Na2SO4+100 ppm | -0.7 | 0.324 mmol·h‒1·cm‒2 | 97.28 | [ | |
| Au/Cu SAA | 0.5 mol/L Na2SO4+100 ppm | -0.8 | 0.193 mmol·h‒1·cm‒2 | 99.69 | [ | |
| VCu⁃Au1Cu SAAs | 0.1 mol/L KOH+7.14 mmol/L | -0.2 | 555 μg·h‒1·cm‒2 | 98.7 | [ | |
| Doped engineering | Zn/Cu⁃2.3 | 0.5 mol/L K2SO4+0.1 mol/L | -0.55 | 5.8 mol·h‒1·g‒1 | 98.4 | [ |
| Co⁃Fe@Fe2O3 | 0.1 mol/L Na2SO4+50 ppm | -0.645 | 1,505.9 μg·h‒1·cm‒2 | 85.2±0.6 | [ | |
| Ru/β⁃Co(OH)2 | 1 mol/L KOH+0.1 mol/L | 0.071 | 0.38 mmol·h‒1·cm‒2 | 98.78 | [ | |
| N⁃C⁃1000 | 0.1 mol/L KOH+0.1 mol/L | -0.7 | 78.2 μmol·h‒1·cm‒2 | 95.0 | [ | |
| Morphological and | ox⁃LIG | 1 mol/L | -0.93 | 2859 µg·h‒1·cm‒2 | ca. 100 | [ |
| structural control | Cu nanosheets | 1 mol/L KOH+0.2 mol/L | -0.59 | 1.41 mmol·h‒1·cm‒2 | 88 | [ |
| Cu@C | 1 mol/L KOH+1 mmol/L | -0.3 | 469.5 µg·h‒1·cm‒2 | 72 | [ |
Table 2 Summary of performance of electrocatalytic NH3 synthesis by NO3RR
| Species | Electrocatalyst | Electrolyte | Potential/V (vs. RHE) | NH3 yield rate | NH3 Faradic efficiency(%) | Ref. |
|---|---|---|---|---|---|---|
| Vacancy engineering | TiO2-x | 0.5 mol/L Na2SO4+50 ppm | -1.36 | 0.045 mmol·h‒1·mg‒1 | 85 | [ |
| P25⁃600 | 0.5 mol/L Na2SO4+100 ppm | -1.0 | 0.052 mmol·h‒1·mg‒1 | 78 | [ | |
| Fe2TiO5 | PBS+0.1 mol/L | -1.0 | 0.77 mmol·h‒1·mg‒1 | 87.5 | [ | |
| PCN⁃600 | 0.5 mol/L Na2SO4+100 ppm | -1.6 | 0.03262 mmol·h‒1·g‒1 | 89.96 | [ | |
| Interface engineering | Ag/Cu2O | 0.5 mol/L Na2SO4+100 ppm | -0.8 | 0.225 mmol·h‒1·cm‒2 | 96.45 | [ |
| Co/CoO NSAs | 0.1 mol/L Na2SO4+200 ppm | -1.06 | 194.46 μmol·h‒1·cm‒2 | 93.8 | [ | |
| CuCl_BEF | 0.5 mol/L Na2SO4+100 ppm | -1.0 | 1.82 mg·h‒1·cm‒2 | 98.6 | [ | |
| Alloy engineering | CuCo alloy | 1 mol/L KOH+100 mmol/L | -0.2 | 960 mmol·h‒1·g‒1 | 100±1 | [ |
| RuFe NFs | 0.5 mol/L Na2SO4+0.1 mol/L | -0.65 | 8.68 mg·h‒1·mg‒1 | 74.4(-0.3 V) | [ | |
