

高等学校化学学报 ›› 2019, Vol. 40 ›› Issue (1): 1.doi: 10.7503/cjcu20180133
张利荣1, 王志鹏1, 刘莹1, 徐超2( ), 陈靖2, 丁颂东1(
), 陈靖2, 丁颂东1( )
)
收稿日期:2018-02-12
出版日期:2019-01-10
发布日期:2018-12-12
作者简介:联系人简介:丁颂东,男,博士,研究员,主要从事核燃料循环与材料方面的研究. E-mail:
基金资助:
ZHANG Lirong1, WANG Zhipeng1, LIU Ying1, XU Chao2,*( ), CHEN Jing2, DING Songdong1,*(
), CHEN Jing2, DING Songdong1,*( )
)
Received:2018-02-12
Online:2019-01-10
Published:2018-12-12
Contact:
XU Chao,DING Songdong
E-mail:xuchao@tsinghua.edu.cn;dsd68@163.com
Supported by:摘要:
以正十二烷作稀释剂, 研究了二(2-乙基己基)二硫代次膦酸(D2EHDTPA)对HNO3溶液中Am3+和Eu3+的萃取行为. 考察了酸度、 萃取剂及N
中图分类号:
TrendMD:
张利荣, 王志鹏, 刘莹, 徐超, 陈靖, 丁颂东. 二(2-乙基己基)二硫代次膦酸对硝酸溶液中Am3+和Eu3+的萃取. 高等学校化学学报, 2019, 40(1): 1.
ZHANG Lirong,WANG Zhipeng,LIU Ying,XU Chao,CHEN Jing,DING Songdong. Extraction of Trivalent Americium and Europiumfrom Nitric Acid Solution with Di(2-ethylhexyl)dithiophosphinic Acid†. Chem. J. Chinese Universities, 2019, 40(1): 1.
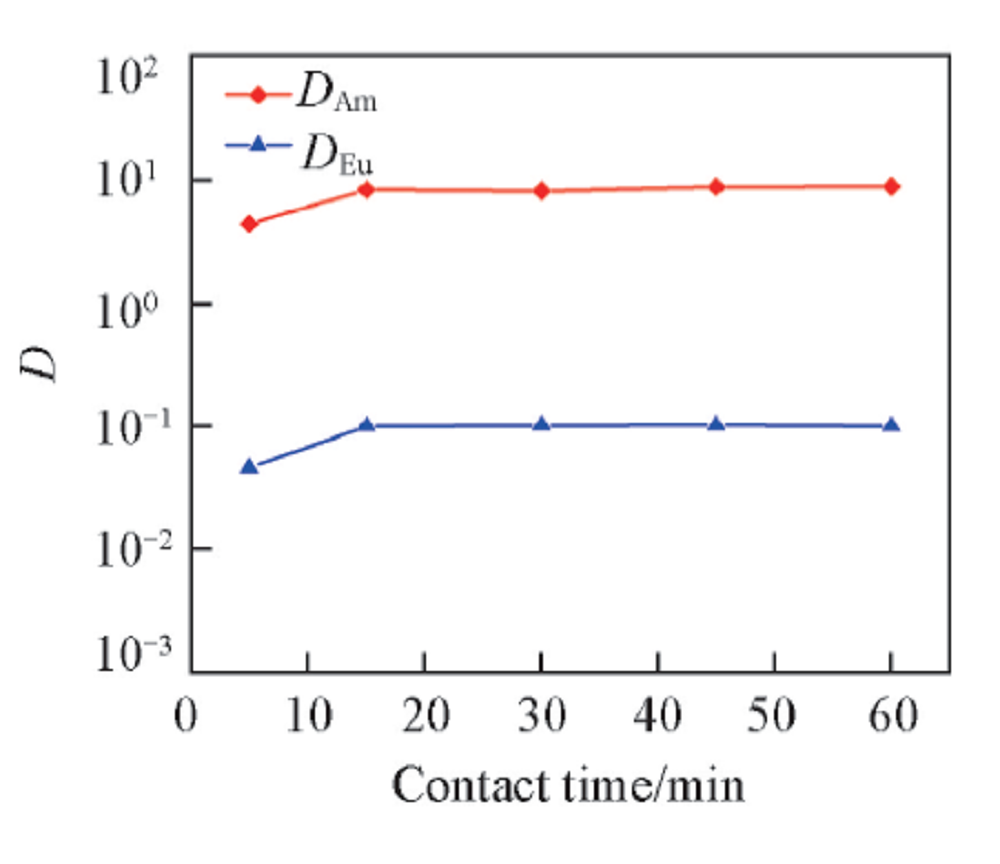
Fig.2 Influence of contact time on the distribution ratio of Am3+ and Eu3+Organic phase: 0.50 mol/L D2EHDTPA in n-dodecane; aqueous phase: trace amount of 241Am3+ or 152,154Eu3+ in 1.0 mol/L NaNO3 solution with pH of 3.35 and 4.05 for Am3+ and Eu3+, respectively.
