

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (8): 2450.doi: 10.7503/cjcu20210107
李鹏杰, 周春妮, 王泽田, 郑子昂, 张玉敏, 王亮( ), 肖标(
), 肖标( )
)
收稿日期:2021-02-22
出版日期:2021-08-10
发布日期:2021-08-05
通讯作者:
王亮
E-mail:wangliang@jhun.edu.cn;biaoxiao@jhun.edu.cn
作者简介:肖 标, 男, 博士, 副教授, 主要从事有机太阳能电池的共轭聚合物设计合成与器件研究. E-mail: 基金资助:
LI Pengjie, ZHOU Chunni, WANG Zetian, ZHENG Ziang, ZHANG Yumin, WANG Liang( ), XIAO Biao(
), XIAO Biao( )
)
Received:2021-02-22
Online:2021-08-10
Published:2021-08-05
Contact:
WANG Liang
E-mail:wangliang@jhun.edu.cn;biaoxiao@jhun.edu.cn
Supported by:摘要:
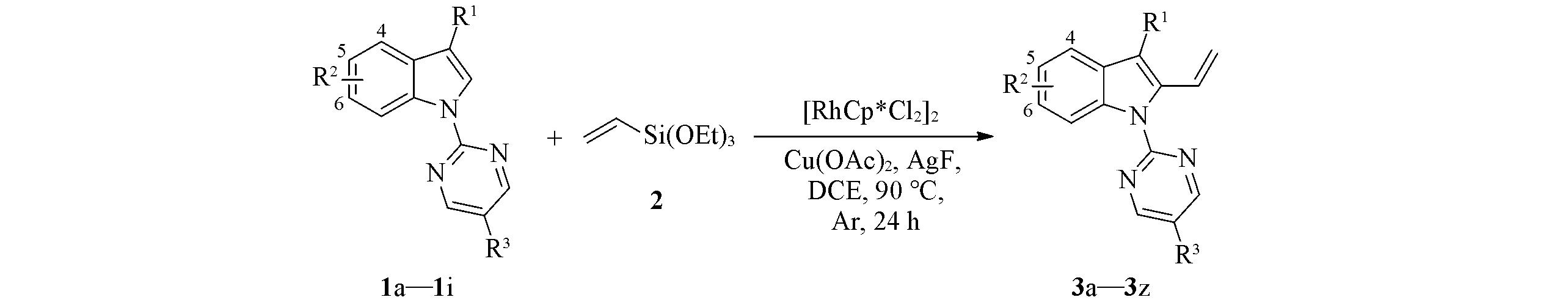
研究了铑催化N-嘧啶吲哚与乙烯基三乙氧基硅烷的C—H烯基化反应. 在以二氯(五甲基环戊二烯基)合铑(Ⅲ)二聚体{[RhCp*Cl2]2(Cp*: 五甲基环戊二烯基)}为催化剂, Cu(OAc)2为氧化剂, AgF为添加剂, 1,2-二氯乙烷为溶剂及反应温度为90 ℃条件下, 以42%~88%的收率得到末端吲哚乙烯衍生物. 动力学同位素效应实验结果为KH/KD=5.7∶1, 表明C—H键断裂可能是反应过程中的决速步骤. 竞争性实验结果表明, 含有供电子取代基的底物比吸电子取代基的底物反应活性高, 反应可能经历亲电性C—H键活化过程. 推测了可能的反应机理, 主要包括配位、 C—H键活化、 转金属化、 还原消除和氧化等步骤. 将此方法应用于一种δ-咔啉衍生物的制备.
中图分类号:
TrendMD:
李鹏杰, 周春妮, 王泽田, 郑子昂, 张玉敏, 王亮, 肖标. 铑催化吲哚与乙烯基三乙氧基硅烷的C—H烯基化反应. 高等学校化学学报, 2021, 42(8): 2450.
LI Pengjie, ZHOU Chunni, WANG Zetian, ZHENG Ziang, ZHANG Yumin, WANG Liang, XIAO Biao. Rhodium⁃catalyzed C—H Alkenylation of Indoles and Vinyltriethoxysilane. Chem. J. Chinese Universities, 2021, 42(8): 2450.
Scheme 2 给出2-乙烯基吲哚-3-甲醛(4)的合成路线. 向干燥的25 mL反应瓶中依次加入1.0 mmol 2-乙烯基-N-嘧啶吲哚-3-甲醛(3t)、 4.0 mmol NaOEt和8 mL DMSO; 将反应混合物于110 ℃反应15 min; 冷却至室温, 加入冰水(20 mL)淬灭; 用乙酸乙酯(20 mL×3)萃取, 用20 mL饱和NaCl溶液洗涤2次, 无水硫酸镁干燥后过滤并浓缩; 粗产品经硅胶柱层析纯化[V(乙酸乙酯)∶V(石油醚)=1∶15~1∶10]得到化合物4.
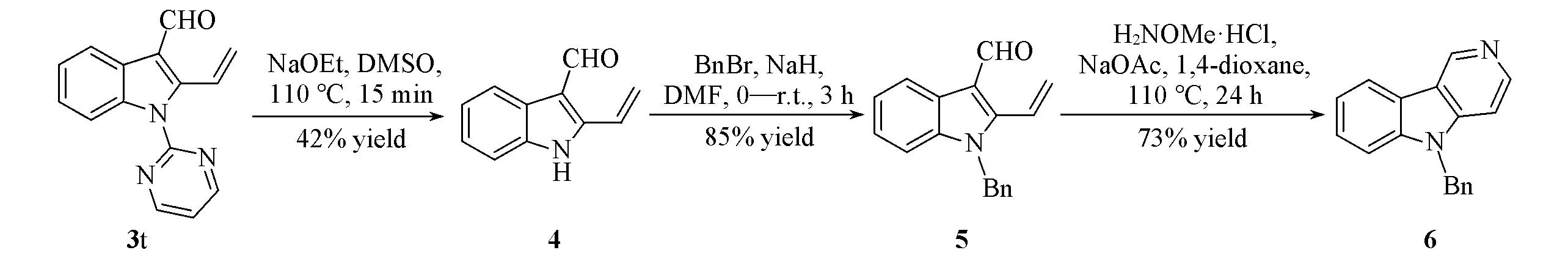
Scheme 2 给出2-乙烯基-N-苄基吲哚-3-甲醛(5)的合成路线. 在氩气保护下, 向干燥的10 mL反应瓶中依次加入0.4 mmol化合物4、 4 mL DMF, 将反应体系冷却至0 ℃; 分批加入0.44 mmol NaH反 应30 min; 再分批加入0.48 mmol溴化苄, 然后升至室温反应3 h; 加入5 mL冰水淬灭反应, 用乙酸乙酯(20 mL×3)萃取, 用20 mL饱和NaCl溶液洗涤2次, 无水硫酸镁干燥后过滤并浓缩; 粗产品经硅胶柱层析纯化[V(乙酸乙酯)∶V(石油醚)=1∶10~1∶5], 得到化合物5.