| PdCu/Cu2O | 0.5 mol/L Na2SO4+100 ppm | -0.8 | 0.19 mmol·h‒1·cm‒2 | 94.32 | [ | |
| PA⁃RhCu cNCs | 0.1 mol/L HClO4+0.05 mol/L KNO3 | 0.05 | 2.4 mg·h‒1·mg‒1 | 93.7 | [ | |
| meso⁃PdN NCs | 0.1 mol/L Na2SO4+5 mmol/L | -0.7 | 3760 μg·h‒1·mg‒1 | 96.1 | [ | |
| Single⁃atom catalyst | Fe SACs | 0.1 mol/L K2SO4+0.5 mol/L | -0.66 | 20 mg·h‒1·mg‒1 | 75 | [ |
| Cu/Ni⁃NC | 0.5 mol/L Na2SO4+100 ppm | -0.7 | 0.324 mmol·h‒1·cm‒2 | 97.28 | [ | |
| Au/Cu SAA | 0.5 mol/L Na2SO4+100 ppm | -0.8 | 0.193 mmol·h‒1·cm‒2 | 99.69 | [ | |
| VCu⁃Au1Cu SAAs | 0.1 mol/L KOH+7.14 mmol/L | -0.2 | 555 μg·h‒1·cm‒2 | 98.7 | [ | |
| Doped engineering | Zn/Cu⁃2.3 | 0.5 mol/L K2SO4+0.1 mol/L | -0.55 | 5.8 mol·h‒1·g‒1 | 98.4 | [ |
| Co⁃Fe@Fe2O3 | 0.1 mol/L Na2SO4+50 ppm | -0.645 | 1,505.9 μg·h‒1·cm‒2 | 85.2±0.6 | [ | |
| Ru/β⁃Co(OH)2 | 1 mol/L KOH+0.1 mol/L | 0.071 | 0.38 mmol·h‒1·cm‒2 | 98.78 | [ | |
| N⁃C⁃1000 | 0.1 mol/L KOH+0.1 mol/L | -0.7 | 78.2 μmol·h‒1·cm‒2 | 95.0 | [ | |
| Morphological and | ox⁃LIG | 1 mol/L | -0.93 | 2859 µg·h‒1·cm‒2 | ca. 100 | [ |
| structural control | Cu nanosheets | 1 mol/L KOH+0.2 mol/L | -0.59 | 1.41 mmol·h‒1·cm‒2 | 88 | [ |
| Cu@C | 1 mol/L KOH+1 mmol/L | -0.3 | 469.5 µg·h‒1·cm‒2 | 72 | [ |
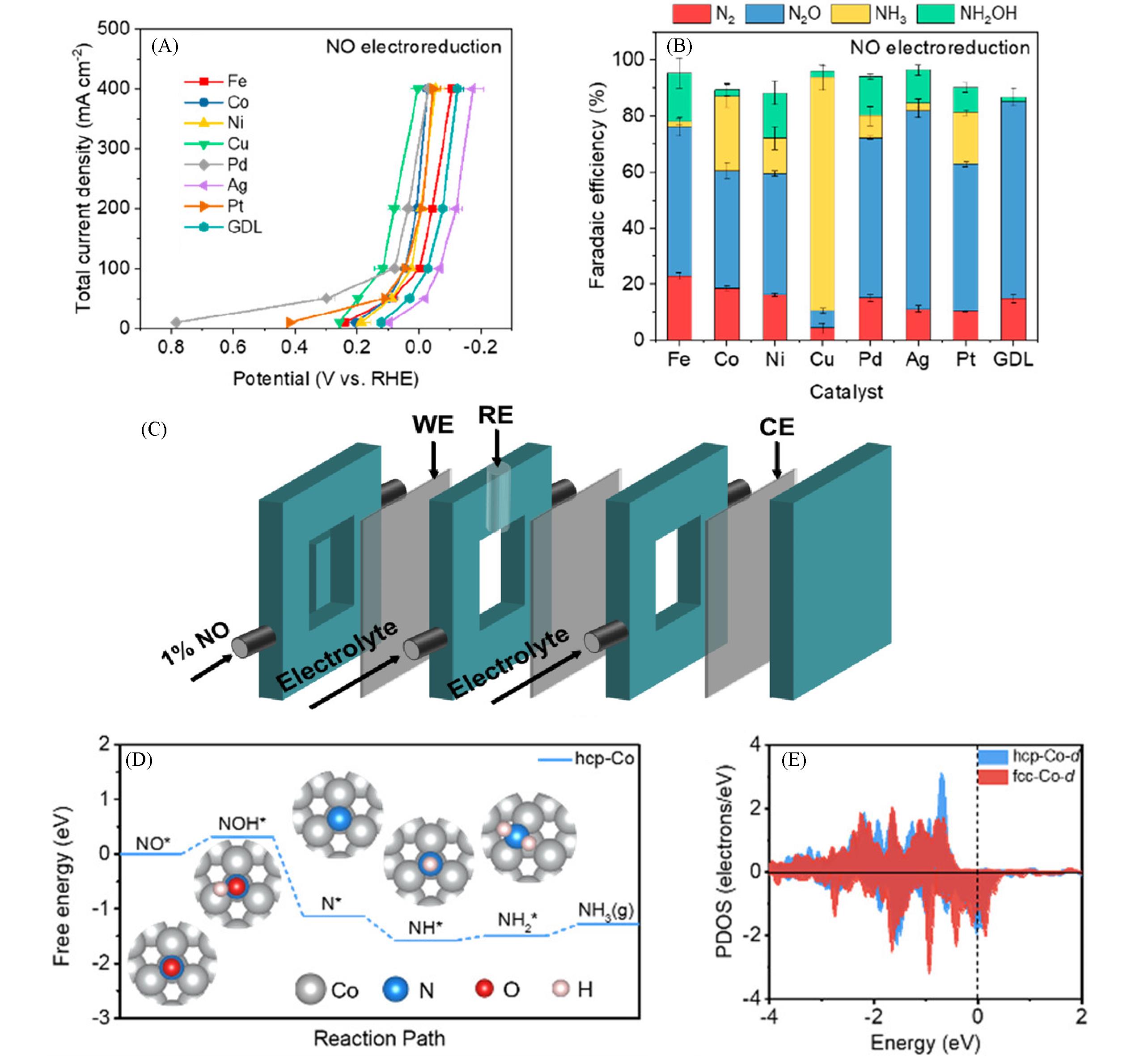
Fig.9 Total current density vs. potential of various catalysts(A), Faradaic efficiency of various catalysts at (0.10±0.02) V(vs. RHE) in NO electroreduction(B)[107], diagram of the flow electrolyzer for NORR over Ru⁃LCN(C)[115], reaction process of NORR on the hcp⁃Co with the reaction free energy and the optimized structures of all reaction intermediates(D), partial density of states(PDOS) of d orbitals in the hcp⁃Co and fcc⁃Co(E)[118](E) The Fermi energy level is set to 0 eV. (A, B) Copyright 2022, American Chemical Society; (C) Copyright 2022, American Chemical Society; (D, E) Copyright 2023, American Chemical Society.