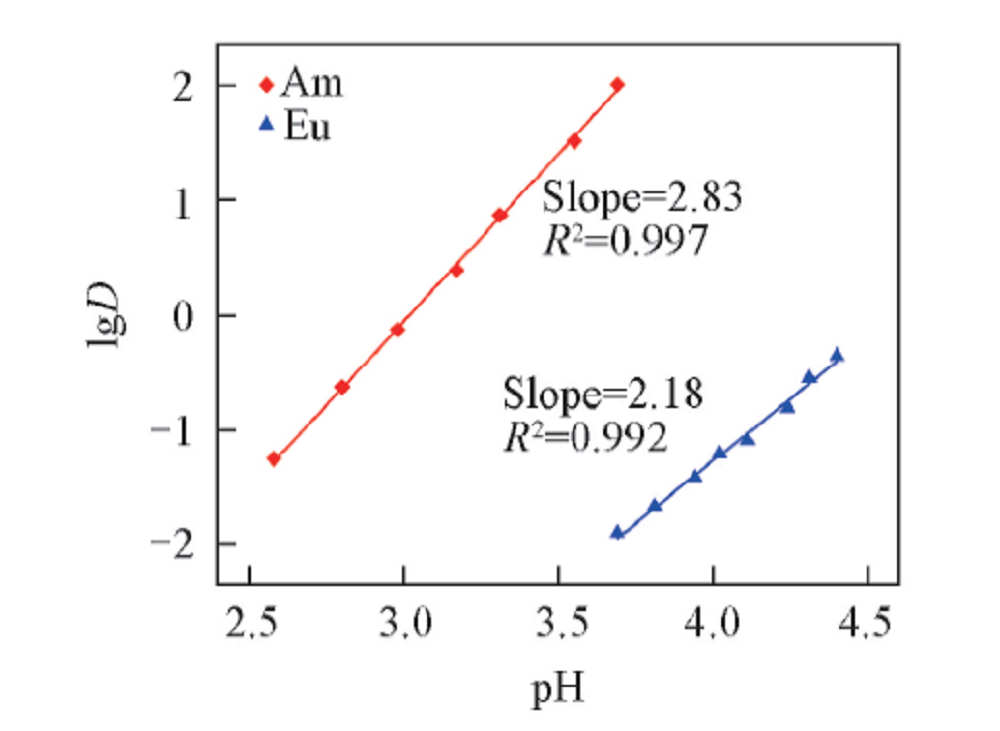
Fig.3 Influence of pH on the distribution ratio of Am3+ and Eu3+Organic phase: 0.50 mol/L D2EHDTPA in n-dodecane; aqueous phase: trace amount of 241Am3+ or 152,154Eu3+ in 1.0 mol/L NaNO3 solution with various pH.