| Compd. | Appearance | m. p./℃ | HRMS(calcd.), m/z [M+H]+ | Compd. | Appearance | m. p./℃ | HRMS(calcd.), m/z [M+H]+ |
|---|---|---|---|---|---|---|---|
| 3a | White solid | 99—100 | 236.1181(236.1182) | 3p | Yellowish oil | — | 278.1659(278.1652) |
| 3b | White solid | 105—106 | 270.0791(270.0793) | 3q | Yellowish oil | — | 264.1496(264.1495) |
| 3c | White solid | 107—108 | 342.1609(342.1601) | 3r | Yellowish oil | — | 262.1346(262.1339) |
| 3d | White solid | 64—65 | 250.1340(250.1339) | 3s | Yellowish oil | — | 356.1763(356.1757) |
| 3e | White solid | 108—109 | 266.1288(266.1288) | 3t | Yellowish oil | — | 370.1558(370.1550) |
| 3f | White solid | 120—121 | 270.0797(270.0793) | 3u | Yellowish oil | — | 280.1088(280.1081) |
| 3g | White solid | 98—99 | 314.0288(314.0287) | 3v | Yellowish oil | — | 247.0978(247.1013) |
| 3h | White solid | 173—174 | 261.1138(261.1135) | 3w | Yellowish solid | 146—147 | 247.0981(247.0978) |
| 3i | Yellow solid | 137—138 | 281.1039(281.1033) | 3x | White solid | 60—61 | 250.1345(250.1339) |
| 3j | White solid | 145—146 | 250.1346(250.1339) | 3y | Yellowish oil | — | 314.0293(314.0287) |
| 3k | White solid | 126—127 | 254.1090(254.1088) | 3z | White solid | 112—113 | 312.1497(312.1495) |
| 3l | White solid | 106—107 | 270.0794(270.0793) | 4 | White solid | 74—75 | 172.0764(172.0757) |
| 3m | White solid | 79—80 | 314.0286(314.0287) | 5 | White solid | 91—92 | 262.1227(262.1226) |
| 3n | White solid | 79—80 | 250.1316(250.1339) | 6 | White solid | 102—103 | 259.1233(259.1230) |
| 3o | Yellowish oil | — | 250.1346(250.1339) |
表1 给出化合物3a~3z和4~6的理化数据, 核磁共振波谱数据见表2和3, 核磁共振谱图见 图S1~S37(见本文支持信息).
Table 1 Appearance, melting points and HRMS data of compounds 3a—3z and 4—6
| Compd. | Appearance | m. p./℃ | HRMS(calcd.), m/z [M+H]+ | Compd. | Appearance | m. p./℃ | HRMS(calcd.), m/z [M+H]+ |
|---|---|---|---|---|---|---|---|
| 3a | White solid | 99—100 | 236.1181(236.1182) | 3p | Yellowish oil | — | 278.1659(278.1652) |
| 3b | White solid | 105—106 | 270.0791(270.0793) | 3q | Yellowish oil | — | 264.1496(264.1495) |
| 3c | White solid | 107—108 | 342.1609(342.1601) | 3r | Yellowish oil | — | 262.1346(262.1339) |
| 3d | White solid | 64—65 | 250.1340(250.1339) | 3s | Yellowish oil | — | 356.1763(356.1757) |
| 3e | White solid | 108—109 | 266.1288(266.1288) | 3t | Yellowish oil | — | 370.1558(370.1550) |
| 3f | White solid | 120—121 | 270.0797(270.0793) | 3u | Yellowish oil | — | 280.1088(280.1081) |
| 3g | White solid | 98—99 | 314.0288(314.0287) | 3v | Yellowish oil | — | 247.0978(247.1013) |
| 3h | White solid | 173—174 | 261.1138(261.1135) | 3w | Yellowish solid | 146—147 | 247.0981(247.0978) |
| 3i | Yellow solid | 137—138 | 281.1039(281.1033) | 3x | White solid | 60—61 | 250.1345(250.1339) |
| 3j | White solid | 145—146 | 250.1346(250.1339) | 3y | Yellowish oil | — | 314.0293(314.0287) |
| 3k | White solid | 126—127 | 254.1090(254.1088) | 3z | White solid | 112—113 | 312.1497(312.1495) |
| 3l | White solid | 106—107 | 270.0794(270.0793) | 4 | White solid | 74—75 | 172.0764(172.0757) |
| 3m | White solid | 79—80 | 314.0286(314.0287) | 5 | White solid | 91—92 | 262.1227(262.1226) |
| 3n | White solid | 79—80 | 250.1316(250.1339) | 6 | White solid | 102—103 | 259.1233(259.1230) |
| 3o | Yellowish oil | — | 250.1346(250.1339) |
| Compd. | 1H NMR(400 MHz, CDCl3), δ |
|---|---|
| 3a[ | 8.78(d, J=4.8 Hz, 1H), 8.27(d, J=7.9 Hz, 0H), 7.58(d, J=7.2 Hz, 1H), 7.32—7.19(m, 1H), 7.15—7.08(m, 1H), 7.08—6.98(m, 0H), 5.53—5.38(m, 1H), 2.45(s, 2H) |
| 3b[ | 8.78(d, J=4.8 Hz, 2H), 7.81(d, J=8.4 Hz, 1H), 7.52(d, J=7.3 Hz, 2H), 7.41(t, J=7.4 Hz, 2H), 7.33(dd, J=8.3, 6.2 Hz, 1H), 7.19—7.07(m, 2H), 6.94(dd, J=17.7, 11.5 Hz, 1H), 6.68(d, J=7.9 Hz, 1H), 5.38(ddd, J=19.3, 14.6, 1.6 Hz, 2H), 5.21(s, 2H), 2.65(s, 3H) |