| Electrocatalyst | Electrolyte | Potential/V (vs. RHE) | NH3 Yield rate | NH3 Faradicefficiency(%) | Reactor | Ref. |
|---|---|---|---|---|---|---|
| Cu Foam | 3 mol/L KCl | -0.9 | 517.1 μmol·cm‒2·h‒1 | 93.5 | H⁃type cell | [ |
| Cu nanoparticle | 0.1 mol/L NaOH+ | 0.03 | 1806 μmol·cm‒2·h‒1 | 78(0.1 V) | Flow cell | [ |
| 0.9 mol/L NaClO4 | ||||||
| Cu1/MoS2 | 0.5 mol/L Na2SO4 | -0.6 | 337.5 μmol·cm‒2·h‒1 | 90.6 | H⁃type cell | [ |
| Ni⁃NCNR700 | 0.1 mol/L HCl | 0.61 | (23.8±2.6) μmol·cm‒2·h‒1 | 85.5±0.8 | H⁃type cell | [ |
| Ni(210) | 0.5 mol/L K2SO4 | -0.68 | 544 μmol·cm‒2·h‒1 | 85 | Flow cell | [ |
| Sb1/a⁃MoO3 | 0.5 mol/L Na2SO4 | -0.6 | 273.5 μmol·cm‒2·h‒1 | 91.7 | H⁃type cell | [ |
| NiNC@CF | 0.5 mol/L PBS | -0.5 | 94 μmol·cm‒2·h‒1 | 87 | H⁃type cell | [ |
| Ru⁃LCN | 0.5 mol/L Na2SO4 | -0.2 | 45.02 μmol·h‒1·mg‒1 | 65.9 | Flow cell | [ |
| Fe/C | 0.5 mol/L H2SO4 | -0.4 | 1239 μmol·cm‒2·h‒1 | 50.4 | Flow cell | [ |
| MoS2/GF | 0.1 mol/L HCl+ | 0.1 | 99.6 μmol·cm‒2·h‒1 | 76.6 | H⁃type cell | [ |
| 0.5 mmol/L Fe(II)SB | ||||||
| hcp⁃Co | 0.1 mol/L Na2SO4 | 0.6 | 439.50 μmol·cm‒2·h‒1 | 72.58 | H⁃type cell | [ |
| Single atom Nb | 0.1 mol/L HCl | -0.6 | 295.2 μmol·cm‒2·h‒1 | 77.1 | H⁃type cell | [ |
| TiO2-x/TP | 0.2 mol/L PBS | -0.7 | 1233.3 μg·h‒1·cm‒2 | 92.5(-0.4 V) | H⁃type cell | [ |
| a⁃B2.6C@TiO2/Ti | 0.1 mol/L Na2SO4+ | -0.9 | 3678.6 μg·h‒1·cm‒2 | 87.6 | H⁃type cell | [ |
| 0.5 mmol/L Fe2+⁃EDTA | ||||||
| Cu6Sn5 | 1 mol/L KOH | -0.23 | 10 mmol·cm‒2·h‒1 | > 96 | Flow cell | [ |
Table 3 Summary of performance of electrocatalytic NH3 synthesis by NORR
| Electrocatalyst | Electrolyte | Potential/V (vs. RHE) | NH3 Yield rate | NH3 Faradicefficiency(%) | Reactor | Ref. |
|---|---|---|---|---|---|---|
| Cu Foam | 3 mol/L KCl | -0.9 | 517.1 μmol·cm‒2·h‒1 | 93.5 | H⁃type cell | [ |
| Cu nanoparticle | 0.1 mol/L NaOH+ | 0.03 | 1806 μmol·cm‒2·h‒1 | 78(0.1 V) | Flow cell | [ |
| 0.9 mol/L NaClO4 | ||||||
| Cu1/MoS2 | 0.5 mol/L Na2SO4 | -0.6 | 337.5 μmol·cm‒2·h‒1 | 90.6 | H⁃type cell | [ |
| Ni⁃NCNR700 | 0.1 mol/L HCl | 0.61 | (23.8±2.6) μmol·cm‒2·h‒1 | 85.5±0.8 | H⁃type cell | [ |
| Ni(210) | 0.5 mol/L K2SO4 | -0.68 | 544 μmol·cm‒2·h‒1 | 85 | Flow cell | [ |
| Sb1/a⁃MoO3 | 0.5 mol/L Na2SO4 | -0.6 | 273.5 μmol·cm‒2·h‒1 | 91.7 | H⁃type cell | [ |
| NiNC@CF | 0.5 mol/L PBS | -0.5 | 94 μmol·cm‒2·h‒1 | 87 | H⁃type cell | [ |
| Ru⁃LCN | 0.5 mol/L Na2SO4 | -0.2 | 45.02 μmol·h‒1·mg‒1 | 65.9 | Flow cell | [ |
| Fe/C | 0.5 mol/L H2SO4 | -0.4 | 1239 μmol·cm‒2·h‒1 | 50.4 | Flow cell | [ |
| MoS2/GF | 0.1 mol/L HCl+ | 0.1 | 99.6 μmol·cm‒2·h‒1 | 76.6 | H⁃type cell | [ |
| 0.5 mmol/L Fe(II)SB | ||||||
| hcp⁃Co | 0.1 mol/L Na2SO4 | 0.6 | 439.50 μmol·cm‒2·h‒1 | 72.58 | H⁃type cell | [ |
| Single atom Nb | 0.1 mol/L HCl | -0.6 | 295.2 μmol·cm‒2·h‒1 | 77.1 | H⁃type cell | [ |
| TiO2-x/TP | 0.2 mol/L PBS | -0.7 | 1233.3 μg·h‒1·cm‒2 | 92.5(-0.4 V) | H⁃type cell | [ |
| a⁃B2.6C@TiO2/Ti | 0.1 mol/L Na2SO4+ | -0.9 | 3678.6 μg·h‒1·cm‒2 | 87.6 | H⁃type cell | [ |
| 0.5 mmol/L Fe2+⁃EDTA | ||||||
| Cu6Sn5 | 1 mol/L KOH | -0.23 | 10 mmol·cm‒2·h‒1 | > 96 | Flow cell | [ |
| 1 | van Langevelde P. H., Katsounaros I., Koper M. T. M., Joule, 2021, 5(2), 290—294 |
| 2 | Tang C., Qiao S. Z., Chem. Soc. Rev., 2019, 48(12), 3166—3180 |
