

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (10): 2230.doi: 10.7503/cjcu20200383
收稿日期:2020-06-23
出版日期:2020-10-10
发布日期:2020-10-08
通讯作者:
刘国凯
E-mail:gkliu@szu.edu.cn
基金资助:
QIN Wenbing, LIN Weifeng, LI Xin, XIONG Wei, LIU Guokai( )
)
Received:2020-06-23
Online:2020-10-10
Published:2020-10-08
Contact:
LIU Guokai
E-mail:gkliu@szu.edu.cn
Supported by:摘要:
以亲电的S-(二氟甲基)硫??盐作为二氟甲基源, 在有机碱作用下, 对1,1-偕二氰基烯烃的二氟甲基化反应进行了研究. 在最优反应条件下, 一系列1,1-偕二氰基烯烃发生二氟甲基化反应, 以43%~99%的分离收率获得结构多样的含有二氟甲基取代全碳中心的目标化合物. 实验结果表明, 该方法底物普适性良好, 不同的给电子和吸电子取代基、 环状及链状底物、 杂环、 苯环、 萘环和菲环等均适用于该反应, 可顺利发生转化得到相应的二氟甲基化目标产物.
中图分类号:
TrendMD:
秦文兵, 林伟锋, 李鑫, 熊威, 刘国凯. 亲电二氟甲基试剂S⁃(二氟甲基)硫𬭩盐对1,1⁃偕二氰基烯烃的二氟甲基化: 二氟甲基取代全碳中心的有效构建. 高等学校化学学报, 2020, 41(10): 2230.
QIN Wenbing, LIN Weifeng, LI Xin, XIONG Wei, LIU Guokai. Difluoromethylation of Dicyanoalkylidenes by Electrophilic S-(Difluoromethyl)sulfonium Salt: Efficient Construction of Difluoromethylated All-carbon-substituted Centers†. Chem. J. Chinese Universities, 2020, 41(10): 2230.
| Compd. | Appearance | m.p./℃(ref.) | MSa(calcd.), m/z |
|---|---|---|---|
| 2a | White solid | 115.1—117.5(115.3—117.8)[ | 194(194) |
| 2b | Yellow solid | — | 225.0(225.1) |
| 2c | Light yellow solid | — | 225.0(225.1) |
| 2d | White solid | — | 225.0(225.1) |
| 2e | Brown solid | — | 255.0(255.1) |
| 2f | White solid | — | 272.9(273.0), 275.0(275.0)b |
| 2g | White solid | — | 208(208) |
| 2h | Off?white solid | 171.9—173.8(172.9—174.0)[ | 208(208) |
| 2i | Colorless oil | — | 146(146) |
| 2j | Yellow solid | 121.0—123.0(120.9—123.2)[ | 274(274), 276(276)b |
| 2k | Yellow solid | 122.6—125.1(122.2—125.6)[ | 212(212) |
| 2m | White solid | 157.1—159.5(157.2—159.3)[ | 180(180) |
| 2n | White solid | 70.2—71.9(70.2—71.9)[ | 194(194) |
| 2o | White solid | — | 194(194) |
| 4a | White solid | — | 168(168) |
| 4b | Light yellow solid | 93.2—94.(93.5—94.5)[ | 246(246), 248(248)b |
| 4c | White solid | 113.2—115.1(113—115)[ | 260(260), 262(262)b |
| 4d | White solid | — | 196(196) |
| 4e | White solid | — | 210(210) |
| 4f | White solid | 84.5—86.8(85.4—88.9)[ | 244(244) |
| 4g | Yellow solid | 130.2—131.9(130—132)[ | 268(268) |
| 4h | Light yellow solid | 114.0—116.3(114.1—116.1)[ | 218(218) |
| 4i | White solid | 86.8—88.3(87—88)[ | 174(174) |
| 4j | Colorless oil | — | 182(182) |
Table 1 Appearance, melting points and MS data for compounds 2a—2k, 2m—2o and 4a—4j
| Compd. | Appearance | m.p./℃(ref.) | MSa(calcd.), m/z |
|---|---|---|---|
| 2a | White solid | 115.1—117.5(115.3—117.8)[ | 194(194) |
| 2b | Yellow solid | — | 225.0(225.1) |
| 2c | Light yellow solid | — | 225.0(225.1) |
| 2d | White solid | — | 225.0(225.1) |
| 2e | Brown solid | — | 255.0(255.1) |
| 2f | White solid | — | 272.9(273.0), 275.0(275.0)b |
| 2g | White solid | — | 208(208) |
| 2h | Off?white solid | 171.9—173.8(172.9—174.0)[ | 208(208) |
| 2i | Colorless oil | — | 146(146) |
| 2j | Yellow solid | 121.0—123.0(120.9—123.2)[ | 274(274), 276(276)b |
| 2k | Yellow solid | 122.6—125.1(122.2—125.6)[ | 212(212) |
| 2m | White solid | 157.1—159.5(157.2—159.3)[ | 180(180) |
| 2n | White solid | 70.2—71.9(70.2—71.9)[ | 194(194) |
| 2o | White solid | — | 194(194) |
| 4a | White solid | — | 168(168) |
| 4b | Light yellow solid | 93.2—94.(93.5—94.5)[ | 246(246), 248(248)b |
| 4c | White solid | 113.2—115.1(113—115)[ | 260(260), 262(262)b |
| 4d | White solid | — | 196(196) |
| 4e | White solid | — | 210(210) |
| 4f | White solid | 84.5—86.8(85.4—88.9)[ | 244(244) |
| 4g | Yellow solid | 130.2—131.9(130—132)[ | 268(268) |
| 4h | Light yellow solid | 114.0—116.3(114.1—116.1)[ | 218(218) |
| 4i | White solid | 86.8—88.3(87—88)[ | 174(174) |
| 4j | Colorless oil | — | 182(182) |
| Compd. | Appearance | m.p./℃ | HRMS(calcd.)a, m/z |
|---|---|---|---|