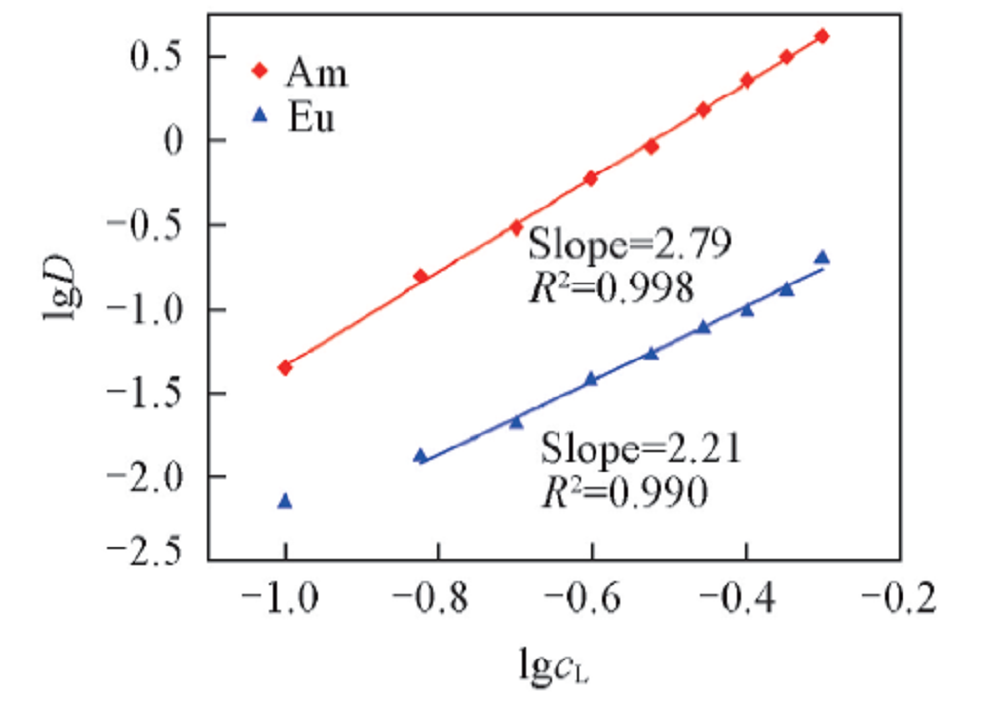
Fig.4 Influence of D2EHDTPA concentration on the extraction of Am3+ and Eu3+Organic phase: various concentration of D2EHDTPA in n-dodecane; aqueous phase: trace amount of 241Am3+ or 152,154Eu3+ in 1.0 mol/L NaNO3 solution with pH of 3.24 and 4.40 for Am3+ and Eu3+, respectively.