| 3 | Foster S. L., Bakovic S. I. P., Duda R. D., Maheshwari S., Milton R. D., Minteer S. D., Janik M. J., Renner J. N., Greenlee L. F., Nat. Catal., 2018, 1(7), 490—500 |
| 4 | Fu X., Pedersen J. B., Zhou Y., Saccoccio M., Li S., Sažinas R., Li K., Andersen S. Z., Xu A., Deissler N. H., Mygind J. B. V., Wei C., Kibsgaard J., Vesborg P. C. K., Nørskov J. K., Chorkendorff I., Science, 2023, 379(6633), 707—712 |
| 5 | Suryanto B. H. R., Matuszek K., Choi J., Hodgetts R. Y., Du H. L., Bakker J. M., Kang C. S. M., Cherepanov P. V., Simonov A. N., MacFarlane D. R., Science, 2021, 372(6547), 1187—1191 |
| 6 | Wang L., Xia M., Wang H., Huang K., Qian C., Maravelias C. T., Ozin G. A., Joule, 2018, 2(6), 1055—1074 |
| 7 | Guang Z. L., He Z., Yu Y. B., Yang Y., Liu K., Shi X. Y., Yan Z. D., Shan W. P., He H., Sci. Adv., 2018, 4(11),eaau4637 |
| 8 | Li Z., Attanayake N. H., Blackburn J. L., Miller E. M., Energy Environ. Sci., 2021, 14(12), 6242—6286 |
| 9 | Zhou F., Azofra L. M., Ali M., Kar M., Simonov A. N., McDonnell⁃Worth C., Sun C., Zhang X., MacFarlane D. R., Energy Environ. Sci., 2017, 10(12), 2516—2520 |
| 10 | Suryanto B. H. R., Du H. L., Wang D., Chen J., Simonov A. N., MacFarlane D. R., Nat. Catal., 2019, 2(4), 290—296 |
| 11 | Wang Q., Guo J., Chen P., J. Energy Chem., 2019, 36, 25—36 |
| 12 | Cheng H., Ding L. X., Chen G. F., Zhang L., Xue J., Wang H., Adv. Mater., 2018, 30(46), 1803694 |
| 13 | Guo C., Ran J., Vasileff A., Qiao S. Z., Energy Environ. Sci., 2018, 11(1), 45—56 |
| 14 | Qu X. Y., Hu S. Z., Li P., Wang F., Zhao Y. F., Wang Q., Chem. J. Chinese Universities, 2017, 38(12), 2280—2288 |
| 曲晓钰, 胡绍争, 李萍, 王菲, 赵艳锋, 王琼. 高等学校化学学报, 2017, 38(12), 2280—2288 | |
| 15 | Sun J., Alam D., Daiyan R., Masood H., Zhang T., Zhou R., Cullen P. J., Lovell E. C., Jalili A., Amal R., Energy Environ. Sci., 2021, 14(2), 865—872 |
| 16 | Hawtof R., Ghosh S., Guarr E., Xu C., Mohan Sankaran R., Renner J. N., Sci. Adv., 2021, 5(1), eaat5778 |
| 17 | Zhang T., Zhou R., Zhang S., Zhou R., Ding J., Li F., Hong J., Dou L., Shao T., Murphy A. B., Ostrikov K., Cullen P. J., Energy Environ. Mater., 2023, 6(2), e12344 |
| 18 | Qing G., Ghazfar R., Jackowski S. T., Habibzadeh F., Ashtiani M. M., Chen C. P., Smith M. R., Hamann T. W. III, Chem. Rev., 2020, 120(12), 5437—5516 |
| 19 | Li K., Andersen S. Z., Statt M. J., Saccoccio M., Bukas V. J., Krempl K., Sažinas R., Pedersen J. B., Shadravan V., Zhou Y., Chakraborty D., Kibsgaard J., Vesborg P. C. K., Nørskov J. K., Chorkendorff I., Science, 2021, 374(6575), 1593—1597 |
| 20 | Shilov A. E., Russ. Chem. Bull., 2003, 52(12), 2555—2562 |
| 21 | Bezdek M. J., Chirik P. J., Angew. Chem. Int. Ed., 2016, 55(28), 7892—7896 |
| 22 | Tang C., Qiao S. Z., Joule, 2019, 3(7), 1573—1575 |
| 23 | Zhao S., Lu X., Wang L., Gale J., Amal R., Adv. Mater., 2019, 31(13), 1805367 |
| 24 | MacFarlane D. R., Choi J., Suryanto B. H. R., Jalili R., Chatti M., Azofra L. M., Simonov A. N., Adv. Mater., 2020, 32(18), 1904804 |
| 25 | Duan G., Chen Y., Tang Y., Gasem K. A. M., Wan P., Ding D., Fan M., Prog. Energy Combust. Sci., 2020, 81, 100860 |
| 26 | Yao Y., Feng Q., Zhu S., Li J., Yao Y., Wang Y., Wang Q., Gu M., Wang H., Li H., Yuan X. Z., Shao M., Small Methods, 2018, 3(6), 1800324 |