| 3c[ | 8.81(d, J=4.8 Hz, 2H), 8.09(dd, J=7.8, 1.4 Hz, 1H), 7.22—7.08(m, 3H), 6.94(dd, J=17.7, 11.5 Hz, 1H), 5.52(dd, J=11.5, 1.6 Hz, 1H), 5.38(dd, J=17.7, 1.6 Hz, 1H), 2.70(s, 3H) |
| 3d[ | 8.77(d, J=4.8 Hz, 2H), 8.19(d, J=8.5 Hz, 1H), 7.36(s, 1H), 7.09(ddd, J=23.5, 12.6, 6.4 Hz, 3H), 5.54—5.35(m, 2H), 2.49(s, 3H), 2.43(s, 3H) |
| 3e[ | 8.73(d, J=4.8 Hz, 2H), 8.22(d, J=9.0 Hz, 1H), 7.04(ddd, J=24.1, 14.8, 6.3 Hz, 3H), 6.89(dd, J=9.0, 2.6 Hz, 1H), 5.53—5.33(m, 2H), 3.88(s, 3H), 2.40(s, 3H) |
| 3f[ | 8.76(d, J=4.8 Hz, 1H), 8.20(d, J=8.8 Hz, 1H), 7.51(d, J=2.0 Hz, 1H), 7.19(dd, J=8.8, 2.1 Hz, 1H), 7.12(t, J=4.8 Hz, 1H), 7.08—6.97(m, 1H), 5.47(ddd, J=19.3, 14.6, 1.6 Hz, 1H), 2.38(s, 2H) |
| 3g[ | 8.80(d, J=4.8 Hz, 2H), 8.19(d, J=8.8 Hz, 1H), 7.70(d, J=1.9 Hz, 1H), 7.36(dd, J=8.8, 1.9 Hz, 1H), 7.17(t, J=4.8 Hz, 1H), 7.06(dd, J=17.7, 11.5 Hz, 1H), 5.50(ddd, J=19.0, 14.6, 1.3 Hz, 2H), 2.41(s, 3H) |
| 3h[ | 8.81(d, J=4.8 Hz, 2H), 8.27(dd, J=8.7, 0.6 Hz, 1H), 7.88(dd, J=1.6, 0.5 Hz, 1H), 7.48(dd, J=8.7, 1.6 Hz, 1H), 7.22(t, J=4.8 Hz, 1H), 7.01(ddd, J=17.7, 11.5, 0.5 Hz, 1H), 5.52(ddd, J=19.2, 14.6, 1.5 Hz, 2H), 2.42(s, 3H) |
| 3i[ | 8.87(d, J=4.8 Hz, 2H), 8.52(d, J=2.2 Hz, 1H), 8.29(d, J=9.2 Hz, 1H), 8.16(dd, J=9.2, 2.3 Hz, 1H), 7.28(t, J=4.8 Hz, 2H), 7.04(dd, J=17.8, 11.6 Hz, 1H), 5.60(dd, J=11.6, 1.4 Hz, 1H), 5.54(dd, J=17.7, 1.4 Hz, 1H), 2.50(s, 3H) |
| 3j[ | 8.76(d, J=4.8 Hz, 2H), 8.08—7.99(m, 1H), 7.42(d, J=8.0 Hz, 1H), 7.09(t, J=4.8 Hz, 1H), 7.06—6.91(m, 2H), 5.45—5.31(m, 2H), 2.46(s, 3H), 2.40(s, 3H) |
| 3k[ | 8.80(d, J=4.8 Hz, 2H), 8.09(dd, J=11.0, 2.3 Hz, 1H), 7.49(dd, J=8.6, 5.5 Hz, 1H), 7.16(t, J=4.8 Hz, 1H), 7.13—6.96(m, 2H), 5.46(ddd, J=19.1, 14.6, 1.4 Hz, 2H), 2.44(s, 3H) |
| Compd. | 1H NMR(400 MHz, CDCl3), δ |
| 3l[ | 8.80(d, J=4.8 Hz, 2H), 8.35(d, J=1.8 Hz, 1H), 7.48(d, J=8.4 Hz, 1H), 7.22(dd, J=8.4, 1.8 Hz, 1H), 7.16(t, J=4.8 Hz, 1H), 7.05(dd, J=17.7, 11.5 Hz, 1H), 5.48(ddd, J=19.2, 14.6, 1.4 Hz, 2H), 2.43(s, 3H) |
| 3m[ | 8.76(d, J=4.8 Hz, 2H), 8.46(d, J=1.6 Hz, 1H), 7.39(d, J=8.3 Hz, 1H), 7.31(dd, J=8.3, 1.7 Hz, 1H), 7.11(t, J=4.8 Hz, 1H), 7.00(dd, J=17.7, 11.5 Hz, 1H), 5.45(ddd, J=19.2, 14.6, 1.5 Hz, 2H), 2.38(s, 3H) |
| 3n[ | 8.89(d, J=4.8 Hz, 2H), 7.48(d, J=7.8 Hz, 1H), 7.34(t, J=4.8 Hz, 1H), 7.13(t, J=7.5 Hz, 1H), 7.01(d, J=7.1 Hz, 1H), 6.71(dd, J=18.1, 11.4 Hz, 1H), 5.42—5.26(m, 2H), 2.46(s, 3H), 1.94(s, 3H) |
| 3o[ | 8.81(d, J=4.8 Hz, 2H), 8.28(d, J=8.1 Hz, 1H), 7.63(d, J=7.5 Hz, 1H), 7.34—7.20(m, 4H), 7.15(t, J=4.8 Hz, 1H), 7.10—6.95(m, 1H), 5.46(s, 1H), 5.42(d, J=4.1 Hz, 1H), 2.94(q, J=7.5 Hz, 2H), 1.36(t, J=7.6 Hz, 3H) |
| 3p[ | 8.76(d, J=4.8 Hz, 2H), 8.22(d, J=8.2 Hz, 1H), 7.58(d, J=7.7 Hz, 1H), 7.23(dt, J=20.1, 7.1 Hz, 2H), 7.09(t, J=4.8 Hz, 1H), 6.97(dd, J=17.6, 11.7 Hz, 1H), 5.38(dd, J=14.8, 7.9 Hz, 2H), 2.91—2.78(m, 2H), 1.69(dt, J=15.4, 7.6 Hz, 2H), 1.47(dq, J=14.6, 7.4 Hz, 2H), 0.96(t, J=7.3 Hz, 3H) |
| 3q[ | 8.78(d, J=4.8 Hz, 1H), 8.21(dd, J=8.3, 0.5 Hz, 1H), 7.80(dd, J=7.8, 0.6 Hz, 1H), 7.28—7.22(m, 1H), 7.22—7.14(m, 1H), 7.12(t, J=4.8 Hz, 0H), 6.98(dd, J=17.7, 11.4 Hz, 1H), 5.47—5.36(m, 1H), 5.25(dd, J=17.7, 1.2 Hz, 1H), 3.51(dt, J=14.3, 7.1 Hz, 1H), 1.50(d, J=0.5 Hz, 2H), 1.48(d, J=0.5 Hz, 2H). |
| 3r[ | 8.80(d, J=4.8 Hz, 2H), 8.31(dd, J=8.3, 0.5 Hz, 1H), 7.59(dd, J=7.7, 0.5 Hz, 1H), 7.30(dd, J=11.4, 4.0 Hz, 2H), 7.24(dd, J=7.6, 7.2 Hz, 1H), 7.13(td, J=4.8, 0.6 Hz, 1H), 7.04(dd, J=17.7, 11.5 Hz, 1H), 6.17—6.03(m, 1H), 5.54—5.41(m, 2H), 5.19—5.04(m, 2H), 3.70—3.62(m, 2H) |
| 3s[ | 8.76(d, J=4.8 Hz, 1H), 8.30(d, J=8.3 Hz, 1H), 7.62(d, J=7.8 Hz, 1H), 7.40—7.24(m, 3H), 7.24—7.16(m, 1H), 7.11(t, J=4.8 Hz, 1H), 7.03(dd, J=17.7, 11.5 Hz, 1H), 5.63(dd, J=17.7, 1.5 Hz, 1H), 5.47(dd, J=11.5, 1.5 Hz, 1H), 5.14(s, 1H), 3.93(s, 1H) |