| 3a | White solid | 95.0—97.1 | 277.1148(277.1147) |
| 3b | White solid | 59.5—62.0 | 275.0987(275.0990) |
| 3c | White solid | 47.0—49.1 | 275.0987(275.0990) |
| 3d | White solid | 79.0—81.2 | 307.1248(307.1253) |
| 3e | White solid | 124.2—126.3 | 305.1098(305.1096) |
| 3f | White solid | 110.2—112.4 | 355.0246(355.0252) |
| 3g | White solid | 84.0—85.6 | 291.1308(291.1303) |
| 3h | White solid | 66.1—67.8 | 291.1306(291.1303) |
| 3i | Colorless oil | — | 229.1147(229.1147) |
| 3j | White solid | 135.0—137.2 | 357.0044(357.0045) |
| 3k | Light yellow solid | 97.1—99.8 | 295.0714(295.0711) |
| 3l | Light yellow solid | 144.7—146.8 | 313.0614(313.0617) |
| 3m | White solid | 85.7—88.0 | 263.0987(263.0990) |
| 3n | White solid | 100.7—102.4 | 277.1144(277.1147) |
| 3o | White solid | 76.5—78.6 | 277.1145(277.1147) |
| 5a | White solid | 37.3—39.0 | 251.0987(251.0990) |
| 5b | White solid | 53.0—55.1 | 329.0096(329.0096) |
| 5c | White solid | 91.7—94.0 | 343.0246(343.0252) |
| 5d | Colorless oil | — | 279.1315(279.1303) |
| 5e | Colorless oil | — | 293.1458(293.1460) |
| 5f | White solid | 81.3—82.4 | 327.1300(327.1303) |
| 5g | White solid | 115.3—117.2 | 351.1301(351.1303) |
| 5h | White solid | 86.8—88.0 | 301.1144(301.1147) |
| 5i | Colorless oil | — | 257.0551(257.0555) |
| 5j | White solid | 37.0—39.3 | 265.1148(265.1147) |
Table 2 Appearance, melting points and HRMS data for compounds 3a—3o and 5a—5j
| Compd. | Appearance | m.p./℃ | HRMS(calcd.)a, m/z |
|---|---|---|---|
| 3a | White solid | 95.0—97.1 | 277.1148(277.1147) |
| 3b | White solid | 59.5—62.0 | 275.0987(275.0990) |
| 3c | White solid | 47.0—49.1 | 275.0987(275.0990) |
| 3d | White solid | 79.0—81.2 | 307.1248(307.1253) |
| 3e | White solid | 124.2—126.3 | 305.1098(305.1096) |
| 3f | White solid | 110.2—112.4 | 355.0246(355.0252) |
| 3g | White solid | 84.0—85.6 | 291.1308(291.1303) |
| 3h | White solid | 66.1—67.8 | 291.1306(291.1303) |
| 3i | Colorless oil | — | 229.1147(229.1147) |
| 3j | White solid | 135.0—137.2 | 357.0044(357.0045) |
| 3k | Light yellow solid | 97.1—99.8 | 295.0714(295.0711) |
| 3l | Light yellow solid | 144.7—146.8 | 313.0614(313.0617) |
| 3m | White solid | 85.7—88.0 | 263.0987(263.0990) |
| 3n | White solid | 100.7—102.4 | 277.1144(277.1147) |
| 3o | White solid | 76.5—78.6 | 277.1145(277.1147) |
| 5a | White solid | 37.3—39.0 | 251.0987(251.0990) |
| 5b | White solid | 53.0—55.1 | 329.0096(329.0096) |
| 5c | White solid | 91.7—94.0 | 343.0246(343.0252) |
| 5d | Colorless oil | — | 279.1315(279.1303) |
| 5e | Colorless oil | — | 293.1458(293.1460) |
| 5f | White solid | 81.3—82.4 | 327.1300(327.1303) |
| 5g | White solid | 115.3—117.2 | 351.1301(351.1303) |
| 5h | White solid | 86.8—88.0 | 301.1144(301.1147) |
| 5i | Colorless oil | — | 257.0551(257.0555) |
| 5j | White solid | 37.0—39.3 | 265.1148(265.1147) |
| Compd. | 1H NMR(500 MHZ, CDCl3), δ | 13C NMR(126 MHZ, CDCl3), δ | 19F NMR(471 MHZ, CDCl3), δ |
|---|---|---|---|
| 3a | 7.57—7.52(m, 1H), 7.32—7.26(m, 3H), 6.86(t, J=4.9 Hz, 1H), 6.39(t, J=54.3 Hz, 1H), 2.84—2.77(m, 2H), 2.51—2.44(m, 2H) | 137.2, 136.5, 129.1, 129.0, 128.5, 127.0, 123.2, 122.5, 110.1(t, J=3.0 Hz), 109.9(t, J=259.3 Hz),46.8(t, J=24.7 Hz), 27.3, 23.5 | -119.1(d, J=54.4 Hz) |
| 3b | 7.26(t, J=8.1 Hz, 1H), 7.18(d, J=7.9 Hz, 1H), 6.92(d, J=8.2 Hz, 1H), 6.86(t, J=4.9 Hz, 1H), 6.40(t, J=54.4 Hz, 1H), 3.86(s, 3H), 2.79(t, J=8.2 Hz, 2H), 2.41(td, J=8.2, 5.0 Hz, 2H) | 156.8, 136.7, 129.4, 127.2, 125.5, 123.1, 115.0, 111.6, 110.2(t, J=2.8 Hz), 109.9(d, J=259.1 Hz), 55.7, 47.1(t, J=24.8 Hz), 23.0, 18.9 | -119.3(d, J=54.5 Hz) |
| Compd. | 1H NMR(500 MHZ, CDCl3), δ | 13C NMR(126 MHZ, CDCl3), δ | 19F NMR(471 MHZ, CDCl3), δ |