| 27 | Wang Y., Wang C., Li M., Yu Y., Zhang B., Chem. Soc. Rev., 2021, 50(12), 6720—6733 |
| 28 | Fan G., Xu W., Li J., Chen J. L., Yu M., Ni Y., Zhu S., Su X. C., Cheng F., Adv. Mater., 2021, 33(42), 2101126 |
| 29 | Montoya J. H., Tsai C., Vojvodic A., Norskov J. K., ChemSusChem, 2015, 8(13), 2180—2186 |
| 30 | Liu C., Li Q., Wu C., Zhang J., Jin Y., MacFarlane D. R., Sun C., J. Am. Chem. Soc., 2019, 141(7), 2884—2888 |
| 31 | Yang X., Nash J., Anibal J., Dunwell M., Kattel S., Stavitski E., Attenkofer K., Chen J. G., Yan Y., Xu B., J. Am. Chem. Soc., 2018, 140(41), 13387—13391 |
| 32 | Deng J., Iñiguez J. A., Liu C., Joule, 2018, 2(5), 846—856 |
| 33 | Ren Y., Yu C., Tan X., Huang H., Wei Q., Qiu J., Energy Environ. Sci., 2021, 14(3), 1176—1193 |
| 34 | Tao H., Choi C., Ding L. X., Jiang Z., Han Z., Jia M., Fan Q., Gao Y., Wang H., Robertson A. W., Hong S., Jung Y., Liu S., Sun Z., Chem, 2019, 5(1), 204—214 |
| 35 | Han L., Liu X., Chen J., Lin R., Liu H., Lü F., Bak S., Liang Z., Zhao S., Stavitski E., Luo J., Adzic R. R., Xin H. L., Angew. Chem. Int. Ed., 2019, 58(8), 2321—2325 |
| 36 | Skulason E., Bligaard T., Gudmundsdottir S., Studt F., Rossmeisl J., Abild⁃Pedersen F., Vegge T., Jonsson H., Norskov J. K., Phys. Chem. Chem. Phys., 2012, 14(3), 1235—1245 |
| 37 | Kim C., Song J. Y., Choi C., Ha J. P., Lee W., Nam Y. T., Lee D. M., Kim G., Gereige I., Jung W. B., Lee H., Jung Y., Jeong H., Jung H. T., Adv. Mater., 2022, 34(40), 2205270 |
| 38 | Kordali V., Kyriacou G., Lambrou C., Chem. Commun., 2000, 17, 1673—1674 |
| 39 | Back S., Jung Y., Phys. Chem. Chem. Phys., 2016, 18(13), 9161—9166 |
| 40 | Bao D., Zhang Q., Meng F. L., Zhong H. X., Shi M. M., Zhang Y., Yan J. M., Jiang Q., Zhang X. B., Adv. Mater., 2017, 29(3), 1604799 |
| 41 | Hu C., Chen X., Jin J., Han Y., Chen S., Ju H., Cai J., Qiu Y., Gao C., Wang C., Qi Z., Long R., Song L., Liu Z., Xiong Y., J. Am. Chem. Soc., 2019, 141(19), 7807—7814 |
| 42 | Liu H. M., Han S. H., Zhao Y., Zhu Y. Y., Tian X. L., Zeng J. H., Jiang J. X., Xia B. Y., Chen Y., J. Mater. Chem. A, 2018, 6(7), 3211—3217 |
| 43 | Yao Y., Zhu S. Q., Wang H. J., Li H., Shao M. H., Angew. Chem. Int. Ed., 2020, 59(26), 10479—10483 |
| 44 | Wang J., Yu L., Hu L., Chen G., Xin H. L., Feng X. F., Nat. Commun., 2018, 9, 1795 |
| 45 | Li X. F., Li Q. K., Cheng J., Liu L., Yan Q., Wu Y., Zhang X. H., Wang Z. Y., Qiu Q., Luo Y., J. Am. Chem. Soc., 2016, 138(28), 8706—8709 |
| 46 | Hu L., Khaniya A., Wang J., Chen G., Kaden W. E., Feng X., ACS Catal., 2018, 8(10), 9312—9319 |
| 47 | Chu K., Cheng Y. H., Li Q. Q., Liu Y. P., Tian Y., J. Mater. Chem. A, 2020, 8(12), 5865—5873 |
| 48 | Yang D., Chen T., Wang Z., J. Mater. Chem. A, 2017, 5(36), 18967—18971 |
| 49 | Zeng L., Chen S., van der Zalm J., Li X., Chen A., Chem. Commun., 2019, 55(51), 7386—7389 |
| 50 | Hui L., Xue Y., Yu H., Liu Y., Fang Y., Xing C., Huang B., Li Y., J. Am. Chem. Soc., 2019, 141(27), 10677—10683 |
| 51 | Zhong X., Yuan E., Yang F., Liu Y., Lu H., Yang J., Gao F., Zhou Y., Pan J., Zhu J., Yua C., Zhug C., Yuan A., Ang E. H., Proc. Natl. Acad. Sci. USA, 2023, 120(40), e2306673120 |
| 52 | Wang X., Xie J., Li S., Yuan Z., Sun Y., Gao X., Tang Z., Zhang H., Li J., Wang S., Yang Z., Yan Y. M., Appl. Catal. B, 2023, 339, 123126 |
