

高等学校化学学报 ›› 2016, Vol. 37 ›› Issue (12): 2159.doi: 10.7503/cjcu20160568
收稿日期:2016-08-11
出版日期:2016-12-10
发布日期:2016-11-15
作者简介:联系人简介: 谢建伟, 男, 博士, 副教授, 主要从事药物化学研究. E-mail:基金资助:
ZHANG Jie, ZHOU Changjian, XIE Jianwei*( ), DAI Bin
), DAI Bin
Received:2016-08-11
Online:2016-12-10
Published:2016-11-15
Contact:
XIE Jianwei
E-mail:cesxjw@foxmail.com
Supported by:摘要:
以大黄酸为原料, 经酯化、 烷基化、 水解及缩合等反应步骤合成了12个大黄酸-缬氨酸加合物. 目标化合物经1H NMR, 13C NMR和HRMS进行了结构确证. 以顺铂和阿霉素为阳性对照药, 采用四甲基偶氮唑盐(MTT)法考察了目标化合物的体外抗肿瘤(Hela, MCF-7, HepG2, KB和HEK293T等5株细胞)活性. 结果表明, 化合物5l显示出较好的抗肿瘤活性, 其IC50值在1.6~9.4 μmol/L之间. 作用机制研究结果表明, 化合物5l能够与DNA发生较强的结合作用.
中图分类号:
TrendMD:
张洁, 周昌健, 谢建伟, 代斌. 大黄酸-缬氨酸加合物的合成及初步抗肿瘤活性. 高等学校化学学报, 2016, 37(12): 2159.
ZHANG Jie, ZHOU Changjian, XIE Jianwei, DAI Bin. Synthesis and Antitumor Activities of Rhein-Valine Adducts†. Chem. J. Chinese Universities, 2016, 37(12): 2159.
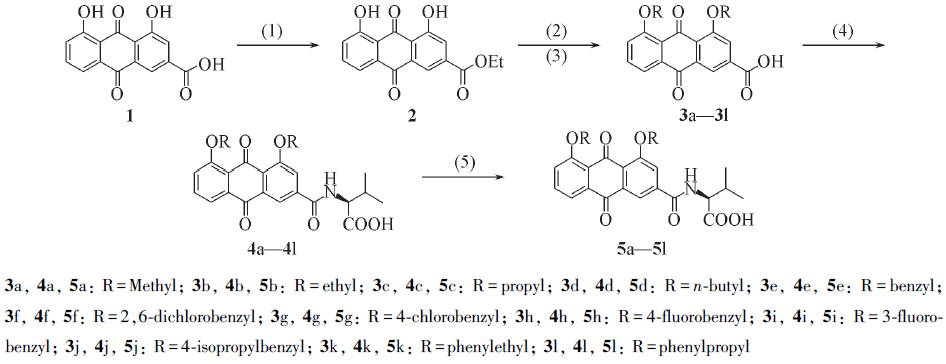
Scheme 1 Synthetic routes of target compounds 5a—5l(1) SOCl2, EtOH, reflux; (2) RX, NaH, DMF; (3) 1 mol/L NaOH(aq), then 1 mol/L HCl(aq);(4) NH2CH(R2)COOEt, EDCI, DMAP, CH2Cl2; (5) 1 mol/L NaOH, then 1 mol/L HCl(aq).