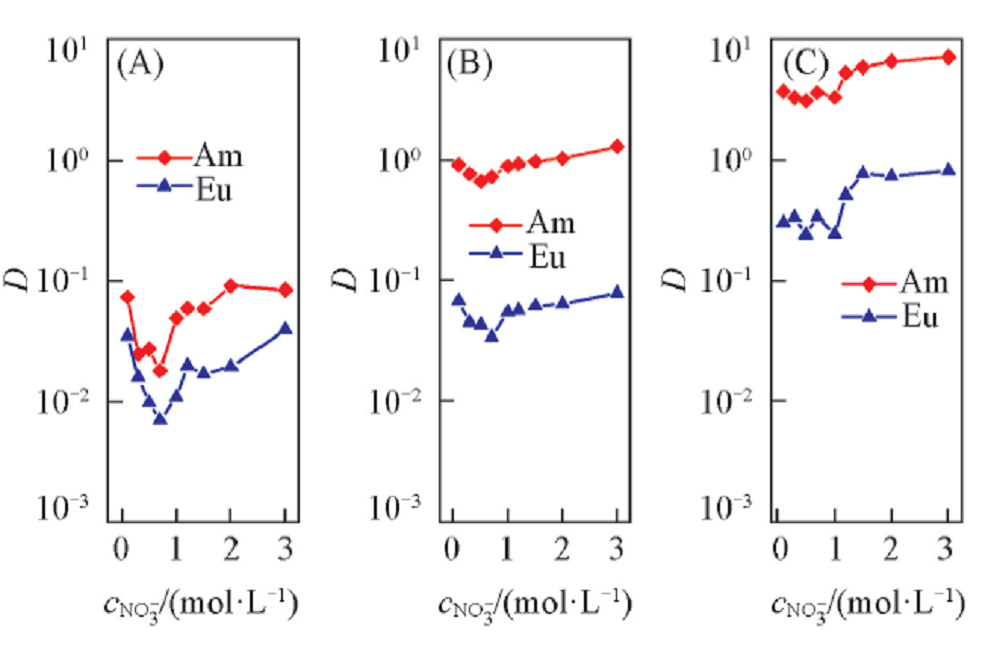
Fig.5 Influence of NO3- concentration on the extraction of Am3+ and Eu3+Organic phase: 0.10(A), 0.30(B) or 0.50(C) mol/L D2EHDTPA in n-dodecane; aqueous phase: trace amount of 241Am3+ or 152,154Eu3+ in various concentration NaNO3 solutions with pH of 3.24 and 4.40 for Am3+ and Eu3+, respectively.
| cL /(mol·L-1) | lg | lg | cL /(mol·L-1) | lg | lg |
|---|---|---|---|---|---|
| 0.10 | -7.22 | -9.00 | 0.35 | -7.21 | -9.16 |
| 0.15 | -7.17 | -9.11 | 0.40 | -7.19 | -9.19 |
| 0.20 | -7.22 | -9.19 | 0.45 | -7.20 | -9.18 |
| 0.25 | -7.21 | -9.15 | 0.50 | -7.20 | -9.09 |
| 0.30 | -7.24 | -9.15 | Average value | -7.21 | -9.14 |
Table 1 Apparent extraction equilibrium constants for Am3+ and Eu3+ by D2EHDTPA at 25 ℃
| cL /(mol·L-1) | lg | lg | cL /(mol·L-1) | lg | lg |
|---|---|---|---|---|---|
| 0.10 | -7.22 | -9.00 | 0.35 | -7.21 | -9.16 |
| 0.15 | -7.17 | -9.11 | 0.40 | -7.19 | -9.19 |
| 0.20 | -7.22 | -9.19 | 0.45 | -7.20 | -9.18 |
| 0.25 | -7.21 | -9.15 | 0.50 | -7.20 | -9.09 |
| 0.30 | -7.24 | -9.15 | Average value | -7.21 | -9.14 |
| Saponification degree(%) | 0 | 1 | 3 | 5 | 10 | 15 | 20 |
|---|---|---|---|---|---|---|---|
| SFAm/Eu | 5.55 | 5.05 | 1.34×104 | 2.51×104 | 1.34×104 | 4.86×103 | 5.07×103 |
Table 2 Influence of the saponification degree of D2EHDTPA on the separation factor*
| Saponification degree(%) | 0 | 1 | 3 | 5 | 10 | 15 | 20 |
|---|---|---|---|---|---|---|---|
| SFAm/Eu | 5.55 | 5.05 | 1.34×104 | 2.51×104 | 1.34×104 | 4.86×103 | 5.07×103 |
| 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
|---|---|---|---|---|---|
| 0.022 | 0.022 | 0.022 | 0.023 | 0.022 |
Table 3 Eu3+ concentration in organic phase after extraction by D2EHDTPAa
| 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
|---|---|---|---|---|---|
| 0.022 | 0.022 | 0.022 | 0.023 | 0.022 |
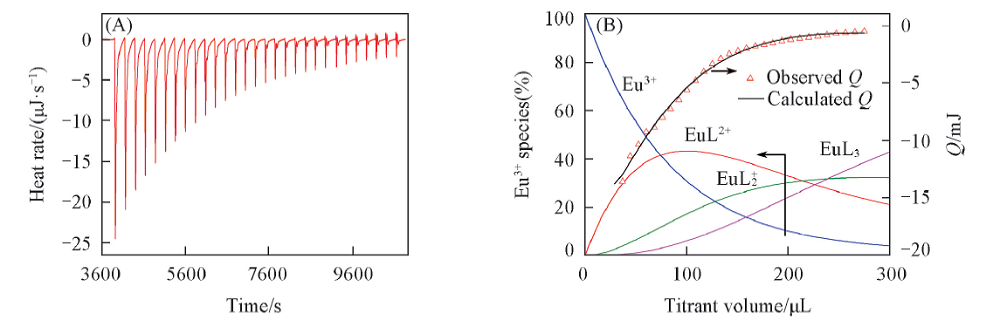