| 53 | Hu C. G., Gao Y. Y., Zhao L. J., Dai L. M., Chem Catal., 2022, 2(9), 2150—2156 |
| 54 | Liu Y., Su Y., Quan X., Fan X., Chen S., Yu H., Zhao H., Zhang Y., Zhao J., ACS Catal., 2018, 8(2), 1186—1191 |
| 55 | Yu X., Han P., Wei Z., Huang L., Gu Z., Peng S., Ma J., Zheng G., Joule, 2018, 2(8), 1610—1622 |
| 56 | Zhang X., Wu T., Wang H., Zhao R., Chen H., Wang T., Wei P., Luo Y., Zhang Y., Sun X., ACS Catal., 2019, 9(5), 4609—4615 |
| 57 | Duca M., Koper M. T. M., Energy Environ. Sci., 2012, 5(12), 9726—9742 |
| 58 | Kim K., Zagalskaya A., Ng J. L., Hong J., Alexandrov V., Pham T. A., Su X., Nat. Commun., 2023, 14(1), 823 |
| 59 | Kim K. H., Lee H., Huang X., Choi J. H., Chen C., Kang J. K., Hare D. O’., Energy Environ. Sci., 2023, 16(2), 663—672 |
| 60 | Zhang X., Wang Y., Liu C., Yu Y., Lu S., Zhang B., Chem. Eng. J., 2021, 403, 126269—126285 |
| 61 | Fu X., Zhang J., Kang Y., Chem. Catal., 2022, 2(10), 2590—2613 |
| 62 | da Cunha M. C. P. M., Weber M., Nart F. C., J. Electroanal. Chem., 1996, 414(2), 163—170 |
| 63 | Zeng Y., Priest C., Wang G., Wu G., Small Methods, 2020, 4(12), 2000672 |
| 64 | Katsounaros I., Kyriacou G., Electrochim. Acta, 2008, 53(17), 5477—5484 |
| 65 | Wu Z., Zhao Y., Jin W., Jia B., Wang J., Ma T., Adv. Funct. Mater., 2021, 31(9), 2009070 |
| 66 | Wu Y., Li Y., Gao J., Zhang Q., SusMat, 2021, 1(1), 66—87 |
| 67 | Jia R., Wang Y., Wang C., Ling Y., Yu Y., Zhang B., ACS Catal., 2020, 10(6), 3533—3540 |
| 68 | Wei Z., Niu X., Yin H., Yu S., Li J., Appl. Catal. A, 2022, 636, 118596 |
| 69 | Du H., Guo H., Wang K., Du X., Beshiwork B. A., Sun S., Luo Y., Liu Q., Li T., Sun X., Angew. Chem. Int. Ed., 2023, 62(5), e202215782 |
| 70 | Huang Y., Long J., Wang Y., Meng N., Yu Y., Lu S., Xiao J., Zhang B., ACS Appl. Mater. Interfaces, 2021, 13(46), 54967—54973 |
| 71 | Guo D., Wang S., Xu J., Zheng W., Wang D., J. Energy Chem., 2022, 65, 448—468 |
| 72 | Bian L., Zhang Z. Y., Tian H., Tian N. N., Ma Z., Wang Z. L., Chinese J. Catal., 2023, 54, 199—211 |
| 73 | Gao T., Tang X., Li X., Wu S., Yu S., Li P., Xiao D., Jin Z., ACS Catal., 2023, 13(1), 49—59 |
| 74 | Yin H. B., Zhao X. G., Xiong S. C., Peng Y., Chen Z., Wang R., Wen M., Luo J. S., Yamashita H., Li J. H., J. Catal., 2022, 406, 39—47 |
| 75 | Yu Y., Wang C., Yu Y., Wang Y., Zhang B., Sci. China Mater., 2020, 63(10), 1469—1476 |
| 76 | Sun W. J., Ji H. Q., Li L. X., Zhang H. Y., Wang Z. K., He J. H., Lu J. M., Angew. Chem. Int. Ed., 2021, 60(42), 22933—22939 |
| 77 | Zhang Z. Y., Tian H., Bian L., Liu S. Z., Liu Y., Wang Z. L., J. Energy Chem., 2023, 83, 90—97 |
| 78 | Luo M., Yang J. T., Li X. G., Eguchi M., Yamauchi Y., Wang Z. L., Chem. Sci., 2023, 14, 3400 |
| 79 | Li Y., Shan W., Zachman M. J., Wang M., Hwang S., Tabassum H., Yang J., Yang X., Karakalos S., Feng Z., Wang G., Wu G., Angew. Chem. Int. Ed., 2022, 61(28), e202205632 |
| 80 | Fang J. Y., Zheng Q. Z., Lou Y. Y., Zhao K. M., Hu S. N., Li G., Akdim O., Huang X. Y., Sun S. G., Nat. Commun., 2022, 13(1), 7899 |
| 81 | Wang Y. H., Sun M. Z., Zhou J. W., Xiong Y. C., Zhang Q. H., Ye C. L., Wang X. X., Lu P. Y., Feng T. Y., Hao F. K., Liu F., Wang J., Ma Y. B., Yin J. W., Chu S. Q., Gu L., Huang B. L., Fan Z. X., Proc. Natl. Acad. Sci. USA, 2023, 120(32), e2306461120 |