| Compd. | Appearance | Yield(%) | m. p./℃ | Compd. | Appearance | Yield(%) | m. p./℃ |
|---|---|---|---|---|---|---|---|
| 3a | Yellow solid | 85 | 301.0—301.7 | 3g | Yellow solid | 90 | 230.1—230.7 |
| 3b | Yellow solid | 72 | 249.9—250.9 | 3h | Yellow solid | 92 | 199.7—200.4 |
| 3c | Yellow solid | 54 | 181.9—182.8 | 3i | Yellow solid | 93 | 222.9—223.7 |
| 3d | Yellow solid | 46 | 231.1—232.1 | 3j | Yellow solid | 95 | 194.3—195.2 |
| 3e | Yellow solid | 86 | 181.1—181.9 | 3k | Yellow solid | 92 | 166.6—167.5 |
| 3f | Yellow solid | 95 | 231.4—232.2 | 3l | Yellow solid | 95 | 179.5—180.3 |
Table 1 Appearances, yields and melting points of compounds 3a—3l
| Compd. | Appearance | Yield(%) | m. p./℃ | Compd. | Appearance | Yield(%) | m. p./℃ |
|---|---|---|---|---|---|---|---|
| 3a | Yellow solid | 85 | 301.0—301.7 | 3g | Yellow solid | 90 | 230.1—230.7 |
| 3b | Yellow solid | 72 | 249.9—250.9 | 3h | Yellow solid | 92 | 199.7—200.4 |
| 3c | Yellow solid | 54 | 181.9—182.8 | 3i | Yellow solid | 93 | 222.9—223.7 |
| 3d | Yellow solid | 46 | 231.1—232.1 | 3j | Yellow solid | 95 | 194.3—195.2 |
| 3e | Yellow solid | 86 | 181.1—181.9 | 3k | Yellow solid | 92 | 166.6—167.5 |
| 3f | Yellow solid | 95 | 231.4—232.2 | 3l | Yellow solid | 95 | 179.5—180.3 |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ |
|---|---|
| 3a | 13.73(s, 1H), 8.17(d, J=1.6 Hz, 1H), 7.90(d, J=1.6 Hz, 1H), 7.78(t, J=8.0 Hz, 1H), 7.70(dd, J=1.2, 7.6 Hz, 1H), 7.56(dd, J=0.8, 8.4 Hz, 1H), 3.98(s, 3H), 3.93(s, 3H) |
| 3b | 13.70(s, 1H), 8.16(d, J=1.2 Hz, 1H), 7.87(d, J=1.2 Hz, 1H), 7.74(t, J=7.6 Hz, 1H), 7.69(dd, J=1.2, 7.6 Hz, 1H), 7.55(dd, J=1.2, 8.0 Hz, 1H), 4.28(q, J=7.2 Hz, 2H), 4.23(q, J=7.2 Hz, 2H), 1.43(t, J=7.2 Hz, 3H), 1.42(t, J=7.2 Hz, 3H) |
| 3c | 8.17(d, J=1.6 Hz, 1H), 7.88(d, J=1.6 Hz, 1H), 7.74(t, J=8.0 Hz, 1H), 7.69(dd, J=1.6, 7.6 Hz, 1H), 7.54(dd, J=1.2, 8.4 Hz, 1H), 4.16(t, J=6.4 Hz, 2H), 4.11(t, J=6.4 Hz, 2H), 1.78—1.84(m, 4H), 1.05—1.10(m, 6H) |
| 3d | 8.15(d, J=1.2 Hz, 1H), 7.86(s, 1H), 7.72(t, J=7.6 Hz, 1H), 7.67(d, J=6.8 Hz, 1H), 7.52(d, J=8.4 Hz, 1H), 4.18(t, J=6.0 Hz, 2H), 4.12(t, J=6.0 Hz, 2H), 1.72—1.80(m, 4H), 1.52—1.59(m, 4H), 0.96(t, J=7.6 Hz, 6H) |
| 3e | 8.25(d, J=1.2 Hz, 1H), 8.05(d, J=1.2 Hz, 1H), 7.73—7.79(m, 2H), 7.63—7.67(m, 5H), 7.35—7.43(m, 6H), 5.37(s, 2H), 5.33(s, 2H) |