| 3c | 7.50(d, J=8.3 Hz, 1H), 6.82(d, J=8.1 Hz, 2H), 6.73(t, J=4.9 Hz, 1H), 6.39(t, J=54.3 Hz, 1H), 3.85(s, 3H), 2.79(t, J=8.0 Hz, 2H), 2.46(td, J=8.0, 4.9 Hz, 2H) | 159.8, 139.4, 133.5, 123.9, 122.9, 121.4, 115.3, 111.3, 110.2(t, J=2.9 Hz), 109.9(t, J=259.3 Hz), 55.4, 46.8(t, J=24.7 Hz), 27.9, 23.4 | -118.6(d, J=53.9 Hz) |
| 3d | 7.18(d, J=8.3 Hz, 1H), 7.13(d, J=1.7 Hz, 1H), 6.87(t, J=4.8 Hz, 1H), 6.83(dd, J=8.3, 2.1 Hz, 1H), 6.40(t, J=54.3 Hz, 1H), 3.82(s, 3H), 2.72(t, J=7.9 Hz, 2H), 2.47—2.41(m, 2H) | 158.5, 137.3, 129.7, 129.4, 129.1, 123.2, 113.5, 110.2(t, J=2.7 Hz), 110.0(t, J=259.4 Hz), 109.9, 55.6, 47.0(t, J=24.7 Hz), 26.5, 24.0 | -119.1(d, J=54.3 Hz) |
| 3e | 7.15(s, 1H), 6.77(s, 1H), 6.71(t, J=4.9 Hz, 1H), 6.32(t, J=54.3 Hz, 1H), 3.91(s, 3H), 3.89(s, 3H), 2.72(t, J=8.1 Hz, 2H), 2.43(td, J=8.1, 7.7, 5.0 Hz, 2H) | 149.2, 147.4, 133.8, 130.6, 122.9, 120.9, 112.1, 110.3(t, J=259.8 Hz), 110.2(t, J=2.8 Hz), 107.2, 56.3, 56.0, 46.7(t, J=24.7 Hz), 27.1, 23.6 | -118.6(d, J=54.4 Hz) |
| 3f | 7.65(s, 1H), 7.43(dd, J=8.0, 1.7 Hz, 1H), 7.14(d, J=8.0 Hz, 1H), 6.92(t, J=4.9 Hz, 1H), 6.35(t, J=54.1 Hz, 1H), 2.75(t, J=8.0 Hz, 2H), 2.47(td, J=8.0, 5.0 Hz, 2H) | 138.2, 136.2, 132.1, 130.6, 130.5, 125.8, 122.6, 120.6, 109.9(t, J=259.8 Hz), 109.9(d, J=4.7 Hz), 26.9, 23.5 | -118.8(d, J=54.1 Hz) |
| 3g | 7.57(d, J=7.5 Hz, 1H), 7.37—7.27(m, 3H), 6.82—6.74(m, 1H), 6.41(t, J=54.3 Hz, 1H), 3.00—2.92(m, 1H), 2.61(ddd, J=17.3, 6.6, 4.2 Hz, 1H), 2.32(dt, J=17.2, 6.1 Hz, 1H), 1.21(d, J=7.0 Hz, 3H) | 142.2, 135.0, 129.5, 127.8, 127.6, 126.8, 122.6, 122.5, 110.1(dd, J=51.3, 4.3 Hz), 109.8(t, J=259.2 Hz), 46.8(t, J=24.8 Hz), 31.5, 31.0, 19.5 | -118.2(d, J=54.1 Hz), -118.8(d, J=54.6 Hz), -118.9(d, J=54.4 Hz), -119.5(d, J=54.0 Hz) |
| 3h | 7.67(dd, J=5.6, 3.5 Hz, 1H), 7.35(dt, J=7.4, 3.7 Hz, 2H), 7.32—7.28(m, 1H), 7.00(t, J=7.6 Hz, 1H), 5.87(t, J=54.1 Hz, 1H), 2.53(t, J=7.1 Hz, 2H), 2.15(p, J=7.1 Hz, 2H), 1.98(q, J=7.3 Hz, 2H) | 141.9, 138.6, 132.8, 129.9, 129.6, 127.1, 126.4, 125.2, 110.9(t, J=258.9 Hz), 110.3(t, J=3.0 Hz),47.7(t, J=24.9 Hz), 33.6, 31.4, 24.7 | -120.8(d, J=53.9 Hz) |
| 3i | 6.41(s, 1H), 5.94(t, J=54.4 Hz, 1H), 2.22(qd, J=8.5, 7.4, 3.2 Hz, 4H), 1.80—1.73(m, 2H), 1.69—1.62(m, 2H) | 134.5, 123.2, 111.2(t, J=260.1 Hz), 109.7(t, J=3.1 Hz), 48.7(t, J=24.2 Hz), 25.7, 25.5, 22.2, 20.9 | -118.8(d, J=54.3 Hz) |
| 3j | 7.62(d, J=2.1 Hz, 1H), 7.42(dd, J=8.7, 2.2 Hz, 1H), 6.88(d, J=8.6 Hz, 1H), 6.59(t, J=4.1 Hz, 1H), 6.34(t, J=54.0 Hz, 1H), 4.89(d, J=4.1 Hz, 2H) | 153.7, 134.3, 129.4, 125.6, 120.2, 119.5, 119.2, 114.2, 109.61(t, J=260.6 Hz), 108.97(t, J=2.9 Hz), 64.6, 45.71(t, J=25.7 Hz) | -117.7(d, J=54.0 Hz) |
| 3k | 7.73—7.69(m, 1H), 7.52—7.46(m, 1H), 7.30—7.25(m, 2H), 6.87(t, J=6.1 Hz, 1H), 6.24(t, J=54.1 Hz, 1H), 3.39(d, J=6.1 Hz, 2H) | 135.8, 129.9, 129.5, 129.12, 128.4, 126.3, 124.8, 124.5, 110.1(t, J=260.1 Hz), 109.9(t, J=2.9 Hz), 47.1(t, J=25.2 Hz), 24.9 | -118.4(d, J=54.0 Hz) |
| 3l | 7.52—7.44(m, 2H), 7.04(td, J=8.3, 2.5 Hz, 1H), 6.94(t, J=6.1 Hz, 1H), 6.23(t, J=54.1 Hz, 1H), 3.38(d, J=6.1 Hz, 2H) | 160.8(d, J=247.0 Hz), 131.6, 130.9(d, J=3.3 Hz), 130.6(d, J=7.9 Hz), 129.8(d, J=7.4 Hz), 124.6, 117.0(d, J=21.8 Hz), 112.3(d, J=24.9 Hz), 110.2(t, J=260.5 Hz), 109.7(t, J=2.9 Hz), 108.1, 47.1(t, J=25.5 Hz), 25.1 | -113.1(m, F), -118.5(d, J=54.1 Hz, 2F) |
| 3m | 7.69(d, J=7.6 Hz, 1H), 7.60(d, J=7.3 Hz, 1H), 7.44(t, J=7.3 Hz, 1H), 7.42—7.38(m, 1H), 7.13(t, J=2.1 Hz, 1H), 6.31(t, J=54.2 Hz, 1H), 3.65—3.59(m, 2H) | 144.2, 139.1, 138.2, 128.3, 127.1, 126.9, 124.8, 120.0, 110.5(t, J=260.7 Hz), 109.2(t, J=3.0 Hz), 43.1(t, J=25.8 Hz), 38.3 | -117.8(d, J=54.1 Hz) |
| 3n | 7.46—7.41(m, 2H), 7.18(d, J=7.8 Hz, 1H), 7.07(t, J=1.9 Hz, 1H), 6.29(t, J=54.2 Hz, 1H), 3.53(s, 2H), 2.45(s, 3H). | 141.4, 139.4, 138.6, 137.1, 128.2, 128.0, 124.6, 120.6, 110.5(t, J=260.6 Hz), 109.4(t, J=2.7 Hz), 43.3(t, J=25.9 Hz), 38.1, 21.8 | -118.0(d, J=54.2 Hz) |