| 3t[ | 8.78(d, J=4.8 Hz, 2H), 8.26(d, J=8.3 Hz, 1H), 7.63(dd, J=7.7, 0.6 Hz, 1H), 7.36(dd, J=7.0, 4.9 Hz, 4H), 7.33—7.20(m, 4H), 7.11(t, J=4.8 Hz, 1H), 6.99(dd, J=17.7, 11.5 Hz, 1H), 5.53—5.36(m, 2H), 4.58(s, 2H), 3.81(t, J=7.7 Hz, 2H), 3.26(t, J=7.7 Hz, 2H) |
| 3u[ | 8.80(d, J=4.8 Hz, 2H), 8.40—8.31(m, 1H), 7.43—7.38(m, 1H), 7.34—7.20(m, 4H), 7.16(t, J=4.8 Hz, 1H), 7.05(dd, J=17.8, 11.7 Hz, 1H), 5.71(dd, J=17.8, 1.6 Hz, 1H), 5.47(dd, J=11.7, 1.6 Hz, 1H), 2.43(s, 3H) |
| 3x[ | 8.59(s, 2H), 8.12(d, J=7.9 Hz, 1H), 7.56(d, J=7.6 Hz, 1H), 7.36—7.10(m, 2H), 7.00(dd, J=17.6, 11.6 Hz, 1H), 5.53—5.34(m, 2H), 2.43(s, 3H), 2.32(s, 3H) |
Table 2 1H NMR and 13C NMR data of compounds 3a—3u and 3x
| Compd. | 1H NMR(400 MHz, CDCl3), δ |
|---|---|
| 3a[ | 8.78(d, J=4.8 Hz, 1H), 8.27(d, J=7.9 Hz, 0H), 7.58(d, J=7.2 Hz, 1H), 7.32—7.19(m, 1H), 7.15—7.08(m, 1H), 7.08—6.98(m, 0H), 5.53—5.38(m, 1H), 2.45(s, 2H) |
| 3b[ | 8.78(d, J=4.8 Hz, 2H), 7.81(d, J=8.4 Hz, 1H), 7.52(d, J=7.3 Hz, 2H), 7.41(t, J=7.4 Hz, 2H), 7.33(dd, J=8.3, 6.2 Hz, 1H), 7.19—7.07(m, 2H), 6.94(dd, J=17.7, 11.5 Hz, 1H), 6.68(d, J=7.9 Hz, 1H), 5.38(ddd, J=19.3, 14.6, 1.6 Hz, 2H), 5.21(s, 2H), 2.65(s, 3H) |
| 3c[ | 8.81(d, J=4.8 Hz, 2H), 8.09(dd, J=7.8, 1.4 Hz, 1H), 7.22—7.08(m, 3H), 6.94(dd, J=17.7, 11.5 Hz, 1H), 5.52(dd, J=11.5, 1.6 Hz, 1H), 5.38(dd, J=17.7, 1.6 Hz, 1H), 2.70(s, 3H) |
| 3d[ | 8.77(d, J=4.8 Hz, 2H), 8.19(d, J=8.5 Hz, 1H), 7.36(s, 1H), 7.09(ddd, J=23.5, 12.6, 6.4 Hz, 3H), 5.54—5.35(m, 2H), 2.49(s, 3H), 2.43(s, 3H) |
| 3e[ | 8.73(d, J=4.8 Hz, 2H), 8.22(d, J=9.0 Hz, 1H), 7.04(ddd, J=24.1, 14.8, 6.3 Hz, 3H), 6.89(dd, J=9.0, 2.6 Hz, 1H), 5.53—5.33(m, 2H), 3.88(s, 3H), 2.40(s, 3H) |
| 3f[ | 8.76(d, J=4.8 Hz, 1H), 8.20(d, J=8.8 Hz, 1H), 7.51(d, J=2.0 Hz, 1H), 7.19(dd, J=8.8, 2.1 Hz, 1H), 7.12(t, J=4.8 Hz, 1H), 7.08—6.97(m, 1H), 5.47(ddd, J=19.3, 14.6, 1.6 Hz, 1H), 2.38(s, 2H) |
| 3g[ | 8.80(d, J=4.8 Hz, 2H), 8.19(d, J=8.8 Hz, 1H), 7.70(d, J=1.9 Hz, 1H), 7.36(dd, J=8.8, 1.9 Hz, 1H), 7.17(t, J=4.8 Hz, 1H), 7.06(dd, J=17.7, 11.5 Hz, 1H), 5.50(ddd, J=19.0, 14.6, 1.3 Hz, 2H), 2.41(s, 3H) |
| 3h[ | 8.81(d, J=4.8 Hz, 2H), 8.27(dd, J=8.7, 0.6 Hz, 1H), 7.88(dd, J=1.6, 0.5 Hz, 1H), 7.48(dd, J=8.7, 1.6 Hz, 1H), 7.22(t, J=4.8 Hz, 1H), 7.01(ddd, J=17.7, 11.5, 0.5 Hz, 1H), 5.52(ddd, J=19.2, 14.6, 1.5 Hz, 2H), 2.42(s, 3H) |
| 3i[ | 8.87(d, J=4.8 Hz, 2H), 8.52(d, J=2.2 Hz, 1H), 8.29(d, J=9.2 Hz, 1H), 8.16(dd, J=9.2, 2.3 Hz, 1H), 7.28(t, J=4.8 Hz, 2H), 7.04(dd, J=17.8, 11.6 Hz, 1H), 5.60(dd, J=11.6, 1.4 Hz, 1H), 5.54(dd, J=17.7, 1.4 Hz, 1H), 2.50(s, 3H) |
| 3j[ | 8.76(d, J=4.8 Hz, 2H), 8.08—7.99(m, 1H), 7.42(d, J=8.0 Hz, 1H), 7.09(t, J=4.8 Hz, 1H), 7.06—6.91(m, 2H), 5.45—5.31(m, 2H), 2.46(s, 3H), 2.40(s, 3H) |
| 3k[ | 8.80(d, J=4.8 Hz, 2H), 8.09(dd, J=11.0, 2.3 Hz, 1H), 7.49(dd, J=8.6, 5.5 Hz, 1H), 7.16(t, J=4.8 Hz, 1H), 7.13—6.96(m, 2H), 5.46(ddd, J=19.1, 14.6, 1.4 Hz, 2H), 2.44(s, 3H) |
| Compd. | 1H NMR(400 MHz, CDCl3), δ |
| 3l[ | 8.80(d, J=4.8 Hz, 2H), 8.35(d, J=1.8 Hz, 1H), 7.48(d, J=8.4 Hz, 1H), 7.22(dd, J=8.4, 1.8 Hz, 1H), 7.16(t, J=4.8 Hz, 1H), 7.05(dd, J=17.7, 11.5 Hz, 1H), 5.48(ddd, J=19.2, 14.6, 1.4 Hz, 2H), 2.43(s, 3H) |
| 3m[ | 8.76(d, J=4.8 Hz, 2H), 8.46(d, J=1.6 Hz, 1H), 7.39(d, J=8.3 Hz, 1H), 7.31(dd, J=8.3, 1.7 Hz, 1H), 7.11(t, J=4.8 Hz, 1H), 7.00(dd, J=17.7, 11.5 Hz, 1H), 5.45(ddd, J=19.2, 14.6, 1.5 Hz, 2H), 2.38(s, 3H) |
| 3n[ | 8.89(d, J=4.8 Hz, 2H), 7.48(d, J=7.8 Hz, 1H), 7.34(t, J=4.8 Hz, 1H), 7.13(t, J=7.5 Hz, 1H), 7.01(d, J=7.1 Hz, 1H), 6.71(dd, J=18.1, 11.4 Hz, 1H), 5.42—5.26(m, 2H), 2.46(s, 3H), 1.94(s, 3H) |