| 3f | 8.27(d, J=1.2 Hz, 1H), 8.06(d, J=1.2 Hz, 1H), 7.79(d, J=1.2 Hz, 1H), 7.78(s, 1H), 7.69(t, J=4.8 Hz, 1H), 7.42—7.50(m, 6H), 5.36(s, 2H), 5.33(s, 2H) |
| 3g | 8.24(d, J=1.6 Hz, 1H), 8.02(d, J=1.6 Hz, 1H), 7.75—7.80(m, 2H), 7.62—7.69(m, 5H), 7.17—7.23(m, 4H), 5.36(s, 2H), 5.30(s, 2H) |
| 3h | 8.23(s, 1H), 8.01(s, 1H), 7.50—7.69(m, 7H), 7.36—7.44(m, 4H), 5.24(s, 4H) |
| 3i | 8.24(d, J=1.6 Hz, 1H), 8.02(d, J=1.2 Hz, 1H), 7.76—7.83(m, 2H), 7.65(dd, J=1.6, 8.0 Hz, 1H), 7.42—7.56(m, 6H), 7.16—7.21(m, 2H), 5.43(s, 2H), 5.37(s, 2H) |
| 3j | 8.22(d, J=1.2 Hz, 1H), 8.03(d, J=1.2 Hz, 1H), 7.73—7.80(m, 2H), 7.64(dd, J=1.2, 7.6 Hz, 1H), 7.57(t, J=8.4 Hz, 4H), 7.29(d, J=7.2 Hz, 4H), 5.34(s, 2H), 5.28(s, 2H), 2.89—2.95(m, 2H), 1.25(s, 6H), 1.23(s, 6H) |
| 3k | 8.17(d, J=1.6 Hz, 1H), 7.89(d, J=1.2 Hz, 1H), 7.68—7.75(m, 2H), 7.50(dd, J=1.6, 8.0 Hz, 4H), 7.29—7.34(m, 5H), 7.20—7.24(m, 2H), 4.41(t, J=6.8 Hz, 2H), 4.35(t, J=6.8 Hz, 2H), 3.16(q, J=6.4 Hz, 4H) |
| 3l | 8.19(d, J=1.6 Hz, 1H), 7.87(d, J=1.6 Hz, 1H), 7.71—7.77(m, 2H), 7.53(dd, J=2.0, 7.6 Hz, 1H), 7.12—7.28(m, 10H), 4.18(t, J=6.0 Hz, 2H), 4.12(t, J=6.0 Hz, 2H), 2.91(t, J=7.2 Hz, 4H), 2.04—2.13(m, 4H) |
Table 2 1H NMR data of compounds 3a—3l
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ |
|---|---|
| 3a | 13.73(s, 1H), 8.17(d, J=1.6 Hz, 1H), 7.90(d, J=1.6 Hz, 1H), 7.78(t, J=8.0 Hz, 1H), 7.70(dd, J=1.2, 7.6 Hz, 1H), 7.56(dd, J=0.8, 8.4 Hz, 1H), 3.98(s, 3H), 3.93(s, 3H) |
| 3b | 13.70(s, 1H), 8.16(d, J=1.2 Hz, 1H), 7.87(d, J=1.2 Hz, 1H), 7.74(t, J=7.6 Hz, 1H), 7.69(dd, J=1.2, 7.6 Hz, 1H), 7.55(dd, J=1.2, 8.0 Hz, 1H), 4.28(q, J=7.2 Hz, 2H), 4.23(q, J=7.2 Hz, 2H), 1.43(t, J=7.2 Hz, 3H), 1.42(t, J=7.2 Hz, 3H) |
| 3c | 8.17(d, J=1.6 Hz, 1H), 7.88(d, J=1.6 Hz, 1H), 7.74(t, J=8.0 Hz, 1H), 7.69(dd, J=1.6, 7.6 Hz, 1H), 7.54(dd, J=1.2, 8.4 Hz, 1H), 4.16(t, J=6.4 Hz, 2H), 4.11(t, J=6.4 Hz, 2H), 1.78—1.84(m, 4H), 1.05—1.10(m, 6H) |
| 3d | 8.15(d, J=1.2 Hz, 1H), 7.86(s, 1H), 7.72(t, J=7.6 Hz, 1H), 7.67(d, J=6.8 Hz, 1H), 7.52(d, J=8.4 Hz, 1H), 4.18(t, J=6.0 Hz, 2H), 4.12(t, J=6.0 Hz, 2H), 1.72—1.80(m, 4H), 1.52—1.59(m, 4H), 0.96(t, J=7.6 Hz, 6H) |
| 3e | 8.25(d, J=1.2 Hz, 1H), 8.05(d, J=1.2 Hz, 1H), 7.73—7.79(m, 2H), 7.63—7.67(m, 5H), 7.35—7.43(m, 6H), 5.37(s, 2H), 5.33(s, 2H) |