| Compd. | 1H NMR(500 MHZ, CDCl3), δ | 13C NMR(126 MHZ, CDCl3), δ | 19F NMR(471 MHZ, CDCl3), δ |
| 3o | 7.68(d, J=7.8 Hz, 1H), 7.45(d, J=7.4 Hz, 1H), 7.34(t, J=7.6 Hz, 1H), 7.26(t, J=7.8 Hz, 1H), 6.23(t, J=54.4 Hz, 1H), 3.54(s, 2H), 2.47(s, 3H) | 150.5, 140.8, 140.7, 126.9, 125.8, 123.9, 119.2, 118.1, 110.6(t, J=260.4 Hz), 109.7(t, J=3.2 Hz), 45.6, 41.7(t, J=26.1 Hz), 15.3 | -118.9(d, J=54.4 Hz) |
| 5a | 7.49—7.42(m, 3H), 7.41—7.37(m, 2H), 6.14(s, 1H), 5.86(t, J=53.9 Hz, 1H), 5.73(s, 1H) | 134.9, 134.4, 130.0, 129.2, 128.7, 125.4, 110.5(t, J=259.2 Hz), 109.5(t, J=2.9 Hz),48.2(t, J=25.1 Hz) | -120.4(d, J=54.3 Hz) |
| 5b | 7.59(d, J=8.4 Hz, 2H), 7.27(d, J=8.3 Hz, 2H), 6.15(s, 1H), 5.87(t, J=53.9 Hz, 1H), 5.75(s, 1H) | 134.0, 133.2, 132.4, 130.3, 126.0, 124.6, 110.5(t, J=259.6 Hz), 109.3(t, J=2.9 Hz), 48.0(t, J=25.3 Hz) | -120.5(d, J=53.8 Hz) |
| 5c | 7.66—7.60(m, 2H), 7.17—7.10(m, 2H), 6.65(q, J=6.8 Hz, 1H), 5.78(t, J=53.9 Hz, 1H), 1.63(d, J=6.8 Hz, 3H) | 136.2, 132.8, 131.6, 131.0, 125.7, 124.2, 110.6(t, J=258.8 Hz), 109.5(t, J=2.9 Hz), 48.9(t, J=25.0 Hz), 15.3 | -120.8(d, J=53.8 Hz) |
| 5d | 7.46(dd, J=5.1, 1.9 Hz, 3H), 7.23(dd, J=6.6, 2.8 Hz, 2H), 6.52(t, J=7.4 Hz, 1H), 5.76(t, J=54.0 Hz, 1H), 1.93(p, J=7.5 Hz, 2H), 0.99(t, J=7.5 Hz, 3H) | 141.9, 132.2, 129.9, 129.6, 129.4, 125.5, 110.6(t, J=258.4 Hz), 109.7(t, J=2.8 Hz), 49.2(t, J=24.9 Hz), 22.9, 13.2 | -121.7(d, J=53.8 Hz) |
| 5e | 7.48—7.44(m, 3H), 7.23(dd, J=6.6, 2.9 Hz, 2H), 6.30(d, J=10.1 Hz, 1H), 5.75(t, J=54.0 Hz, 1H), 2.15(dp, J=10.1, 6.6 Hz, 1H), 0.97(d, J=6.6 Hz, 6H) | 146.9, 132.3, 129.8, 129.6, 129.3, 123.9, 110.6(t, J=258.4 Hz), 109.7(t, J=2.9 Hz), 49.2(t, J=24.8 Hz), 29.0, 22.0, 21.7 | -121.8(d, J=54.0 Hz) |
| 5f | 7.57—7.50(m, 3H), 7.40—7.33(m, 3H), 7.26(t, J=7.3 Hz, 1H), 7.19(t, J=7.6 Hz, 2H), 6.97(d, J=7.6 Hz, 2H), 5.89(t, J=53.9 Hz, 1H) | 137.3, 133.2, 132.7, 130.1, 130.1, 130.0, 129.8, 129.3, 128.5, 125.4, 110.8(t, J=258.9 Hz),109.7(t, J=2.8 Hz), 50.2(t, J=25.0 Hz) | -121.5(d, J=54.0 Hz) |
| 5g | 8.76(s, 1H), 8.69(d, J=8.2 Hz, 1H), 7.99(d, J=8.2 Hz, 1H), 7.96(d, J=7.8 Hz, 1H), 7.87(d, J=8.8 Hz, 1H), 7.79(d, J=8.8 Hz, 1H), 7.74(t, J=7.6 Hz, 1H), 7.69(t, J=7.0 Hz, 1H), 7.63(dd, J=8.2, 1.5 Hz, 2H), 6.31(s, 1H), 5.95(t, J=53.8 Hz, 1H), 5.92(s, 1H) | 135.2, 132.6, 132.4, 132.3, 130.3, 129.8, 129.6, 128.9, 128.8, 127.5, 127.3, 126.3, 126.1, 125.8, 123.3, 122.6, 110.7(t, J=259.3 Hz), 109.7(t, J=2.9 Hz), 48.5(t, J=25.1 Hz) | -120.8(d, J=53.8 Hz) |
| 5h | 7.91(d, J=8.5 Hz, 1H), 7.90—7.87(m, 3H), 7.61—7.54(m, 2H), 7.47(dd, J=8.4, 1.9 Hz, 1H), 6.22(d, J=1.0 Hz, 1H), 5.90(t, J=53.9 Hz, 1H), 5.83(s, 1H) | 135.0, 133.5, 132.9, 131.7, 129.2, 128.5, 128.4, 127.8, 127.6, 127.3, 125.7, 125.5, 110.6(t, J=259.3 Hz), 109.6(t, J=2.9 Hz), 48.3(t, J=25.2 Hz) | -120.8(d, J=53.9 Hz) |
| 5i | 7.43—7.39(m, 1H), 7.35(d, J=3.6 Hz, 1H), 7.10(dd, J=5.2, 3.7 Hz, 1H), 6.09(s, 1H), 6.08(t, J=54.2 Hz, 1H), 5.95(d, J=1.5 Hz, 1H) | 135.0, 128.2, 128.0, 127.9, 127.8, 124.9, 110.4(t, J=260.0 Hz), 109.4(t, J=2.9 Hz), 48.1(d, J=25.3 Hz) | -119.5(d, J=54.5 Hz) |
| 5j | 7.47—7.33(m, 5H), 6.17(t, J=54.2 Hz, 1H), 5.67—5.53(m, 1H), 2.47(d, J=1.4 Hz, 3H) | 150.9, 140.6, 129.4, 128.8, 126.1, 111.3(t, J=259.1 Hz), 109.7(t, J=3.1 Hz), 109.2(t, J=2.7 Hz), 40.1(t, J=25.1 Hz), 18.5 | -120.5(d, J=54.0 Hz) |
Table 3 1H NMR, 13C NMR and 19F NMR data for compounds 3a—3o and 5a—5j
| Compd. | 1H NMR(500 MHZ, CDCl3), δ | 13C NMR(126 MHZ, CDCl3), δ | 19F NMR(471 MHZ, CDCl3), δ |
|---|---|---|---|
| 3a | 7.57—7.52(m, 1H), 7.32—7.26(m, 3H), 6.86(t, J=4.9 Hz, 1H), 6.39(t, J=54.3 Hz, 1H), 2.84—2.77(m, 2H), 2.51—2.44(m, 2H) | 137.2, 136.5, 129.1, 129.0, 128.5, 127.0, 123.2, 122.5, 110.1(t, J=3.0 Hz), 109.9(t, J=259.3 Hz),46.8(t, J=24.7 Hz), 27.3, 23.5 | -119.1(d, J=54.4 Hz) |