| 82 | Yin H. B., Chen Z., Xiong S. C., Chen J. J., Wang C., Wang R., Kuwahara Y., Luo J. S., Yamashita H., Peng Y., Li J. H., Chem Catal., 2021, 1(5), 1088—1103 |
| 83 | Gao W. S., Xie K. F., Xie J., Wang X. M., Zhang H., Chen S. Q., Wang H., Li Z. L., Li C., Adv. Mater., 2023, 35(1), 2202952 |
| 84 | Sun L., Liu B., Adv. Mater., 2023, 35(1), e2207305 |
| 85 | Pi Y. C., Zhang Y., Cheng Z. F., Huang X. Q., Chem. J. Chinese Universities, 2021, 42(2), 456—474 |
| 皮业灿, 张应, 成子方, 黄小青. 高等学校化学学报, 2021, 42(2), 456—474 | |
| 86 | Zhuo H. Y., Zhang X., Liang J. X., Yu Q., Xiao H., Li J., Chem. Rev., 2020, 120(21), 12315—12341 |
| 87 | Wu Z. Y., Karamad M., Yong X., Huang Q., Cullen D. A., Zhu P., Xia C., Xiao Q., Shakouri M., Chen F. Y., Kim J. Y., Xia Y., Heck K., Hu Y., Wong M. S., Li Q., Gates I., Siahrostami S., Wang H., Nat. Commun., 2021, 12(1), 2870 |
| 88 | Wang Y., Yin H., Dong F., Zhao X., Qu Y., Wang L., Peng Y., Wang D., Fang W., Li J., Small, 2023, 19(20), e2207695 |
| 89 | Yin H., Peng Y., Li J., Environ. Sci. Technol., 2023, 57(8), 3134—3144 |
| 90 | Zhang Y., Chen X., Wang W., Yin L., Crittenden J. C., Appl. Catal. B, 2022, 310, 121346 |
| 91 | Wu L., Feng J., Zhang L., Jia S., Song X., Zhu Q., Kang X., Xing X., Sun X., Han B., Angew. Chem. Int. Ed., 2023, 62(43), e202307952 |
| 92 | Zhang S., Li M., Li J., Song Q., Liu X., Proc. Natl. Acad. Sci. USA, 2022, 119(6), e2115504119 |
| 93 | Zhu W., Yao F., Wu Q., Jiang Q., Wang J., Wang Z., Liang H., Energy Environ. Sci., 2023, 16(6), 2483—2493 |
| 94 | Li R., Gao T., Wang P., Qiu W., Liu K., Liu Y., Jin Z., Li P., Appl. Catal. B, 2023, 331, 122677 |
| 95 | Huang L., Cheng L., Ma T., Zhang J. J., Wu H., Su J., Song Y., Zhu H., Liu Q., Zhu M., Zeng Z., He Q., Tse M. K., Yang D. T., Yakobson B. I., Tang B. Z., Ren Y., Ye R., Adv. Mater., 2023, 35(24), e2211856 |
| 96 | Fu Y. F., Wang S., Wang Y., Wei P. F., Shao J. Q., Liu T. F., Wang G. X., Bao X. H., Angew. Chem. Int. Ed., 2023, 62, e202303327 |
| 97 | Song Z., Liu Y., Zhong Y., Guo Q., Zeng J., Geng Z., Adv. Mater., 2022, 34(36), e2204306 |
| 98 | Islam A., Teo S. H., Ng C. H., Taufiq⁃Yap Y. H., Choong S. Y. T., Awual M. R., Prog. Mater. Sci., 2023, 132, 101033 |
| 99 | Liu Z., Li J., Woo S. I., Energy Environ. Sci., 2012, 5(10), 8799—8814 |
| 100 | Perry R. A., Siebers D. L., Nature, 1986, 324, 657—658 |
| 101 | Kelly S., Bogaerts A., Joule, 2021, 5(11), 3006—3030 |
| 102 | Cui F., Sun Y., Xie J., Li D., Wu M., Song L., Hu Y., Tian Y., Eur. Respir. J., 2023, 61(2), 2200777 |
| 103 | Han L., Cai S., Gao M., Hasegawa J. Y., Wang P., Zhang J., Shi L., Zhang D., Chem. Rev., 2019, 119(19), 10916—10976 |
| 104 | Beale A. M., Gao F., Lezcano⁃Gonzalez I., Peden C. H. F., Szanyi J., Chem. Soc. Rev., 2015, 44(20), 7371—7405 |
| 105 | Duan Y., Wang Y., Gan L., Meng J., Feng Y., Wang K., Zhou K., Wang C., Han X., Zhou X., Adv. Energy Mater., 2021, 11(19), 2004001 |
| 106 | Long J., Chen S., Zhang Y., Guo C., Fu X., Deng D., Xiao J., Angew. Chem. Int. Ed., 2020, 59(24), 9711—9718 |
| 107 | Ko B. H., Hasa B., Shin H., Zhao Y., Jiao F., J. Am. Chem. Soc., 2022, 144(3), 1258—1266 |
| 108 | Chen K., Zhang G., Li X., Zhao X., Chu K., Nano Research, 2022, 144, 1—7 |
| 109 | Dhanabal D., Markandaraj S. S., Shanmugam S., ACS Catal., 2023, 13(13), 9136—9149 |