Fig.9 Microcalorimentric titration of Eu(ClO4)3 with D2EHDTPA in EtOH-H2O(volume ratio 99:1)(A) and the observed and fitted cumulative heat and the speciation of Eu3+ along the titration(B)Initial conditions: [Eu3+]=1.0×10-3 mol/L, volume=1.40 mL; Titration conditions: [D2EHDTPA]=1.0×10-2 mol/L, 30 additions of 0.01 mL each.
| Reaction | lgβ | ΔH/(kJ·mol-1) | ΔS/(J·mol-1·K-1) | ΔG/(kJ·mol-1) |
|---|---|---|---|---|
| Eu3++L-=EuL2+ | 3.56 | 12.7 | 72.3 | -8.85 |
| Eu3++2L-=Eu | 6.57 | 16.6 | 113 | -17.2 |
| Eu3++3L-=EuL3 | 9.52 | 18.5 | 162 | -29.8 |
Table 4 Thermodynamic parameters for the complexation of Eu3+ with D2EHDTPA at 25 ℃
| Reaction | lgβ | ΔH/(kJ·mol-1) | ΔS/(J·mol-1·K-1) | ΔG/(kJ·mol-1) |
|---|---|---|---|---|
| Eu3++L-=EuL2+ | 3.56 | 12.7 | 72.3 | -8.85 |
| Eu3++2L-=Eu | 6.57 | 16.6 | 113 | -17.2 |
| Eu3++3L-=EuL3 | 9.52 | 18.5 | 162 | -29.8 |
| [1] | Bhattacharyya A., Ghanty T. K., Mohapatra P. K., Manchanda V. K., Inorg.Chem.,2011, 50, 3913—3921 |
| [2] | Xue W. J., Zhang A. Y., Chai Z. F., China Science Paper.,2012, 7(9), 657—665 |
| (薛文静, 张安运, 柴之芳. 中国科技论文, 2012, 7(9), 657—665) | |
| [3] | Mathur J. N., Murali M. S., Nash K. L., Solvent Extr. IonExch.,2001, 19(3), 357—390 |
| [4] | Nash K. L., Solvent Extr. Ion Exch., 1993, 11(4), 729—768 |
| [5] | Mincher B. J., Modolo G., Mezyk S. P., Solvent Extr. Ion Exch.,2010, 28(4), 415—436 |
| [6] | Chen J., Wang F., He X. H., Pan D. F., Prog.Chem.,2011, 23(7), 1338—1344 |
| (陈靖, 王芳, 何喜红, 盘登芳. 化学进展, 2011, 23(7), 1338—1344) | |
| [7] | Dam H. H., Reinhoudt D. N., Verboom W., Chem. Soc.Rev.,2007, 36, 367—377 |
| [8] | Jensen M. P., Bond A. H., J. Am. Chem.Soc.,2002, 124, 9870—9877 |
| [9] | Kolarik Z., Müllich U., Gassner F., Solvent Extr. Ion Exch.,1999, 17(1), 23—32 |
| [10] | Trumm S., Geist A., Panak P. J., Fanghänel T., Solvent Extr. Ion Exch., 2011, 29(4), 213—229 |
| [11] | Foreman M. R. S., Hudson M. J., Geist A., Madic C., Weigl M., Solvent Extr. Ion Exch., 2005, 23, 645—662 |
| [12] | Retegan T., Ekberg C., Dubois I., Fermvik A., Wass T. J., Skarnemark G., Solvent Extr. Ion Exch., 2007, 25(4), 417—431 |