| 3b | 7.26(t, J=8.1 Hz, 1H), 7.18(d, J=7.9 Hz, 1H), 6.92(d, J=8.2 Hz, 1H), 6.86(t, J=4.9 Hz, 1H), 6.40(t, J=54.4 Hz, 1H), 3.86(s, 3H), 2.79(t, J=8.2 Hz, 2H), 2.41(td, J=8.2, 5.0 Hz, 2H) | 156.8, 136.7, 129.4, 127.2, 125.5, 123.1, 115.0, 111.6, 110.2(t, J=2.8 Hz), 109.9(d, J=259.1 Hz), 55.7, 47.1(t, J=24.8 Hz), 23.0, 18.9 | -119.3(d, J=54.5 Hz) |
| Compd. | 1H NMR(500 MHZ, CDCl3), δ | 13C NMR(126 MHZ, CDCl3), δ | 19F NMR(471 MHZ, CDCl3), δ |
| 3c | 7.50(d, J=8.3 Hz, 1H), 6.82(d, J=8.1 Hz, 2H), 6.73(t, J=4.9 Hz, 1H), 6.39(t, J=54.3 Hz, 1H), 3.85(s, 3H), 2.79(t, J=8.0 Hz, 2H), 2.46(td, J=8.0, 4.9 Hz, 2H) | 159.8, 139.4, 133.5, 123.9, 122.9, 121.4, 115.3, 111.3, 110.2(t, J=2.9 Hz), 109.9(t, J=259.3 Hz), 55.4, 46.8(t, J=24.7 Hz), 27.9, 23.4 | -118.6(d, J=53.9 Hz) |
| 3d | 7.18(d, J=8.3 Hz, 1H), 7.13(d, J=1.7 Hz, 1H), 6.87(t, J=4.8 Hz, 1H), 6.83(dd, J=8.3, 2.1 Hz, 1H), 6.40(t, J=54.3 Hz, 1H), 3.82(s, 3H), 2.72(t, J=7.9 Hz, 2H), 2.47—2.41(m, 2H) | 158.5, 137.3, 129.7, 129.4, 129.1, 123.2, 113.5, 110.2(t, J=2.7 Hz), 110.0(t, J=259.4 Hz), 109.9, 55.6, 47.0(t, J=24.7 Hz), 26.5, 24.0 | -119.1(d, J=54.3 Hz) |
| 3e | 7.15(s, 1H), 6.77(s, 1H), 6.71(t, J=4.9 Hz, 1H), 6.32(t, J=54.3 Hz, 1H), 3.91(s, 3H), 3.89(s, 3H), 2.72(t, J=8.1 Hz, 2H), 2.43(td, J=8.1, 7.7, 5.0 Hz, 2H) | 149.2, 147.4, 133.8, 130.6, 122.9, 120.9, 112.1, 110.3(t, J=259.8 Hz), 110.2(t, J=2.8 Hz), 107.2, 56.3, 56.0, 46.7(t, J=24.7 Hz), 27.1, 23.6 | -118.6(d, J=54.4 Hz) |
| 3f | 7.65(s, 1H), 7.43(dd, J=8.0, 1.7 Hz, 1H), 7.14(d, J=8.0 Hz, 1H), 6.92(t, J=4.9 Hz, 1H), 6.35(t, J=54.1 Hz, 1H), 2.75(t, J=8.0 Hz, 2H), 2.47(td, J=8.0, 5.0 Hz, 2H) | 138.2, 136.2, 132.1, 130.6, 130.5, 125.8, 122.6, 120.6, 109.9(t, J=259.8 Hz), 109.9(d, J=4.7 Hz), 26.9, 23.5 | -118.8(d, J=54.1 Hz) |
| 3g | 7.57(d, J=7.5 Hz, 1H), 7.37—7.27(m, 3H), 6.82—6.74(m, 1H), 6.41(t, J=54.3 Hz, 1H), 3.00—2.92(m, 1H), 2.61(ddd, J=17.3, 6.6, 4.2 Hz, 1H), 2.32(dt, J=17.2, 6.1 Hz, 1H), 1.21(d, J=7.0 Hz, 3H) | 142.2, 135.0, 129.5, 127.8, 127.6, 126.8, 122.6, 122.5, 110.1(dd, J=51.3, 4.3 Hz), 109.8(t, J=259.2 Hz), 46.8(t, J=24.8 Hz), 31.5, 31.0, 19.5 | -118.2(d, J=54.1 Hz), -118.8(d, J=54.6 Hz), -118.9(d, J=54.4 Hz), -119.5(d, J=54.0 Hz) |
| 3h | 7.67(dd, J=5.6, 3.5 Hz, 1H), 7.35(dt, J=7.4, 3.7 Hz, 2H), 7.32—7.28(m, 1H), 7.00(t, J=7.6 Hz, 1H), 5.87(t, J=54.1 Hz, 1H), 2.53(t, J=7.1 Hz, 2H), 2.15(p, J=7.1 Hz, 2H), 1.98(q, J=7.3 Hz, 2H) | 141.9, 138.6, 132.8, 129.9, 129.6, 127.1, 126.4, 125.2, 110.9(t, J=258.9 Hz), 110.3(t, J=3.0 Hz),47.7(t, J=24.9 Hz), 33.6, 31.4, 24.7 | -120.8(d, J=53.9 Hz) |
| 3i | 6.41(s, 1H), 5.94(t, J=54.4 Hz, 1H), 2.22(qd, J=8.5, 7.4, 3.2 Hz, 4H), 1.80—1.73(m, 2H), 1.69—1.62(m, 2H) | 134.5, 123.2, 111.2(t, J=260.1 Hz), 109.7(t, J=3.1 Hz), 48.7(t, J=24.2 Hz), 25.7, 25.5, 22.2, 20.9 | -118.8(d, J=54.3 Hz) |
| 3j | 7.62(d, J=2.1 Hz, 1H), 7.42(dd, J=8.7, 2.2 Hz, 1H), 6.88(d, J=8.6 Hz, 1H), 6.59(t, J=4.1 Hz, 1H), 6.34(t, J=54.0 Hz, 1H), 4.89(d, J=4.1 Hz, 2H) | 153.7, 134.3, 129.4, 125.6, 120.2, 119.5, 119.2, 114.2, 109.61(t, J=260.6 Hz), 108.97(t, J=2.9 Hz), 64.6, 45.71(t, J=25.7 Hz) | -117.7(d, J=54.0 Hz) |
| 3k | 7.73—7.69(m, 1H), 7.52—7.46(m, 1H), 7.30—7.25(m, 2H), 6.87(t, J=6.1 Hz, 1H), 6.24(t, J=54.1 Hz, 1H), 3.39(d, J=6.1 Hz, 2H) | 135.8, 129.9, 129.5, 129.12, 128.4, 126.3, 124.8, 124.5, 110.1(t, J=260.1 Hz), 109.9(t, J=2.9 Hz), 47.1(t, J=25.2 Hz), 24.9 | -118.4(d, J=54.0 Hz) |
| 3l | 7.52—7.44(m, 2H), 7.04(td, J=8.3, 2.5 Hz, 1H), 6.94(t, J=6.1 Hz, 1H), 6.23(t, J=54.1 Hz, 1H), 3.38(d, J=6.1 Hz, 2H) | 160.8(d, J=247.0 Hz), 131.6, 130.9(d, J=3.3 Hz), 130.6(d, J=7.9 Hz), 129.8(d, J=7.4 Hz), 124.6, 117.0(d, J=21.8 Hz), 112.3(d, J=24.9 Hz), 110.2(t, J=260.5 Hz), 109.7(t, J=2.9 Hz), 108.1, 47.1(t, J=25.5 Hz), 25.1 | -113.1(m, F), -118.5(d, J=54.1 Hz, 2F) |