| 3f | 8.27(d, J=1.2 Hz, 1H), 8.06(d, J=1.2 Hz, 1H), 7.79(d, J=1.2 Hz, 1H), 7.78(s, 1H), 7.69(t, J=4.8 Hz, 1H), 7.42—7.50(m, 6H), 5.36(s, 2H), 5.33(s, 2H) |
| 3g | 8.24(d, J=1.6 Hz, 1H), 8.02(d, J=1.6 Hz, 1H), 7.75—7.80(m, 2H), 7.62—7.69(m, 5H), 7.17—7.23(m, 4H), 5.36(s, 2H), 5.30(s, 2H) |
| 3h | 8.23(s, 1H), 8.01(s, 1H), 7.50—7.69(m, 7H), 7.36—7.44(m, 4H), 5.24(s, 4H) |
| 3i | 8.24(d, J=1.6 Hz, 1H), 8.02(d, J=1.2 Hz, 1H), 7.76—7.83(m, 2H), 7.65(dd, J=1.6, 8.0 Hz, 1H), 7.42—7.56(m, 6H), 7.16—7.21(m, 2H), 5.43(s, 2H), 5.37(s, 2H) |
| 3j | 8.22(d, J=1.2 Hz, 1H), 8.03(d, J=1.2 Hz, 1H), 7.73—7.80(m, 2H), 7.64(dd, J=1.2, 7.6 Hz, 1H), 7.57(t, J=8.4 Hz, 4H), 7.29(d, J=7.2 Hz, 4H), 5.34(s, 2H), 5.28(s, 2H), 2.89—2.95(m, 2H), 1.25(s, 6H), 1.23(s, 6H) |
| 3k | 8.17(d, J=1.6 Hz, 1H), 7.89(d, J=1.2 Hz, 1H), 7.68—7.75(m, 2H), 7.50(dd, J=1.6, 8.0 Hz, 4H), 7.29—7.34(m, 5H), 7.20—7.24(m, 2H), 4.41(t, J=6.8 Hz, 2H), 4.35(t, J=6.8 Hz, 2H), 3.16(q, J=6.4 Hz, 4H) |
| 3l | 8.19(d, J=1.6 Hz, 1H), 7.87(d, J=1.6 Hz, 1H), 7.71—7.77(m, 2H), 7.53(dd, J=2.0, 7.6 Hz, 1H), 7.12—7.28(m, 10H), 4.18(t, J=6.0 Hz, 2H), 4.12(t, J=6.0 Hz, 2H), 2.91(t, J=7.2 Hz, 4H), 2.04—2.13(m, 4H) |
| Compd. | Appearance | Yield(%) | m. p./℃ | Compd. | Appearance | Yield(%) | m. p./℃ |
|---|---|---|---|---|---|---|---|
| 4a | Yellow solid | 68 | 223.5—224.3 | 4g | Yellow solid | 70 | 216.8—217.6 |
| 4b | Yellow solid | 96 | 184.5—185.1 | 4h | Yellow solid | 44 | 221.8—222.6 |
| 4c | Yellow solid | 89 | 151.9—152.8 | 4i | Yellow solid | 49 | 193.7—194.6 |
| 4d | Yellow solid | 79 | 161.2—162.4 | 4j | Yellow solid | 39 | 98.7—99.4 |
| 4e | Yellow solid | 97 | 172.3—173.1 | 4k | Yellow solid | 72 | 72.3—73.1 |
| 4f | Yellow solid | 51 | 100.2—101.1 | 4l | Yellow solid | 81 | 125.5—126.4 |
Table 3 Appearances, yields and melting points of compounds 4a—4l
| Compd. | Appearance | Yield(%) | m. p./℃ | Compd. | Appearance | Yield(%) | m. p./℃ |
|---|---|---|---|---|---|---|---|
| 4a | Yellow solid | 68 | 223.5—224.3 | 4g | Yellow solid | 70 | 216.8—217.6 |
| 4b | Yellow solid | 96 | 184.5—185.1 | 4h | Yellow solid | 44 | 221.8—222.6 |
| 4c | Yellow solid | 89 | 151.9—152.8 | 4i | Yellow solid | 49 | 193.7—194.6 |