| 3o[ | 8.81(d, J=4.8 Hz, 2H), 8.28(d, J=8.1 Hz, 1H), 7.63(d, J=7.5 Hz, 1H), 7.34—7.20(m, 4H), 7.15(t, J=4.8 Hz, 1H), 7.10—6.95(m, 1H), 5.46(s, 1H), 5.42(d, J=4.1 Hz, 1H), 2.94(q, J=7.5 Hz, 2H), 1.36(t, J=7.6 Hz, 3H) |
| 3p[ | 8.76(d, J=4.8 Hz, 2H), 8.22(d, J=8.2 Hz, 1H), 7.58(d, J=7.7 Hz, 1H), 7.23(dt, J=20.1, 7.1 Hz, 2H), 7.09(t, J=4.8 Hz, 1H), 6.97(dd, J=17.6, 11.7 Hz, 1H), 5.38(dd, J=14.8, 7.9 Hz, 2H), 2.91—2.78(m, 2H), 1.69(dt, J=15.4, 7.6 Hz, 2H), 1.47(dq, J=14.6, 7.4 Hz, 2H), 0.96(t, J=7.3 Hz, 3H) |
| 3q[ | 8.78(d, J=4.8 Hz, 1H), 8.21(dd, J=8.3, 0.5 Hz, 1H), 7.80(dd, J=7.8, 0.6 Hz, 1H), 7.28—7.22(m, 1H), 7.22—7.14(m, 1H), 7.12(t, J=4.8 Hz, 0H), 6.98(dd, J=17.7, 11.4 Hz, 1H), 5.47—5.36(m, 1H), 5.25(dd, J=17.7, 1.2 Hz, 1H), 3.51(dt, J=14.3, 7.1 Hz, 1H), 1.50(d, J=0.5 Hz, 2H), 1.48(d, J=0.5 Hz, 2H). |
| 3r[ | 8.80(d, J=4.8 Hz, 2H), 8.31(dd, J=8.3, 0.5 Hz, 1H), 7.59(dd, J=7.7, 0.5 Hz, 1H), 7.30(dd, J=11.4, 4.0 Hz, 2H), 7.24(dd, J=7.6, 7.2 Hz, 1H), 7.13(td, J=4.8, 0.6 Hz, 1H), 7.04(dd, J=17.7, 11.5 Hz, 1H), 6.17—6.03(m, 1H), 5.54—5.41(m, 2H), 5.19—5.04(m, 2H), 3.70—3.62(m, 2H) |
| 3s[ | 8.76(d, J=4.8 Hz, 1H), 8.30(d, J=8.3 Hz, 1H), 7.62(d, J=7.8 Hz, 1H), 7.40—7.24(m, 3H), 7.24—7.16(m, 1H), 7.11(t, J=4.8 Hz, 1H), 7.03(dd, J=17.7, 11.5 Hz, 1H), 5.63(dd, J=17.7, 1.5 Hz, 1H), 5.47(dd, J=11.5, 1.5 Hz, 1H), 5.14(s, 1H), 3.93(s, 1H) |
| 3t[ | 8.78(d, J=4.8 Hz, 2H), 8.26(d, J=8.3 Hz, 1H), 7.63(dd, J=7.7, 0.6 Hz, 1H), 7.36(dd, J=7.0, 4.9 Hz, 4H), 7.33—7.20(m, 4H), 7.11(t, J=4.8 Hz, 1H), 6.99(dd, J=17.7, 11.5 Hz, 1H), 5.53—5.36(m, 2H), 4.58(s, 2H), 3.81(t, J=7.7 Hz, 2H), 3.26(t, J=7.7 Hz, 2H) |
| 3u[ | 8.80(d, J=4.8 Hz, 2H), 8.40—8.31(m, 1H), 7.43—7.38(m, 1H), 7.34—7.20(m, 4H), 7.16(t, J=4.8 Hz, 1H), 7.05(dd, J=17.8, 11.7 Hz, 1H), 5.71(dd, J=17.8, 1.6 Hz, 1H), 5.47(dd, J=11.7, 1.6 Hz, 1H), 2.43(s, 3H) |
| 3x[ | 8.59(s, 2H), 8.12(d, J=7.9 Hz, 1H), 7.56(d, J=7.6 Hz, 1H), 7.36—7.10(m, 2H), 7.00(dd, J=17.6, 11.6 Hz, 1H), 5.53—5.34(m, 2H), 2.43(s, 3H), 2.32(s, 3H) |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
|---|---|---|
| 3v | 10.18(s, 1H), 8.87(d, J=4.8 Hz, 2H), 8.47(dd, J=7.5, 1.7 Hz, 1H), 8.24—8.13(m, 1H), 7.41—7.34(m, 2H), 7.31(t, J=4.9 Hz, 1H), 7.28—7.22(m, 1H), 5.78(dd, J=11.3, 1.4 Hz, 1H), 5.68(dd, J=17.4, 1.4 Hz, 1H) | 187.6, 158.5 157.2, 148.6, 136.0, 126.4, 126.2, 125.3, 124.8, 124.2, 122.0, 118.7, 118.5, 113.7 |
| 3w | 8.88(d, J=4.8 Hz, 2H), 8.22—8.09(m, 1H), 7.82—7.69(m, 1H), 7.40—7.35(m, 2H), 7.35—7.31(m, 1H), 7.11(dd, J=17.7, 11.6 Hz, 1H), 6.34(d, J=17.7 Hz, 1H), 5.74(d, J=11.7 Hz, 1H) | 158.6, 156.8, 144.5, 135.5, 127.9, 125.9, 125.4, 123.8, 121.9, 119.3, 119.0, 116.1, 114.3, 89.0 |
| 3y | 8.77(d, J=4.8 Hz, 2H), 8.26(dd, J=8.6, 0.6 Hz, 1H), 7.61—7.51(m, 1H), 7.31—7.20(m, 2H), 7.05(dd, J=18.2, 12.1 Hz, 1H), 5.50—5.39(m, 2H), 2.44(s, 3H) | 158.2, 158.1, 136.2, 133.9, 130.7, 128.8, 123.9, 121.7, 118.8, 116.8, 116.7, 115.4, 113.7, 10.3 |
| 3z | 8.98(s, 2H), 8.32(d, J=8.2 Hz, 1H), 7.67—7.56(m, 3H), 7.55—7.49(m, 2H), 7.48—7.43(m, 1H), 7.34—7.24(m, 2H), 7.10(dd, J=17.7, 11.6 Hz, 1H), 5.61—5.37(m, 2H), 2.46(s, 3H) | 157.2, 156.0, 136.2, 134.1, 134.0, 130.7, 129.6, 129.4, 128.9, 128.6, 126.6, 123.9, 121.8, 118.8, 116.8, 115.5, 113.8, 10.3 |
| 4 | 12.27(s, 1H), 10.25(s, 1H), 8.11(dd, J=7.8, 1.0 Hz, 1H), 7.46(dt, J=8.1, 0.8 Hz, 1H), 7.40(dd, J=17.6, 11.4 Hz, 1H), 7.30—7.26(m, 1H), 7.22—7.18(m, 1H), 6.21(dd, J=17.6, 0.5 Hz, 1H), 5.72(d, J=11.8 Hz, 1H) | 184.9, 144.6, 136.3, 125.5, 124.2, 122.2, 120. 9, 120.3, 114.1, 111.7 |
| 5 | 10.12(s, 1H), 8.43(dd, J=6.8, 1.2 Hz, 1H), 7.37—7.28(m, 4H), 7.27—7.24(m, 2H), 7.11—7.00(m, 2H), 6.83(dd, J=17.4, 11.5 Hz, 1H), 5.83(ddd, J=18.6, 14.5, 1.1 Hz, 2H), 5.38(s, 2H) | 185.9, 148.0, 136.9, 135.8, 129.0, 127.9, 127.2, 126.0, 125.6, 124.3, 123.7, 123.2, 122.2, 115.5, 110.0, 47.4 |