| 3m | 7.69(d, J=7.6 Hz, 1H), 7.60(d, J=7.3 Hz, 1H), 7.44(t, J=7.3 Hz, 1H), 7.42—7.38(m, 1H), 7.13(t, J=2.1 Hz, 1H), 6.31(t, J=54.2 Hz, 1H), 3.65—3.59(m, 2H) | 144.2, 139.1, 138.2, 128.3, 127.1, 126.9, 124.8, 120.0, 110.5(t, J=260.7 Hz), 109.2(t, J=3.0 Hz), 43.1(t, J=25.8 Hz), 38.3 | -117.8(d, J=54.1 Hz) |
| 3n | 7.46—7.41(m, 2H), 7.18(d, J=7.8 Hz, 1H), 7.07(t, J=1.9 Hz, 1H), 6.29(t, J=54.2 Hz, 1H), 3.53(s, 2H), 2.45(s, 3H). | 141.4, 139.4, 138.6, 137.1, 128.2, 128.0, 124.6, 120.6, 110.5(t, J=260.6 Hz), 109.4(t, J=2.7 Hz), 43.3(t, J=25.9 Hz), 38.1, 21.8 | -118.0(d, J=54.2 Hz) |
| Compd. | 1H NMR(500 MHZ, CDCl3), δ | 13C NMR(126 MHZ, CDCl3), δ | 19F NMR(471 MHZ, CDCl3), δ |
| 3o | 7.68(d, J=7.8 Hz, 1H), 7.45(d, J=7.4 Hz, 1H), 7.34(t, J=7.6 Hz, 1H), 7.26(t, J=7.8 Hz, 1H), 6.23(t, J=54.4 Hz, 1H), 3.54(s, 2H), 2.47(s, 3H) | 150.5, 140.8, 140.7, 126.9, 125.8, 123.9, 119.2, 118.1, 110.6(t, J=260.4 Hz), 109.7(t, J=3.2 Hz), 45.6, 41.7(t, J=26.1 Hz), 15.3 | -118.9(d, J=54.4 Hz) |
| 5a | 7.49—7.42(m, 3H), 7.41—7.37(m, 2H), 6.14(s, 1H), 5.86(t, J=53.9 Hz, 1H), 5.73(s, 1H) | 134.9, 134.4, 130.0, 129.2, 128.7, 125.4, 110.5(t, J=259.2 Hz), 109.5(t, J=2.9 Hz),48.2(t, J=25.1 Hz) | -120.4(d, J=54.3 Hz) |
| 5b | 7.59(d, J=8.4 Hz, 2H), 7.27(d, J=8.3 Hz, 2H), 6.15(s, 1H), 5.87(t, J=53.9 Hz, 1H), 5.75(s, 1H) | 134.0, 133.2, 132.4, 130.3, 126.0, 124.6, 110.5(t, J=259.6 Hz), 109.3(t, J=2.9 Hz), 48.0(t, J=25.3 Hz) | -120.5(d, J=53.8 Hz) |
| 5c | 7.66—7.60(m, 2H), 7.17—7.10(m, 2H), 6.65(q, J=6.8 Hz, 1H), 5.78(t, J=53.9 Hz, 1H), 1.63(d, J=6.8 Hz, 3H) | 136.2, 132.8, 131.6, 131.0, 125.7, 124.2, 110.6(t, J=258.8 Hz), 109.5(t, J=2.9 Hz), 48.9(t, J=25.0 Hz), 15.3 | -120.8(d, J=53.8 Hz) |
| 5d | 7.46(dd, J=5.1, 1.9 Hz, 3H), 7.23(dd, J=6.6, 2.8 Hz, 2H), 6.52(t, J=7.4 Hz, 1H), 5.76(t, J=54.0 Hz, 1H), 1.93(p, J=7.5 Hz, 2H), 0.99(t, J=7.5 Hz, 3H) | 141.9, 132.2, 129.9, 129.6, 129.4, 125.5, 110.6(t, J=258.4 Hz), 109.7(t, J=2.8 Hz), 49.2(t, J=24.9 Hz), 22.9, 13.2 | -121.7(d, J=53.8 Hz) |
| 5e | 7.48—7.44(m, 3H), 7.23(dd, J=6.6, 2.9 Hz, 2H), 6.30(d, J=10.1 Hz, 1H), 5.75(t, J=54.0 Hz, 1H), 2.15(dp, J=10.1, 6.6 Hz, 1H), 0.97(d, J=6.6 Hz, 6H) | 146.9, 132.3, 129.8, 129.6, 129.3, 123.9, 110.6(t, J=258.4 Hz), 109.7(t, J=2.9 Hz), 49.2(t, J=24.8 Hz), 29.0, 22.0, 21.7 | -121.8(d, J=54.0 Hz) |
| 5f | 7.57—7.50(m, 3H), 7.40—7.33(m, 3H), 7.26(t, J=7.3 Hz, 1H), 7.19(t, J=7.6 Hz, 2H), 6.97(d, J=7.6 Hz, 2H), 5.89(t, J=53.9 Hz, 1H) | 137.3, 133.2, 132.7, 130.1, 130.1, 130.0, 129.8, 129.3, 128.5, 125.4, 110.8(t, J=258.9 Hz),109.7(t, J=2.8 Hz), 50.2(t, J=25.0 Hz) | -121.5(d, J=54.0 Hz) |
| 5g | 8.76(s, 1H), 8.69(d, J=8.2 Hz, 1H), 7.99(d, J=8.2 Hz, 1H), 7.96(d, J=7.8 Hz, 1H), 7.87(d, J=8.8 Hz, 1H), 7.79(d, J=8.8 Hz, 1H), 7.74(t, J=7.6 Hz, 1H), 7.69(t, J=7.0 Hz, 1H), 7.63(dd, J=8.2, 1.5 Hz, 2H), 6.31(s, 1H), 5.95(t, J=53.8 Hz, 1H), 5.92(s, 1H) | 135.2, 132.6, 132.4, 132.3, 130.3, 129.8, 129.6, 128.9, 128.8, 127.5, 127.3, 126.3, 126.1, 125.8, 123.3, 122.6, 110.7(t, J=259.3 Hz), 109.7(t, J=2.9 Hz), 48.5(t, J=25.1 Hz) | -120.8(d, J=53.8 Hz) |
| 5h | 7.91(d, J=8.5 Hz, 1H), 7.90—7.87(m, 3H), 7.61—7.54(m, 2H), 7.47(dd, J=8.4, 1.9 Hz, 1H), 6.22(d, J=1.0 Hz, 1H), 5.90(t, J=53.9 Hz, 1H), 5.83(s, 1H) | 135.0, 133.5, 132.9, 131.7, 129.2, 128.5, 128.4, 127.8, 127.6, 127.3, 125.7, 125.5, 110.6(t, J=259.3 Hz), 109.6(t, J=2.9 Hz), 48.3(t, J=25.2 Hz) | -120.8(d, J=53.9 Hz) |
| 5i | 7.43—7.39(m, 1H), 7.35(d, J=3.6 Hz, 1H), 7.10(dd, J=5.2, 3.7 Hz, 1H), 6.09(s, 1H), 6.08(t, J=54.2 Hz, 1H), 5.95(d, J=1.5 Hz, 1H) | 135.0, 128.2, 128.0, 127.9, 127.8, 124.9, 110.4(t, J=260.0 Hz), 109.4(t, J=2.9 Hz), 48.1(d, J=25.3 Hz) | -119.5(d, J=54.5 Hz) |