| 4d | Yellow solid | 79 | 161.2—162.4 | 4j | Yellow solid | 39 | 98.7—99.4 |
| 4e | Yellow solid | 97 | 172.3—173.1 | 4k | Yellow solid | 72 | 72.3—73.1 |
| 4f | Yellow solid | 51 | 100.2—101.1 | 4l | Yellow solid | 81 | 125.5—126.4 |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ |
|---|---|
| 4a | 8.10(d, J=1.6 Hz, 1H), 7.88(d, J=1.6 Hz, 1H), 7.86(dd, J=0.8, 7.6 Hz, 1H), 7.66(t, J=8.0 Hz, 1H), 7.32(dd, J=0.8, 8.4 Hz, 1H), 6.84(d, J=8.4 Hz, 1H), 4.77(q, J=5.2 Hz, 1H), 4.23—4.31(m, 2H), 4.06(s, 3H), 4.01(s, 3H), 2.28—2.37(m, 1H), 1.33(t, J=7.2 Hz, 3H), 1.06(d, J=4.4 Hz, 3H), 1.04(d, J=4.0 Hz, 3H) |
| 4b | 8.08(d, J=1.6 Hz, 1H), 7.86(d, J=1.2 Hz, 1H), 7.84(dd, J=0.8, 7.6 Hz, 1H), 7.63(t, J=8.0 Hz, 1H), 7.31(d, J=8.4 Hz, 1H), 6.86(d, J=8.4 Hz, 1H), 4.78(q, J=4.8 Hz, 1H), 4.23—4.34(m, 6H), 2.28—2.36(m, 1H), 1.56(t, J=6.8 Hz, 6H), 1.33(t, J=6.8 Hz, 3H), 1.06(d, J=5.2 Hz, 3H), 1.04(d, J=4.8 Hz, 3H) |
| 4c | 8.07(d, J=1.6 Hz, 1H), 7.85(d, J=1.6 Hz, 1H), 7.83(dd, J=0.8, 7.6 Hz, 1H), 7.62(t, J=8.0 Hz, 1H), 7.30(dd, J=1.2, 8.4 Hz, 1H), 6.85(d, J=8.4 Hz, 1H), 4.78(q, J=4.8 Hz, 1H), 4.23—4.31(m, 2H), 4.17(t, J=6.4 Hz, 2H), 4.11(t, J=6.4 Hz, 2H), 2.28—2.36(m, 1H), 1.90—2.00(m, 4H), 1.33(t, J=7.2 Hz, 3H), 1.10—1.15(m, 6H), 1.05(d, J=4.8 Hz, 3H), 1.03(d, J=4.8 Hz, 3H) |
| 4d | 8.07(d, J=1.6 Hz, 1H), 7.85(d, J=1.6 Hz, 1H), 7.82(dd, J=1.2, 7.6 Hz, 1H), 7.62(t, J=8.0 Hz, 1H), 7.30(dd, J=0.8, 8.4 Hz, 1H), 6.85(d, J=8.4 Hz, 1H), 4.77(q, J=4.8 Hz, 1H), 4.23—4.30(m, 2H), 4.20(t, J=6.4 Hz, 2H), 4.15(t, J=6.4 Hz, 2H), 2.93—2.36(m, 1H), 1.87—1.94(m, 4H), 1.58—1.66(m, 4H), 1.33(t, J=7.2 Hz, 3H), 0.99—1.06(m, 12H) |
| 4e | 8.13(d, J=1.6 Hz, 1H), 7.94(d, J=1.6 Hz, 1H), 7.88(dd, J=0.8, 7.6 Hz, 1H), 7.61—7.67(m, 5H), 7.33—7.42(m, 7H), 6.84(d, J=8.4 Hz, 1H), 5.38(s, 2H), 5.33(s, 2H), 4.77(q, J=5.2 Hz, 1H), 4.26—4.29(m, 2H), 2.28—2.36(m, 1H), 1.33(t, J=7.2 Hz, 3H), 1.05(d, J=4.4 Hz, 3H), 1.03(d, J=4.8 Hz, 3H) |
| 4f | 8.18(s, 1H), 7.99(s, 1H), 7.92(d, J=8.0 Hz, 1H), 7.63(t, J=8.0 Hz, 1H), 7.44(d, J=8.4 Hz, 1H), 7.27—7.32(m, 4H), 7.18—7.24(m, 2H), 6.81(d, J=8.4 Hz, 1H), 5.49(s, 2H), 5.42(s, 2H), 4.80(q, J=5.2 Hz, 1H), 3.80(s, 3H), 2.28—2.36(m, 1H), 1.05(d, J=4.4 Hz, 3H), 1.03(d, J=4.4 Hz, 3H) |