| 6 | 9.35(d, J=0.8 Hz, 1H), 8.53(d, J=5.8 Hz, 1H), 8.19(d, J=7.8 Hz, 1H), 7.51—7.47(m, 1H), 7.41(d, J=8.2 Hz, 1H), 7.38—7.31(m, 1H), 7.30—7.26(m, 4H), 7.13(dd, J=5.4, 2.5 Hz, 2H), 5.51(s, 2H) | 145.2, 144.8, 142.9 140.6, 136.1, 129.0, 127.9, 127.0, 126.4, 121.6, 120.9, 120.8, 119.9, 109.5, 104.4, 46.7 |
Table 3 1H NMR and 13C NMR data of compounds 3v, 3w, 3y, 3z and 4—6
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
|---|---|---|
| 3v | 10.18(s, 1H), 8.87(d, J=4.8 Hz, 2H), 8.47(dd, J=7.5, 1.7 Hz, 1H), 8.24—8.13(m, 1H), 7.41—7.34(m, 2H), 7.31(t, J=4.9 Hz, 1H), 7.28—7.22(m, 1H), 5.78(dd, J=11.3, 1.4 Hz, 1H), 5.68(dd, J=17.4, 1.4 Hz, 1H) | 187.6, 158.5 157.2, 148.6, 136.0, 126.4, 126.2, 125.3, 124.8, 124.2, 122.0, 118.7, 118.5, 113.7 |
| 3w | 8.88(d, J=4.8 Hz, 2H), 8.22—8.09(m, 1H), 7.82—7.69(m, 1H), 7.40—7.35(m, 2H), 7.35—7.31(m, 1H), 7.11(dd, J=17.7, 11.6 Hz, 1H), 6.34(d, J=17.7 Hz, 1H), 5.74(d, J=11.7 Hz, 1H) | 158.6, 156.8, 144.5, 135.5, 127.9, 125.9, 125.4, 123.8, 121.9, 119.3, 119.0, 116.1, 114.3, 89.0 |
| 3y | 8.77(d, J=4.8 Hz, 2H), 8.26(dd, J=8.6, 0.6 Hz, 1H), 7.61—7.51(m, 1H), 7.31—7.20(m, 2H), 7.05(dd, J=18.2, 12.1 Hz, 1H), 5.50—5.39(m, 2H), 2.44(s, 3H) | 158.2, 158.1, 136.2, 133.9, 130.7, 128.8, 123.9, 121.7, 118.8, 116.8, 116.7, 115.4, 113.7, 10.3 |
| 3z | 8.98(s, 2H), 8.32(d, J=8.2 Hz, 1H), 7.67—7.56(m, 3H), 7.55—7.49(m, 2H), 7.48—7.43(m, 1H), 7.34—7.24(m, 2H), 7.10(dd, J=17.7, 11.6 Hz, 1H), 5.61—5.37(m, 2H), 2.46(s, 3H) | 157.2, 156.0, 136.2, 134.1, 134.0, 130.7, 129.6, 129.4, 128.9, 128.6, 126.6, 123.9, 121.8, 118.8, 116.8, 115.5, 113.8, 10.3 |
| 4 | 12.27(s, 1H), 10.25(s, 1H), 8.11(dd, J=7.8, 1.0 Hz, 1H), 7.46(dt, J=8.1, 0.8 Hz, 1H), 7.40(dd, J=17.6, 11.4 Hz, 1H), 7.30—7.26(m, 1H), 7.22—7.18(m, 1H), 6.21(dd, J=17.6, 0.5 Hz, 1H), 5.72(d, J=11.8 Hz, 1H) | 184.9, 144.6, 136.3, 125.5, 124.2, 122.2, 120. 9, 120.3, 114.1, 111.7 |
| 5 | 10.12(s, 1H), 8.43(dd, J=6.8, 1.2 Hz, 1H), 7.37—7.28(m, 4H), 7.27—7.24(m, 2H), 7.11—7.00(m, 2H), 6.83(dd, J=17.4, 11.5 Hz, 1H), 5.83(ddd, J=18.6, 14.5, 1.1 Hz, 2H), 5.38(s, 2H) | 185.9, 148.0, 136.9, 135.8, 129.0, 127.9, 127.2, 126.0, 125.6, 124.3, 123.7, 123.2, 122.2, 115.5, 110.0, 47.4 |
| 6 | 9.35(d, J=0.8 Hz, 1H), 8.53(d, J=5.8 Hz, 1H), 8.19(d, J=7.8 Hz, 1H), 7.51—7.47(m, 1H), 7.41(d, J=8.2 Hz, 1H), 7.38—7.31(m, 1H), 7.30—7.26(m, 4H), 7.13(dd, J=5.4, 2.5 Hz, 2H), 5.51(s, 2H) | 145.2, 144.8, 142.9 140.6, 136.1, 129.0, 127.9, 127.0, 126.4, 121.6, 120.9, 120.8, 119.9, 109.5, 104.4, 46.7 |
| Entry | Solvent | Temperature | "F" salt | n(2)∶ n(1a) | Yieldb(%) | Entry | Solvent | Temperature | "F" salt | n(2)∶ n(1a) | Yieldb(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MeCN | 100 | AgF | 3∶1 | 51 | 13 | DCE | 80 | AgF | 3∶1 | 43 |
| 2 | DMF | 100 | AgF | 3∶1 | 51 | 14 | DCE | 90 | AgBF4 | 3∶1 | 67 |
| 3 | Toluene | 100 | AgF | 3∶1 | 42 | 15 | DCE | 90 | KHF2 | 3∶1 | 60 |
| 4 | DMSO | 100 | AgF | 3∶1 | 70 | 16 | DCE | 90 | LiF | 3∶1 | 0 |
| 5 | DCE | 100 | AgF | 3∶1 | 72 | 17 | DCE | 90 | TBAF | 3∶1 | 0 |
| 6 | THF | 100 | AgF | 3∶1 | 0 | 18 | DCE | 90 | — | 3∶1 | 0 |
| 7 | t?BuOH | 100 | AgF | 3∶1 | 0 | 19 | DCE | 90 | AgF | 1∶1 | 82 |
| 8 | 1,4?Dioxane | 100 | AgF | 3∶1 | 0 | 20 | DCE | 90 | AgF | 1.5∶1 | 82 |
| 9c | DCE/DMF | 100 | AgF | 3∶1 | 65 | 21 | DCE | 90 | AgF | 2∶1 | 74 |
| 10 | DCE | 110 | AgF | 3∶1 | 74 | 22 | DCE | 90 | AgF | 2.5∶1 | 85 |
| 11 | DCE | 120 | AgF | 3∶1 | 55 | 23 | DCE | 90 | AgF | 3.5∶1 | 64 |
| 12 | DCE | 90 | AgF | 3∶1 | 88 | 24 | DCE | 90 | AgF | 4∶1 | 52 |
Table 4 Optimization of the reaction conditionsa