| 5j | 7.47—7.33(m, 5H), 6.17(t, J=54.2 Hz, 1H), 5.67—5.53(m, 1H), 2.47(d, J=1.4 Hz, 3H) | 150.9, 140.6, 129.4, 128.8, 126.1, 111.3(t, J=259.1 Hz), 109.7(t, J=3.1 Hz), 109.2(t, J=2.7 Hz), 40.1(t, J=25.1 Hz), 18.5 | -120.5(d, J=54.0 Hz) |
| Entry | Product | Yieldb(%) | Entry | Product | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | 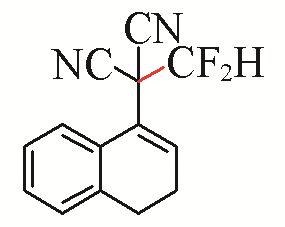 | 94 | 4 | 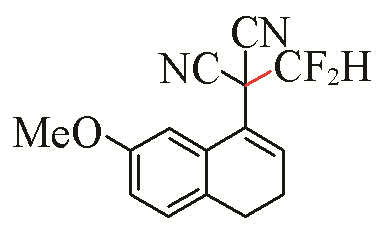 | 99 |
| 3a | 3d | ||||
| 2 | 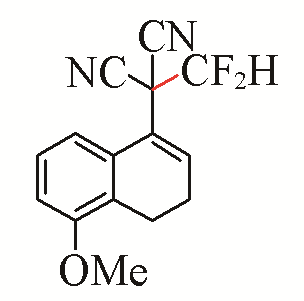 | 79 | 5 | 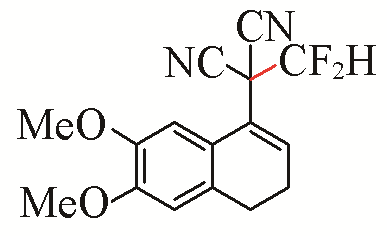 | 79 |
| 3b | 3e | ||||
| 3 | 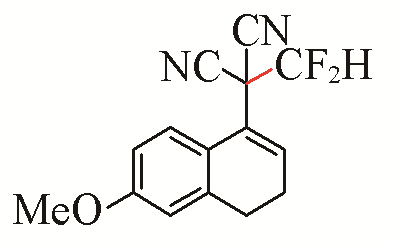 | 72 | 6 | 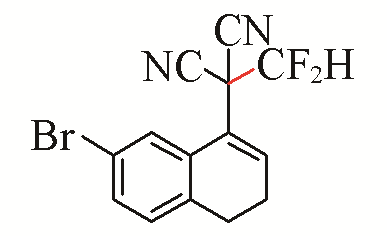 | 81 |
| 3c | 3f | ||||
| Entry | Product | Yieldb(%) | Entry | Product | Yieldb(%) |
| 7 | 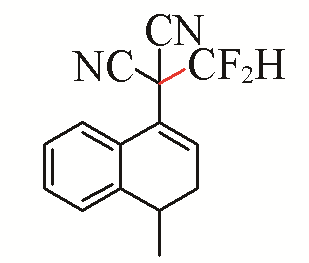 | 92 | 17 | 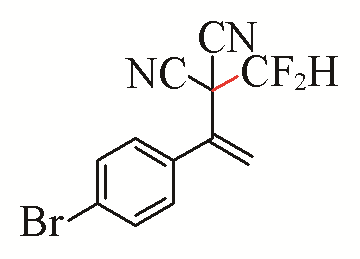 | 54 |
| 3g | 5b | ||||
| 8 | 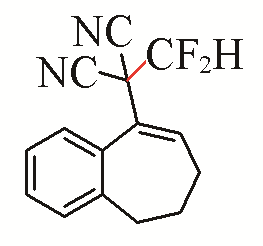 | 85 | 18 | 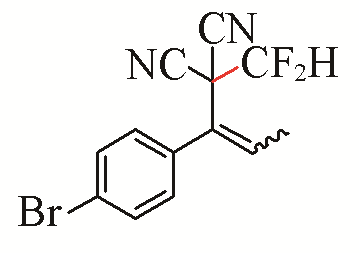 | 81 Z/E=4:1 |
| 3h | 5c | ||||
| 9 | 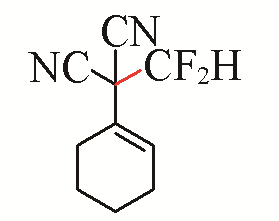 | 51 | 19 | 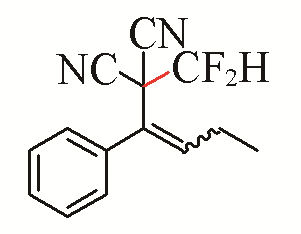 | 77 Z/E=9:1 |
| 3i | 5d | ||||
| 10 | 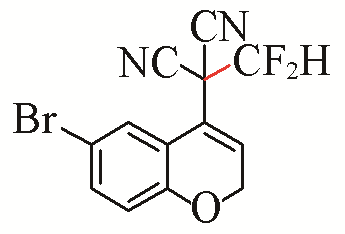 | 66 | 20 | 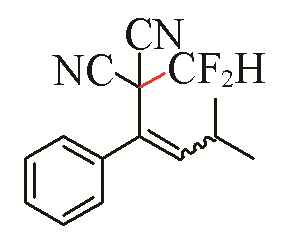 | 79 Z/E=8:1 |
| 3j | 5e | ||||
| 11 | 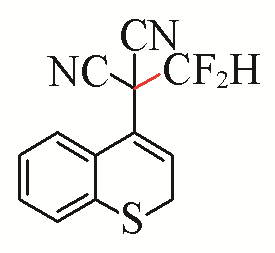 | 82 | 21 | 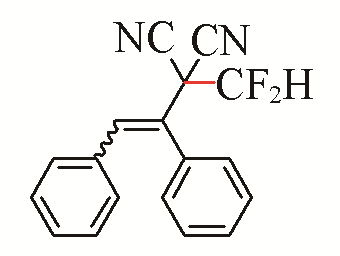 | 55 E/Z>99:1 |
| 3k | 5f | ||||
| 12 | 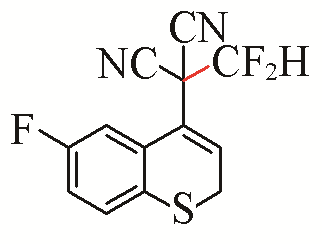 | 74 | 22 | 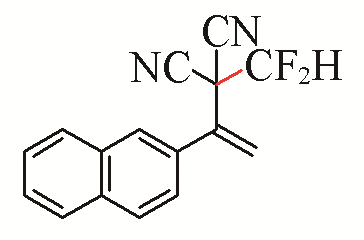 | 51 |
| 3l | 5g | ||||
| 13 | 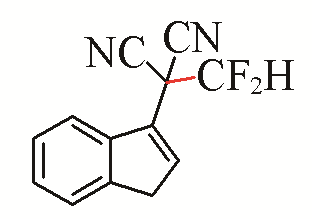 | 62 | 23 | 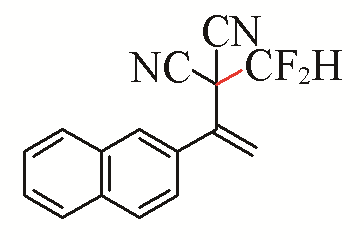 | 44 |
| 3m | 5h | ||||
| 14 | 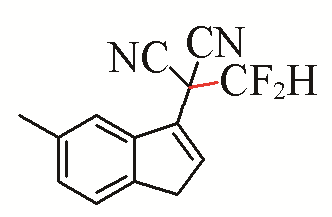 | 49 | 24 | 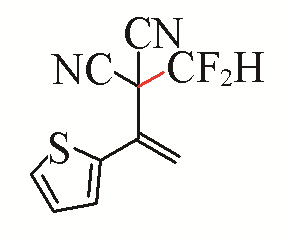 | 48 |
| 3n | 5i | ||||
| 15 | 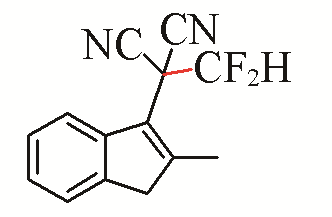 | 72 | 25 | 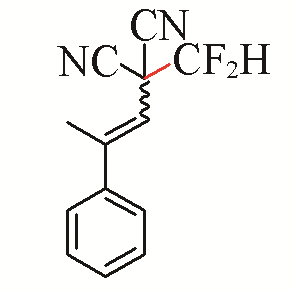 | 43 E/Z>99:1 |
| 3o | 5j | ||||
| 16 | 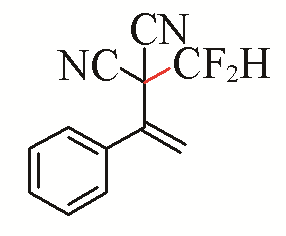 | 55 | |||
| 5a |
Table 5 Substrate scope of difluoromethylation of dicyanoalkylidenesa
| Entry | Product | Yieldb(%) | Entry | Product | Yieldb(%) |
|---|---|---|---|---|---|
| 1 |  | 94 | 4 |  | 99 |
| 3a | 3d | ||||
| 2 |  | 79 | 5 |  | 79 |
| 3b | 3e | ||||
| 3 |  | 72 | 6 |  | 81 |
| 3c | 3f | ||||
| Entry | Product | Yieldb(%) | Entry | Product | Yieldb(%) |
| 7 |  | 92 | 17 |  | 54 |
| 3g | 5b | ||||
| 8 |  | 85 | 18 |  | 81 Z/E=4:1 |
| 3h | 5c | ||||
| 9 |  | 51 | 19 |  | 77 Z/E=9:1 |
| 3i | 5d | ||||
| 10 |  | 66 | 20 |  | 79 Z/E=8:1 |
| 3j | 5e | ||||
| 11 |  | 82 | 21 |  | 55 E/Z>99:1 |
| 3k | 5f | ||||
| 12 |  | 74 | 22 |  | 51 |
| 3l | 5g | ||||
| 13 |  | 62 | 23 |  | 44 |
| 3m | 5h | ||||
| 14 |  | 49 | 24 |  | 48 |
| 3n | 5i | ||||
| 15 |  | 72 | 25 |  | 43 E/Z>99:1 |
| 3o | 5j | ||||
| 16 |  | 55 | |||
| 5a |
| 1 | Smart B. E., Chem. Rev.,1996, 96, 1555—1556 |
| 2 | Furuya T., Kuttruff C., Ritter T., Curr. Opin. Drug Discovery Dev.,2008, 11, 803—819 |
| 3 | Ojima I., Fluorine in Medicinal Chemistry and Chemical Biology, Blackwell, Oxford, 2009,199—212 |
| 4 | Wang J., Sánchez⁃Roselló M., Aceña J. L., del Pozo C., Sorochinsky A. E., Fustero S., Soloshonok V. A., Liu H., Chem. Rev., 2014, 114, 2432—2506 |
| 5 | Bassetto M., Ferla S., Pertusati F., Future Med. Chem.,2015, 7, 527—546 |
| 6 | Goure W. F., Leschinsky K. L., Wratten S. J., Chupp J. P., J. Agric. Food Chem.,1991, 39, 981—986 |
| 7 | Pérez R. A., Sánchez⁃Brunete C., Miguel E., Tadeo J. L., J. Agric. Food Chem.,1998, 46, 1864—1869 |
| 8 | Kirsch P., Bremer B., Angew. Chem. Int. Ed.,2000, 39, 4216—4239 |
| 9 | Tasaka T., Takenaka S., Kabu K., Morita Y., Okamoto H., Ferroelectronics, 2002, 276, 83—92 |