| [13] | Galletta M., Scaravaggi S., Macerata E., Famulari A., Mele A., Panzeri W., Sansone F., Casnati A., Mariani M., Dalton Trans.,2013, 42, 16930—16938 |
| [14] | Edwards A. C., Wagner C., Geist A., Burton N. A., Sharrad C. A., Adams R. W., Pritchard R. G., Panak P. J., Whitehead R. C., Harwood L. M., Dalton Trans.,2016, 45, 18102—18112 |
| [15] | Bremer A., Ruff C. M., Girnt D., Müllich U., Rothe J., Roesky P. W., Panak P. J., Karpov A., Müllich T. J. J., Denecke M. A., Geist A., Inorg.Chem., 2012, 51, 5199—5207 |
| [16] | Wang J. R., Su D. P., Wang D. Q., Ding S. D., Huang C., Huang H., Hu X. Y., Wang Z. P., Li S. M., Inorg.Chem., 2015, 54, 10648—10655 |
| [17] | Su D.P., Liu Y., Li S. M., Ding S. D., Jin Y. D., Wang Z. P., Hu X. Y., Zhang L. R.,Eur. J. Inorg. Chem., 2017, 651—658 |
| [18] | Hu X. Y., Su D. P., Li S. M., Wang Z. P., Zhang L. R., Liu Y., Song L. J., Chen Z. L., Ding S. D., Chem. J. Chinese Universities.,2017, 38(8), 1324—1333 |
| (胡晓阳, 苏冬萍, 李诗萌, 王志鹏, 张利荣, 刘莹, 宋莲君, 陈志力, 丁颂东. 高等学校化学学报. , 2017, 38(8), 1324—1333) | |
| [19] | Geist A., Hill C., Modold G., Foreman M. R. St. J., Weigl M., Gompper K., Hudson M. J., Solvent Extr. Ion Exch., 2007, 24, 463—483 |
| [20] | Zhu Y. J., Chen J., Jiao R. Z., Solvent Extr. IonExch., 1996, 14(1), 61—68 |
| [21] | Wang X. H., Zhu Y. J., Jiao R. Z., J. Radioanal. Nucl. Chem.,., 2002, 251 487—492 |
| [22] | Bhattacharyya A., Mohapatra P. K., Manchanda V. K., Solvent Extr. Ion Exch.,2006, 24(1), 1—17 |
| [23] | Bhattacharyya A., Mohapatra P. K., Manchanda V. K., Solvent Extr. Ion Exch.,2007, 25(1), 27—39 |
| [24] | Ionova G., Rabbe C., Hill C., Madic C., Guillaumont R., Krupa C., Solvent Extr. Ion Exch., 2001, 19(3), 391—414 |
| [25] | Cao X. Y., Heidelberg D., Ciupka J., Dolg M., Inorg.Chem.,2010, 49(22), 10307—10315 |
| [26] | Tian G. X., Zhu Y. J., Xu J. M., Hu T. D., Xie Y. N., J. Alloys Compd.,2002, 334(1/2), 86—91 |
| [27] | Tian G. X., Zhu Y. J., Xu J. M., Zhang P., Hu T. D., Xie Y. N., Zhang J., Inorg.Chem. ,2003, 42, 735—741 |