| Entry | Solvent | Temperature | "F" salt | n(2)∶ n(1a) | Yieldb(%) | Entry | Solvent | Temperature | "F" salt | n(2)∶ n(1a) | Yieldb(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MeCN | 100 | AgF | 3∶1 | 51 | 13 | DCE | 80 | AgF | 3∶1 | 43 |
| 2 | DMF | 100 | AgF | 3∶1 | 51 | 14 | DCE | 90 | AgBF4 | 3∶1 | 67 |
| 3 | Toluene | 100 | AgF | 3∶1 | 42 | 15 | DCE | 90 | KHF2 | 3∶1 | 60 |
| 4 | DMSO | 100 | AgF | 3∶1 | 70 | 16 | DCE | 90 | LiF | 3∶1 | 0 |
| 5 | DCE | 100 | AgF | 3∶1 | 72 | 17 | DCE | 90 | TBAF | 3∶1 | 0 |
| 6 | THF | 100 | AgF | 3∶1 | 0 | 18 | DCE | 90 | — | 3∶1 | 0 |
| 7 | t?BuOH | 100 | AgF | 3∶1 | 0 | 19 | DCE | 90 | AgF | 1∶1 | 82 |
| 8 | 1,4?Dioxane | 100 | AgF | 3∶1 | 0 | 20 | DCE | 90 | AgF | 1.5∶1 | 82 |
| 9c | DCE/DMF | 100 | AgF | 3∶1 | 65 | 21 | DCE | 90 | AgF | 2∶1 | 74 |
| 10 | DCE | 110 | AgF | 3∶1 | 74 | 22 | DCE | 90 | AgF | 2.5∶1 | 85 |
| 11 | DCE | 120 | AgF | 3∶1 | 55 | 23 | DCE | 90 | AgF | 3.5∶1 | 64 |
| 12 | DCE | 90 | AgF | 3∶1 | 88 | 24 | DCE | 90 | AgF | 4∶1 | 52 |
| Entry | Product | R1 | R2 | R3 | Yield*(%) | Entry | Product | R1 | R2 | R3 | Yield*(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3a | Me | H | H | 88 | 14 | 3n | Me | 7?Me | H | 68 |
| 2 | 3b | Me | 4?OBn | H | 60 | 15 | 3o | H | Et | H | 65 |
| 3 | 3c | Me | 4?Cl | H | 58 | 16 | 3p | H | n?Bu | H | 38 |
| 4 | 3d | Me | 5?Me | H | 69 | 17 | 3q | H | i?Pr | H | 42 |
| 5 | 3e | Me | 5?OMe | H | 63 | 18 | 3r | H | Allyl | H | 51 |
| 6 | 3f | Me | 5?Cl | H | 72 | 19 | 3s | H | CH2CH2OBn | H | 61 |
| 7 | 3g | Me | 5?Br | H | 79 | 20 | 3t | H | CH2CO2Bn | H | 59 |
| 8 | 3h | Me | 5?CN | H | 66 | 21 | 3u | H | OAc | H | 50 |
| 9 | 3i | Me | 5?NO2 | H | 56 | 22 | 3v | H | CHO | H | 42 |
| 10 | 3j | Me | 6?Me | H | 71 | 23 | 3w | H | CN | H | 44 |
| 11 | 3k | Me | 6?F | H | 53 | 24 | 3x | H | H | Me | 58 |
| 12 | 3l | Me | 6?Cl | H | 64 | 25 | 3y | H | H | Br | 47 |
| 13 | 3m | Me | 6?Br | H | 61 | 26 | 3z | H | H | Ph | 72 |
Table 5 Substrate scope of C—H alkenylation of indoles and vinyltriethoxysilane
| Entry | Product | R1 | R2 | R3 | Yield*(%) | Entry | Product | R1 | R2 | R3 | Yield*(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3a | Me | H | H | 88 | 14 | 3n | Me | 7?Me | H | 68 |
| 2 | 3b | Me | 4?OBn | H | 60 | 15 | 3o | H | Et | H | 65 |
| 3 | 3c | Me | 4?Cl | H | 58 | 16 | 3p | H | n?Bu | H | 38 |
| 4 | 3d | Me | 5?Me | H | 69 | 17 | 3q | H | i?Pr | H | 42 |
| 5 | 3e | Me | 5?OMe | H | 63 | 18 | 3r | H | Allyl | H | 51 |
| 6 | 3f | Me | 5?Cl | H | 72 | 19 | 3s | H | CH2CH2OBn | H | 61 |
| 7 | 3g | Me | 5?Br | H | 79 | 20 | 3t | H | CH2CO2Bn | H | 59 |
| 8 | 3h | Me | 5?CN | H | 66 | 21 | 3u | H | OAc | H | 50 |
| 9 | 3i | Me | 5?NO2 | H | 56 | 22 | 3v | H | CHO | H | 42 |
| 10 | 3j | Me | 6?Me | H | 71 | 23 | 3w | H | CN | H | 44 |
| 11 | 3k | Me | 6?F | H | 53 | 24 | 3x | H | H | Me | 58 |
| 12 | 3l | Me | 6?Cl | H | 64 | 25 | 3y | H | H | Br | 47 |
| 13 | 3m | Me | 6?Br | H | 61 | 26 | 3z | H | H | Ph | 72 |
| 1 | Hao Y., Yu H., Liao P., Wang W., Chem. Res. Chinese Universities, 2020, 36(5), 847—852 |
| 2 | Guo L., Xu M., Jian Y., Liu S., Pan W., Duan L., Chem. Res. Chinese Universities, 2019, 35(4), 621—626 |
| 3 | Zhang Y. C., Jiang F., Shi F., Acc. Chem. Res., 2020, 53(2), 425—446 |
| 4 | Lu L. Q., Chen J. R., Xiao W. J., Acc. Chem. Res., 2012, 45(8), 1278—1293 |
| 5 | Gandeepan P., Müller T., Zell D., Cera G., Warratz S., Ackermann L., Chem. Rev., 2019, 119(4), 2192—2452 |
| 6 | Rej S., Ano Y., Chatani N., Chem. Rev., 2020, 120(3), 1788—1887 |
| 7 | Liao G., Zhang T., Lin Z. K., Shi B. F., Angew. Chem. Int. Ed., 2020, 59, 19773—19786 |
| 8 | Cai Y., Jiao L., Qiu X., Du Z., Chem. Res. Chinese Universities, 2020, 36(5), 843—846 |
| 9 | Liu Z., Cheng H., Zhou Q., Chem. Res. Chinese Universities, 2020, 36(4), 727—852 |