| 4g | 8.13(d, J=1.2 Hz, 1H), 7.90(dd, J=1.6, 6.4 Hz, 2H), 7.65(t, J=8.0 Hz, 1H), 7.55—7.59(m, 4H), 7.33—7.38(m, 5H), 6.86(d, J=8.4 Hz, 1H), 5.31(s, 2H), 5.27(s, 2H), 4.79(q, J=5.2 Hz, 1H), 3.81(s, 3H), 2.28—2.36(m, 1H), 1.05(d, J=3.2 Hz, 3H), 1.03(d, J=3.2 Hz, 3H) |
| 4h | 8.13(d, J=1.6 Hz, 1H), 7.92(d, J=1.6 Hz, 1H), 7.90(dd, J=0.8, 7.6 Hz, 1H), 7.58—7.67(m, 5H), 7.36(dd, J=1.2, 8.4 Hz, 1H), 7.05—7.10(m, 4H), 6.88(d, J=8.4 Hz, 1H), 5.31(s, 2H), 5.27(s, 2H), 4.79(q, J=5.2 Hz, 1H), 3.81(s, 3H), 2.30—2.35(m, 1H), 1.05(d, J=3.2 Hz, 3H), 1.03(d, J=3.2 Hz, 3H) |
| 4i | 8.14(d, J=1.6 Hz, 1H), 7.89—7.91(m, 2H), 7.65(t, J=8.0 Hz, 1H), 7.34—7.45(m, 7H), 7.00—7.06(m, 2H), 6.91(d, J=8.8 Hz, 1H), 5.34(s, 2H), 5.29(s, 2H), 4.79(q, J=5.2 Hz, 1H), 3.82(s, 3H), 2.30—2.35(m, 1H), 1.05(d, J=2.4 Hz, 3H), 1.03(d, J=2.4 Hz, 3H) |
| 4j | 8.12(d, J=1.6 Hz, 1H), 7.94(d, J=1.6 Hz, 1H), 7.87(dd, J=0.8, 7.6 Hz, 1H), 7.55—7.64(m, 5H), 7.37(dd, J=1.2, 8.4 Hz, 1H), 7.28(s, 2H), 7.26(s, 2H), 6.79(d, J=8.8 Hz, 1H), 5.35(s, 2H), 5.30(s, 2H), 4.79(q, J=4.8 Hz, 1H), 3.80(s, 3H), 2.89—2.97(m, 2H), 2.27—2.35(m, 1H), 1.28(d, J=2.0 Hz, 6H), 1.26(d, J=2.4 Hz, 6H), 1.04(d, J=4.4 Hz, 3H), 1.02(d, J=4.4 Hz, 3H) |
| 4k | 8.07(s, 1H), 7.72—7.89(m, 2H), 7.57(t, J=8.0 Hz, 1H), 7.41—7.45(m, 4H), 7.32(q, J=7.2 Hz, 4H), 7.20—7.26(m, 3H), 7.02(d, J=8.8 Hz, 1H), 4.75—4.80(m, 1H), 4.39(t, J=7.2 Hz, 2H), 4.32(t, J=7.2 Hz, 2H), 4.23—4.29(m, 2H), 3.28(t, J=6.8 Hz, 4H), 2.28—2.36(m, 1H), 1.32(t, J=7.2 Hz, 3H), 1.05(d, J=4.8 Hz, 3H), 1.03(d, J=4.8 Hz, 3H) |
| 4l | 8.09(d, J=1.6 Hz, 1H), 7.85(dd, J=0.8, 7.6 Hz, 1H), 7.82(d, J=1.6 Hz, 1H), 7.62(t, J=8.0 Hz, 1H), 7.28(d, J=0.8 Hz, 1H), 7.20—7.26(m, 8H), 7.14—7.18(m, 2H), 6.81(d, J=8.4 Hz, 1H), 4.79(q, J=5.2 Hz, 1H), 4.19(t, J=6.0 Hz, 2H), 4.13(t, J=6.0 Hz, 2H), 3.80(s, 3H), 2.95—3.00(m, 4H), 2.29—2.33(m, 1H), 2.20—2.27(m, 4H), 1.04(d, J=3.6 Hz, 3H), 1.02(d, J=3.6 Hz, 3H) |
Table 4 1H NMR data of compounds 4a—4l
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ |
|---|---|
| 4a | 8.10(d, J=1.6 Hz, 1H), 7.88(d, J=1.6 Hz, 1H), 7.86(dd, J=0.8, 7.6 Hz, 1H), 7.66(t, J=8.0 Hz, 1H), 7.32(dd, J=0.8, 8.4 Hz, 1H), 6.84(d, J=8.4 Hz, 1H), 4.77(q, J=5.2 Hz, 1H), 4.23—4.31(m, 2H), 4.06(s, 3H), 4.01(s, 3H), 2.28—2.37(m, 1H), 1.33(t, J=7.2 Hz, 3H), 1.06(d, J=4.4 Hz, 3H), 1.04(d, J=4.0 Hz, 3H) |