| [28] | Tian G. X., Kimura T., Yoshida Z., Zhu Y. J., Rao L. F., Radiochim .Acta.,2004, 92(8), 495—499 |
| [29] | Sole K. C., Hiskey J. B., Ferguson T. L., Solvent Extr. IonExch.,1993, 11(5), 783—796 |
| [30] | Chen J., The Separation of Amerieium from Lanthanides by Bis(2,4,4-trimethylpentyl)dithiophosphinic Acid Extraction, Tsinghua University, Beijing, 1996 |
| (陈靖. 二(2,4,4-三甲基戊基)二硫代膦酸萃取分离镅与镧系元素, 北京: 清华大学, 1996) | |
| [31] | Chen J., Wang S. W., Xu C., Wang X. H., Feng X. G., Procedia Chemistry.,2012, 7, 172—177 |
| [32] | Sole K. C., Hiskey J. B., Hydrometallurgy.,1995, 37, 129—147 |
| [33] | Tian G. X., Zhu Y. J., Xu J. M., Solvent Extr. Ion Exch., 2001, 19(6), 993—1005 |
| [34] | Xu Q. C., Wu J. F., Chang Y. Z., Zhang L. X., Yang Y. S., Radiochim.Acta.,2008, 96, 771—779 |
| [35] | Xu C., Rao L. F., Chem. Eur.J.,2014, 20, 14807—14815 |
| [36] | Xu G.X., Wang W. Q., Wu J. G., Gao H. C., Shi N., Extraction Chemistry Principle., Shanghai Science and Technology Press, Shanghai, 1985, 87—91 |
| (徐光宪, 王文清, 吴瑾光, 高宏成, 施鼐. 萃取化学原理., 上海: 上海科学技术出版社, 1985, 87—91) | |
| [37] | Peterman D. R., Greenhalgh M. R., Tillotson R. D., Klaehn J. R., Harrup M. K., Luther T. A., Law J. D., Sep. Sci.Technol.,2010, 45, 1711—1717 |
| [38] | Jensen M. P., Chiarizia R., Urban V., Solvent Extr. Ion Exch., 2001, 19(5), 865—884 |
| [39] | Pattee D., Musikas C.,Faure A., Chachaty C., J. Less-Common Metals.,1986, 122, 295—302 |
| [40] | Horwitz E. P., Muscatello A. L., Kalina D. G., Kaplan L., Sep. Sci.Technol.,1981, 16(4), 417—437 |
| [41] | Chen J., Zhu Y. J., Jiao R. Z., Solvent Extr. Ion Exch., 1996, 31(19), 2723—2731 |
| [42] | Chen H. H., Zhao X., Luan X. H., Yu G. L., Chem. J. Chinese Universities.,2015, 36(1), 1—8 |
| (陈欢欢, 赵峡, 栾晓红, 于广利. 高等学校化学学报., 2015, 36(1), 1—8) | |
| [43] | Zhang L., Li H. F., Chen P., Sun W. B., Yan P. F., Chem. Res. Chinese Universities.,2016, 32(4), 534—538 |
| [44] | Ferraro J. R., J. Inorg. Nucl.Chem., 1959, 10, 319—324 |
| [45] | Bernardo P. D., Melchior A., Tolazzi M., Zanonato P. L., Coord. Chem.Rev., 2012, 256, 328—351 |