| 10 | Petrini M., Chem. Eur. J., 2017, 23(64), 16115—16151 |
| 11 | Chen W. L., Gao Y. R., Mao S., Zhang Y. L., Wang Y. F., Wang Y. Q., Org. Lett., 2012, 14(23), 5920—5923 |
| 12 | Chen H., Lin C., Xiong C., Liu Z., Zhang Y., Org. Chem. Front., 2017, 4(3), 455—459 |
| 13 | Gong B., Shi J., Wang X., Yan Y., Li Q., Meng Y., Xu E. H., Yi W., Adv. Synth. Catal., 2014, 356(1), 137—143 |
| 14 | Yang L., Zhang G., Huang H., Adv. Synth. Catal., 2014, 356(7), 1509—1515 |
| 15 | García⁃Rubia A., Urones B., Arrayás R. G., Carretero J. C., Chem. Eur. J., 2010, 16(31), 9676—9685 |
| 16 | Patureau W. F., Glorius F., J. Am. Chem. Soc., 2010, 132(29), 9982—9983 |
| 17 | Kong L., Zhou X., Li X., Org. Lett., 2016, 18(24), 6320—6323 |
| 18 | Cai S. H., Ye L., Wang D. X., Wang Y. Q., Lai L. J., Zhu C., Feng C., Loh T. P., Chem. Commun., 2017, 53(62), 8731—8734 |
| 19 | Moselage M., Sauermann N., Richter S. C., Ackermann L., Angew. Chem. Int. Ed., 2015, 54, 6352—6355 |
| 20 | Zhang L., Qiu R., Xue X., Pan Y., Xu C., Wang D., Wang X., Xu L., Li H., Chem. Commun., 2014, 50(82), 12385—12388 |
| 21 | Jagtap A. R., Vinod P. C., Punji B., ACS Catal., 2019, 9(1), 431—441 |
| 22 | Zhou C. N., Xie H. H., Zheng Z. A., Xiao Y. C., Li G., Shen Y. H., Peng W. M., Wang L., Chem. Eur. J., 2018, 24(21), 5469—5473 |
| 23 | Zhou C. N., Zheng Z. A., Peng W. M., Wang H. B., Zhang Y. M., Wang L., Xiao B., Chem. J. Chinese Universities, 2020, 41(4), 726—734(周春妮, 郑子昂, 彭望明, 王洪波, 张玉敏, 王亮, 肖标. 高等学校化学学报, 2020, 41(4), 726—734) |
| 24 | Wang L., Li Z., Qu X., Peng W. M., Tetrahedron Lett., 2015, 56(24), 3754—3757 |
| 25 | Wang L., Li Z., Qu X., Peng W. M., Hu S. Q., Tetrahedron Lett., 2015, 56(45), 6214—6218 |
| 26 | Wang L., Qu X., Fang L., Hu S., Wang F., Eur. J. Org. Chem., 2016, 33(33), 5494—5501 |
| 27 | Armarego W. L. F., Chai C. L. L.; Translated by Lin Y. J., Liu W., Wang H. P., Sun X. B., Li Q. S., Cao J. G., Purification of Laboratory Chemicals, Chemical Industry Press, Beijing, 2007, 65—365(Armarego W. L. F., Chai C. L. L.; 林英杰, 刘伟, 王会萍, 孙晓波, 李青山, 曹军刚[译]. 实验室化学品纯化手册, 北京: 化学工业出版社, 2007, 65—365) |
| 28 | Shan C., Bai R., Lan Y., Acta Phys. Chim. Sin., 2019, 35(9), 940—953 |
| 29 | Lu M. Z., Wang C. Q., Song S. J., Loh T. P., Org. Chem. Front., 2017, 4(2), 303—307 |
| [1] | 黄秋红, 李文军, 李鑫. 有机催化靛红衍生酮亚胺与噁唑酮的不对称Mannich型加成反应[J]. 高等学校化学学报, 2022, 43(8): 20220131. |
| [2] | 金睿明, 穆晓清, 徐岩. 生物-化学法合成黑色素前体5, 6-二羟基吲哚[J]. 高等学校化学学报, 2022, 43(8): 20220134. |
| [3] | 穆宏文, 吴昊, 高莹莹, 金瑛, 王黎明. 有机催化吲哚碳环上的不对称Friedel⁃Crafts烷基化反应[J]. 高等学校化学学报, 2022, 43(2): 20210571. |
| [4] | 刘苗, 刘瑞波, 刘巴蒂, 钱鹰. 溶酶体靶向吲哚氟硼二吡咯光敏剂的合成、 双光子荧光成像及光动力治疗[J]. 高等学校化学学报, 2022, 43(10): 20220326. |
| [5] | 田胜侨, 韦美菊. Rh(Ⅱ)催化吲哚衍生物[3+3]环化机理及产物性质分析[J]. 高等学校化学学报, 2021, 42(6): 1899. |
| [6] | 马静雨, 刘双磊, 张振国, 金俊阳, 贾振华. B(C6F5)3催化合成二吲哚甲烷类化合物的研究[J]. 高等学校化学学报, 2020, 41(10): 2225. |
| [7] | 苏秋铭, 原安莹, 邝福儿, 陈志强, 白呈超, 辛伟贤. CM⁃Phos配体在钯催化交叉偶联反应中的应用[J]. 高等学校化学学报, 2020, 41(10): 2185. |
| [8] | 李冰, 王学敏, 白凤英, 刘淑清. 稀土氮杂环配合物的合成、 结构及抑菌活性[J]. 高等学校化学学报, 2019, 40(4): 632. |
| [9] | 刘天伟, 张苏韬, 何江华, 张越涛. B(C6F5)3催化吲哚与苯乙炔的区域选择性加成[J]. 高等学校化学学报, 2019, 40(4): 719. |
| [10] | 张莹莹,黄译文,赵冰,王丽艳,宋波. Cr 3+比色荧光探针的合成及细胞成像应用[J]. 高等学校化学学报, 2019, 40(12): 2486. |
| [11] | 谷耀华 薛屏. 离子液体再生纤维素微球共固定漆酶-介体体系对吲哚降解的研究[J]. 高等学校化学学报, 2018, 39(8): 1846. |
| [12] | 郭明, 张新鸽, 曾楚楚, 殷欣欣. 基于Diels-Alder反应的智能分子印迹聚合物制备及性能表征[J]. 高等学校化学学报, 2018, 39(3): 566. |
| [13] | 刘笑宇, 徐议, 唐良富. 3-烷基膦酸酯基取代的异吲哚啉酮衍生物的合成及生物活性[J]. 高等学校化学学报, 2018, 39(11): 2433. |
| [14] | 张金, 刘佳, 马养民, 杨秀芳, 程佩, 范超, 卢萍. 纳米TiO2催化一锅法合成喹唑啉酮并酞嗪酮及3-酰胺基异吲哚酮并喹唑啉酮类化合物[J]. 高等学校化学学报, 2016, 37(9): 1629. |
| [15] | 林海, 李亚巍, 林华宽. 吲哚-3-醛-邻硝基苯基半卡巴腙的阴离子识别[J]. 高等学校化学学报, 2016, 37(3): 480. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||