| 4b | 8.08(d, J=1.6 Hz, 1H), 7.86(d, J=1.2 Hz, 1H), 7.84(dd, J=0.8, 7.6 Hz, 1H), 7.63(t, J=8.0 Hz, 1H), 7.31(d, J=8.4 Hz, 1H), 6.86(d, J=8.4 Hz, 1H), 4.78(q, J=4.8 Hz, 1H), 4.23—4.34(m, 6H), 2.28—2.36(m, 1H), 1.56(t, J=6.8 Hz, 6H), 1.33(t, J=6.8 Hz, 3H), 1.06(d, J=5.2 Hz, 3H), 1.04(d, J=4.8 Hz, 3H) |
| 4c | 8.07(d, J=1.6 Hz, 1H), 7.85(d, J=1.6 Hz, 1H), 7.83(dd, J=0.8, 7.6 Hz, 1H), 7.62(t, J=8.0 Hz, 1H), 7.30(dd, J=1.2, 8.4 Hz, 1H), 6.85(d, J=8.4 Hz, 1H), 4.78(q, J=4.8 Hz, 1H), 4.23—4.31(m, 2H), 4.17(t, J=6.4 Hz, 2H), 4.11(t, J=6.4 Hz, 2H), 2.28—2.36(m, 1H), 1.90—2.00(m, 4H), 1.33(t, J=7.2 Hz, 3H), 1.10—1.15(m, 6H), 1.05(d, J=4.8 Hz, 3H), 1.03(d, J=4.8 Hz, 3H) |
| 4d | 8.07(d, J=1.6 Hz, 1H), 7.85(d, J=1.6 Hz, 1H), 7.82(dd, J=1.2, 7.6 Hz, 1H), 7.62(t, J=8.0 Hz, 1H), 7.30(dd, J=0.8, 8.4 Hz, 1H), 6.85(d, J=8.4 Hz, 1H), 4.77(q, J=4.8 Hz, 1H), 4.23—4.30(m, 2H), 4.20(t, J=6.4 Hz, 2H), 4.15(t, J=6.4 Hz, 2H), 2.93—2.36(m, 1H), 1.87—1.94(m, 4H), 1.58—1.66(m, 4H), 1.33(t, J=7.2 Hz, 3H), 0.99—1.06(m, 12H) |
| 4e | 8.13(d, J=1.6 Hz, 1H), 7.94(d, J=1.6 Hz, 1H), 7.88(dd, J=0.8, 7.6 Hz, 1H), 7.61—7.67(m, 5H), 7.33—7.42(m, 7H), 6.84(d, J=8.4 Hz, 1H), 5.38(s, 2H), 5.33(s, 2H), 4.77(q, J=5.2 Hz, 1H), 4.26—4.29(m, 2H), 2.28—2.36(m, 1H), 1.33(t, J=7.2 Hz, 3H), 1.05(d, J=4.4 Hz, 3H), 1.03(d, J=4.8 Hz, 3H) |
| 4f | 8.18(s, 1H), 7.99(s, 1H), 7.92(d, J=8.0 Hz, 1H), 7.63(t, J=8.0 Hz, 1H), 7.44(d, J=8.4 Hz, 1H), 7.27—7.32(m, 4H), 7.18—7.24(m, 2H), 6.81(d, J=8.4 Hz, 1H), 5.49(s, 2H), 5.42(s, 2H), 4.80(q, J=5.2 Hz, 1H), 3.80(s, 3H), 2.28—2.36(m, 1H), 1.05(d, J=4.4 Hz, 3H), 1.03(d, J=4.4 Hz, 3H) |
| 4g | 8.13(d, J=1.2 Hz, 1H), 7.90(dd, J=1.6, 6.4 Hz, 2H), 7.65(t, J=8.0 Hz, 1H), 7.55—7.59(m, 4H), 7.33—7.38(m, 5H), 6.86(d, J=8.4 Hz, 1H), 5.31(s, 2H), 5.27(s, 2H), 4.79(q, J=5.2 Hz, 1H), 3.81(s, 3H), 2.28—2.36(m, 1H), 1.05(d, J=3.2 Hz, 3H), 1.03(d, J=3.2 Hz, 3H) |
| 4h | 8.13(d, J=1.6 Hz, 1H), 7.92(d, J=1.6 Hz, 1H), 7.90(dd, J=0.8, 7.6 Hz, 1H), 7.58—7.67(m, 5H), 7.36(dd, J=1.2, 8.4 Hz, 1H), 7.05—7.10(m, 4H), 6.88(d, J=8.4 Hz, 1H), 5.31(s, 2H), 5.27(s, 2H), 4.79(q, J=5.2 Hz, 1H), 3.81(s, 3H), 2.30—2.35(m, 1H), 1.05(d, J=3.2 Hz, 3H), 1.03(d, J=3.2 Hz, 3H) |