| 110 | Zhao S., Liu J., Zhang Z., Zhu C., Shi G., Wu J., Yang C., Wang Q., Chang M., Liu K., Li S., Zhang L., Chem, 2023, 155, 9136—9149 |
| 111 | Chen K., Zhang Y., Xiang J., Zhao X., Li X., Chu K., ACS Energy Lett., 2023, 8(3), 1281—1288 |
| 112 | Langer S. H., Pate K. T., Nature, 1980, 284, 434—435 |
| 113 | Colucci J. A., Foral M. J., Langer S. H., Electrochim. Acta, 1985, 30(12), 1675—1685 |
| 114 | Muthusamy T., Markandaraj S. S., Shanmugam S., J. Mater. Chem. A, 2022, 10(12), 6470—6474 |
| 115 | Li Y., Cheng C., Han S., Huang Y., Du X., Zhang B., Yu Y., ACS Energy Lett., 2022, 7(3), 1187—1194 |
| 116 | Cheon S., Kim W. J., Kim D. Y., Kwon Y., Han J. I., ACS Energy Lett., 2022, 7(3), 958—965 |
| 117 | Zhang L., Liang J., Wang Y., Mou T., Lin Y., Yue L., Li T., Liu Q., Luo Y., Li N., Tang B., Liu Y., Gao S., Alshehri A. Guo A., X., Ma D., Sun X., Angew. Chem. Int. Ed., 2021, 60(48), 25263—25268 |
| 118 | Wang D., Chen Z. W., Gu K., Chen C., Liu Y., Wei X., Singh C. V., Wang S., J. Am. Chem. Soc., 2023, 145(12), 6899—6940 |
| 119 | Peng X., Mi Y., Bao H., Liu Y., Qi D., Qiu Y., Zhuo L., Zhao S., Sun J., Tang X., Luo J., Liu X., Nano Energy, 2020, 78, 105321 |
| 120 | Li Z., Zhou Q., Liang J., Zhang L., Fan X., Zhao D., Cai Z., Li J., Zheng D., He X., Luo Y., Wang Y., Ying B., Yan H., Sun S., Zhang J., Alshehri A. A., Gong F., Zheng Y., Sun X., Small, 2023, 19(24), 2300291 |
| 121 | Liang J., Liu P., Li Q., Li T., Yue L., Luo Y., Liu Q., Li N., Tang B., Alshehri A. A., Shakir I., Agboola P. O., Sun C., Sun X., Angew. Chem. Int. Ed., 2022, 61(18), 6720—6733 |
| 122 | Shao J., Jing H., Wei P., Fu X., Pang L., Song Y., Ye K., Li M., Jiang L., Ma J., Li R., Si R., Peng Z., Wang G., Xiao J., Nat. Energy, 2023, 8, 1273—1283 |
| [1] | 成施雨, 杨灵, 包芮于, 陈宸, 崔萌萌, 张谷令, 李华. 非贵金属催化电极Ni/C@CF的制备及绿色类Fenton性能[J]. 高等学校化学学报, 2024, 45(2): 20230382. |
| [2] | 赵环宇, 米洪田, 常月琪, 周冬雪, 张立国, 杨穆. Ni/TiO2-VO纳米线自支撑薄膜的界面工程与电催化产氢性能[J]. 高等学校化学学报, 2023, 44(8): 20230057. |
| [3] | 卞宣昂, 周超, 赵运宣, 张铁锐. 基于水滑石衍生钌催化剂光驱动合成氨的研究[J]. 高等学校化学学报, 2023, 44(6): 20230095. |
| [4] | 池丽萍, 牛壮壮, 廖洁, 唐凯斌, 高敏锐. 过渡金属氧化物插层化学及其电催化应用的新进展[J]. 高等学校化学学报, 2023, 44(5): 20220740. |
| [5] | 徐佳宁, 白文静, 楼雨寒, 于海鹏, 窦烁. 电催化氧化木质素解聚: 温和高效的生物质增值策略[J]. 高等学校化学学报, 2023, 44(5): 20220749. |
| [6] | 李轩, 亓帅, 周伟良, 李小杰, 景玲胭, 冯超, 蒋兴星, 杨恒攀, 胡琪, 何传新. 纤维基氧化还原电催化剂的研究进展[J]. 高等学校化学学报, 2023, 44(5): 20220770. |
| [7] | 张潇然, 郑建云, 吕艳红, 王双印. 绿色路径C-N偶联合成尿素的最新研究进展[J]. 高等学校化学学报, 2023, 44(5): 20220717. |
| [8] | 胡宏苏, 申传喆, 王宇航, 王青青, 何士龙, 李鹏. 石墨烯复合多孔碳毡气体扩散阴极的H2O2催生性能与机理[J]. 高等学校化学学报, 2023, 44(11): 20230245. |
| [9] | 匡华艺, 陈晨. 贵金属纳米框架设计合成及电催化性能的研究进展[J]. 高等学校化学学报, 2023, 44(1): 20220586. |
| [10] | 杨庆凤, 吕良, 赖小勇. 中空MOFs材料制备及电催化应用的研究进展[J]. 高等学校化学学报, 2023, 44(1): 20220666. |
| [11] | 刘至辰, 张宏伟, 张博稳, 陈鹏, 袁珮. 吸附法制备金属/碳催化剂用于5-羟基甲基糠醛高效电催化氧化的研究[J]. 高等学校化学学报, 2023, 44(1): 20220631. |
| [12] | 林高鑫, 王家成. 单原子掺杂二硫化钼析氢催化的进展和展望[J]. 高等学校化学学报, 2022, 43(9): 20220321. |
| [13] | 汪思聪, 庞贝贝, 刘潇康, 丁韬, 姚涛. XAFS技术在单原子电催化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220487. |
| [14] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [15] | 姚青, 俞志勇, 黄小青. 单原子催化剂的合成及其能源电催化应用的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220323. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||