| 10 | Boltalina O. V., Nakajima T., New Fluorinated Carbons: Fundamentals and Applications, Elsevier, Amsterdam, 2016, 305—323 |
| 11 | Prakash G. K. S., Mandal M., Schweizer S., Petasis N. A., Olah G. A., J. Org. Chem., 2002, 67, 3718—3723 |
| 12 | Narjes F., Koehler K. F., Koch U., Gerlach B., Colarusso S., Steink_hler C., Brunetti M., Altamura S., de Francesco R., Matassa V. G., Bioorg. Med. Chem. Lett., 2002, 12(4), 701—704 |
| 13 | Hu J., Zhang W., Wang F., Chem. Commun., 2009, 7465—7578 |
| 14 | Li Y., Hu J., Angew. Chem. Int. Ed., 2005, 44, 5882—5886 |
| 15 | Erickson J. A., McLoughlin J. I., J. Org. Chem., 1995, 60, 1626—1631 |
| 16 | Zafrani Y., Yeffet D., Sod⁃Moriah G., Berliner A., Amir D., Marciano D., Gershonov E., Saphier S., J. Med. Chem.,2017, 60, 797—804 |
| 17 | Prakash G. K. S., Weber C., Chacko S., Olah G. A., Org. Lett.,2007, 9(10), 1863—1866 |
| 18 | Prakash G. K. S., Zhang Z., Wang F., Ni C., Olah G. A., J. Fluorine Chem., 2011, 132(10), 792—798 |
| 19 | Zhang W., Wang F., Hu J., Org. Lett., 2009, 11(10), 2109—2112 |
| 20 | Zhu J., Liu Y., Shen Q., Angew. Chem. Int. Ed., 2016, 55, 9050—9054 |
| 21 | Lu S. L., Li X., Qin W. B., Liu J. J., Huang Y. Y., Wong H. N. C., Liu G. K, Org. Lett., 2018, 20, 6925—6929 |
| 22 | Liu G. K., Li X., Qin W. B., Peng X. S., Wong H. N. C., Zhang L., Zhang X., Chem. Commun., 2019, 55, 7446—7449 |
| 23 | Liu G. K., Qin W. B., Li X., Lin L. T., Wong H. N. C., J. Org. Chem., 2019, 84(24), 15948—15957 |
| 24 | Liu G. K., Li X., Qin W. B., Lin W. F., Lin L. T., Chen J. Y., Liu J. J., Chin. Chem. Lett., 2019, 30, 1515—1518 |
| 25 | Xue D., Chen Y. C., Cui X., Wang Q. W., Zhu J., Deng J. G., J. Org. Chem.,2005, 70, 3584—3591 |
| 26 | Liu G. K., Wang X., Lu X., Xu X. H., Tokunaga E., Shibata N., Chemistry Open, 2012, 1, 227—231 |
| 27 | Barnes D. M., Haight A. R., Hameury T., McLaughlin M. A., Mei J., Tedrow J. S., Riva Toma J. D., Tetrahedron, 2006, 62, 11311—11319 |
| 28 | Chen H., Zhao S., Cheng S., Dai X., Xu X., Yuan W., Zhang X., J. Heterocyclic Chem.,2019, 56, 1672—1683 |
| 29 | Aksu K., Özgeriş B., Tümer F., Org. Commun.,2019, 12, 38—42 |
| 30 | Matsnev A., Noritake S., Nomura Y., Tokunaga E., Nakamura S., Shibata N., Angew. Chem. Int. Ed., 2010, 49, 572—576 |
| [1] | 何贝贝, 杨葵华, 吕瑞. 锰-铜双金属层状硅酸盐纳米酶的构筑及类酶活性[J]. 高等学校化学学报, 2022, 43(8): 20220150. |
| [2] | 林中樵, 陈佩佩, 王蕾. 不同化学结构盐皮质激素受体拮抗剂在心血管疾病中的应用[J]. 高等学校化学学报, 2022, 43(6): 20220059. |
| [3] | 陈瀚翔, 边绍菊, 胡斌, 李武. LiCl-NaCl-KCl-H2O溶液体系渗透压的分子动力学模拟[J]. 高等学校化学学报, 2022, 43(3): 20210727. |
| [4] | 周宁, 唐小华, 曹红, 查飞, 李春, 谢春燕, 徐明平, 孙艺格. 石榴状凝胶微球固定化漆酶的制备、 表征及降解双酚A[J]. 高等学校化学学报, 2022, 43(2): 20210705. |
| [5] | 朱浩天, 金美秀, 唐文思, 苏芳, 李阳光. 过渡金属-联咪唑-Dawson型钨磷酸盐杂化化合物的酶固定化性能[J]. 高等学校化学学报, 2022, 43(11): 20220328. |
| [6] | 张太文, 郭军, 张丹, 袁常梅, 邱双艳. trz-Cl-Cu-PMo12的合成、 表征及催化氧化碘离子性能[J]. 高等学校化学学报, 2022, 43(10): 20220215. |
| [7] | 李伦, 张静妍, 罗静, 刘仁, 朱乙. UV/Vis-LED激发的香豆素吡啶鎓盐光引发剂的合成及性能[J]. 高等学校化学学报, 2022, 43(10): 20220178. |
| [8] | 李波, 孟禹汐, 王雯雯, 臧宏瑛. 多核多氧硫钼酸盐化合物的合成及质子传导性能[J]. 高等学校化学学报, 2022, 43(1): 20210657. |
| [9] | 王进, 石文杰, 金邻豫, 马鹏涛, 王敬平, 牛景杨. 两种砷钼杂化多酸盐的合成、 结构及变色性质[J]. 高等学校化学学报, 2022, 43(1): 20210600. |
| [10] | 陈慧娜, 李新雄, 郑寿添. 铌多酸三维框架材料的研究进展[J]. 高等学校化学学报, 2022, 43(1): 20210625. |
| [11] | 梁宇, 刘欢, 宫丽阁, 王春晓, 王春梅, 于凯, 周百斌. 联咪唑修饰{SiW12O40}杂化物的合成及超级电容性能[J]. 高等学校化学学报, 2022, 43(1): 20210556. |
| [12] | 蒋君, 宫田田, 张成鹏, 刘晓倩, 赵俊伟. 吡啶二羧酸修饰的稀土嵌入碲钨酸盐的合成及电化学生物识别性质[J]. 高等学校化学学报, 2022, 43(1): 20210561. |
| [13] | 初明月, 李峰博, 高宁, 杨昕, 于婷婷, 马慧媛, 杨桂欣, 庞海军. 轮型多金属氧酸盐复合物膜的制备及在检测亚硝酸盐中的应用[J]. 高等学校化学学报, 2022, 43(1): 20210579. |
| [14] | 杨英杰, 张晓蓉, 孙玉雪, 刘军, 谢海明. 一种双锂盐梳状聚合物电解质的制备及电化学性能[J]. 高等学校化学学报, 2021, 42(9): 2861. |
| [15] | 鲍俊全, 郑仕兵, 苑旭明, 史金强, 孙田将, 梁静. 有机盐PTO(KPD)2作为高性能锂离子电池正极材料的研究[J]. 高等学校化学学报, 2021, 42(9): 2911. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||