| [46] | Xu C., Sun T. X., Rao L. F., Inorg.Chem.,2017, 56, 2556—2565 |
| [47] | Wang L. F., He D. Q., Chen W., Yu H. Q., Water Research.,2015, 81, 325—332 |
| [48] | Shi X. L., Zhang T., Li X. W., Feng Y., Tan X., Jin Y. R., Chem. Res. Chinese Universities.,2016, 32(4), 556—560 |
| [49] | Wang L.F., Interactions Between Natural Organic MatterNOM) and Metal Ions/Nanoparticles and Their Effects in Membrane Fouling Process, University of Science and Technology of China,Hefei, 2016 |
| (王龙飞. 天然有机脂与金属离子/纳米颗粒的相互作用及其对膜污染过程的影响, 合肥: 中国科学技术大学, 2016) |
| [1] | 蒋小康, 周琦, 周恒为. Gd2ZnTiO6∶Dy3+, Eu3+单基质白光荧光粉的制备与发光性能[J]. 高等学校化学学报, 2022, 43(6): 20220029. |
| [2] | 宋有为, 安江伟, 王征, 王旭慧, 权燕红, 任军, 赵金仙. Ag,Zn,Pd掺杂对铜基催化剂草酸二甲酯选择性加氢反应的影响[J]. 高等学校化学学报, 2022, 43(6): 20210842. |
| [3] | 宋颖颖, 黄琳, 李庆森, 陈立妙. CuO/BiVO4光催化剂的制备及光催化CO2还原性能[J]. 高等学校化学学报, 2022, 43(6): 20220126. |
| [4] | 闭格宁, 肖小华, 李攻科. 微波辅助萃取多物理场耦合模型的构建及验证[J]. 高等学校化学学报, 2022, 43(3): 20210739. |
| [5] | 李学宇, 王朝, 陈雅, 李可可, 李建全, 金顺敬, 陈丽华, 苏宝连. 等离激元共振光转热增强负载纳米金对丁二烯选择性加氢的催化性能[J]. 高等学校化学学报, 2022, 43(10): 20220174. |
| [6] | 魏李娜, 彭莉, 朱锋, 顾鹏飞, 顾学红. 中空纤维Au-CeZr/FAU催化膜的制备及在富氢气氛CO选择性氧化反应中的应用[J]. 高等学校化学学报, 2022, 43(10): 20220175. |
| [7] | 柳雪广, 杨晓珊, 马菁菁, 刘伟生. 铕基金属有机框架材料从混合染料中选择性分离亚甲基蓝[J]. 高等学校化学学报, 2022, 43(1): 20210715. |
| [8] | 李晨晨, 那永. 双功能复合材料g-C3N4/CdS/Ni催化光解水产氢和5-羟甲基糠醛氧化性能[J]. 高等学校化学学报, 2021, 42(9): 2896. |
| [9] | 史歌, 徐茜, 代枭, 张洁, 沈军, 宛新华. 芳香取代基结构对螺旋聚乙炔高效液相色谱手性固定相手性识别性能的影响[J]. 高等学校化学学报, 2021, 42(8): 2673. |
| [10] | 王高博, 马晶. 重氮苯与不同亲核试剂结合选择性:共价与非共价作用分析[J]. 高等学校化学学报, 2021, 42(7): 2238. |
| [11] | 腾云洋, 曲泽星, 周中军, 黄旭日. 光诱导分步分态的苯型脱芳构化反应的理论研究[J]. 高等学校化学学报, 2021, 42(3): 752. |
| [12] | 郑海娇, 姜丽艳, 贾琼. 精氨酸功能化磁性纳米材料的制备及在磷酸化肽富集中的应用[J]. 高等学校化学学报, 2021, 42(3): 717. |
| [13] | 宋文尧, 周张浪, 杨鑫莉, 陈岚, 葛广路. 介孔二氧化硅对映选择性吸附的手性印迹调控[J]. 高等学校化学学报, 2021, 42(10): 3144. |
| [14] | 赵淑芳, 黄骏. 分子筛材料的酸性和择形选择性的固体核磁共振研究[J]. 高等学校化学学报, 2021, 42(1): 165. |
| [15] | 邓洁薇, 杨运云, 林里, 栾天罡. 表面修饰探针纳升电喷雾质谱脂质组学对大型溞和蚤状溞的快速鉴别[J]. 高等学校化学学报, 2020, 41(9): 2011. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||