| 4i | 8.14(d, J=1.6 Hz, 1H), 7.89—7.91(m, 2H), 7.65(t, J=8.0 Hz, 1H), 7.34—7.45(m, 7H), 7.00—7.06(m, 2H), 6.91(d, J=8.8 Hz, 1H), 5.34(s, 2H), 5.29(s, 2H), 4.79(q, J=5.2 Hz, 1H), 3.82(s, 3H), 2.30—2.35(m, 1H), 1.05(d, J=2.4 Hz, 3H), 1.03(d, J=2.4 Hz, 3H) |
| 4j | 8.12(d, J=1.6 Hz, 1H), 7.94(d, J=1.6 Hz, 1H), 7.87(dd, J=0.8, 7.6 Hz, 1H), 7.55—7.64(m, 5H), 7.37(dd, J=1.2, 8.4 Hz, 1H), 7.28(s, 2H), 7.26(s, 2H), 6.79(d, J=8.8 Hz, 1H), 5.35(s, 2H), 5.30(s, 2H), 4.79(q, J=4.8 Hz, 1H), 3.80(s, 3H), 2.89—2.97(m, 2H), 2.27—2.35(m, 1H), 1.28(d, J=2.0 Hz, 6H), 1.26(d, J=2.4 Hz, 6H), 1.04(d, J=4.4 Hz, 3H), 1.02(d, J=4.4 Hz, 3H) |
| 4k | 8.07(s, 1H), 7.72—7.89(m, 2H), 7.57(t, J=8.0 Hz, 1H), 7.41—7.45(m, 4H), 7.32(q, J=7.2 Hz, 4H), 7.20—7.26(m, 3H), 7.02(d, J=8.8 Hz, 1H), 4.75—4.80(m, 1H), 4.39(t, J=7.2 Hz, 2H), 4.32(t, J=7.2 Hz, 2H), 4.23—4.29(m, 2H), 3.28(t, J=6.8 Hz, 4H), 2.28—2.36(m, 1H), 1.32(t, J=7.2 Hz, 3H), 1.05(d, J=4.8 Hz, 3H), 1.03(d, J=4.8 Hz, 3H) |
| 4l | 8.09(d, J=1.6 Hz, 1H), 7.85(dd, J=0.8, 7.6 Hz, 1H), 7.82(d, J=1.6 Hz, 1H), 7.62(t, J=8.0 Hz, 1H), 7.28(d, J=0.8 Hz, 1H), 7.20—7.26(m, 8H), 7.14—7.18(m, 2H), 6.81(d, J=8.4 Hz, 1H), 4.79(q, J=5.2 Hz, 1H), 4.19(t, J=6.0 Hz, 2H), 4.13(t, J=6.0 Hz, 2H), 3.80(s, 3H), 2.95—3.00(m, 4H), 2.29—2.33(m, 1H), 2.20—2.27(m, 4H), 1.04(d, J=3.6 Hz, 3H), 1.02(d, J=3.6 Hz, 3H) |
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd.), m/z[M-H]- |
|---|---|---|---|---|
| 5a | Yellow solid | 88 | 159.2—159.8 | 410.1246(410.1245) |
| 5b | Yellow solid | 86 | 102.6—103.2 | 438.1561(438.1558) |
| 5c | Yellow solid | 83 | 183.3—184.2 | 466.1873(466.1871) |
| 5d | Yellow solid | 92 | 169.8—170.4 | 494.2178(494.2184) |
| 5e | Yellow solid | 74 | 132.5—133.4 | 562.1865(562.1871) |
| 5f | Yellow solid | 85 | 153.9—154.8 | 698.0313(698.0312) |
| 5g | Yellow solid | 81 | 229.9—230.7 | 630.1085(630.1092) |
| 5h | Yellow solid | 83 | 181.3—182.2 | 598.1677(598.1683) |
| 5i | Yellow solid | 80 | 227.1—227.9 | 598.1675(598.1677) |
| 5j | Yellow solid | 67 | 109.4—110.3 | 646.2802(646.2810) |
| 5k | Yellow solid | 93 | 79.1—80.0 | 590.2174(590.2184) |
| 5l | Yellow solid | 93 | 127.1—128.4 | 618.2491(618.2497) |
Table 5 Appearances, yields, melting points and HRMS data of target compounds 5a—5l
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd.), m/z[M-H]- |
|---|---|---|---|---|
| 5a | Yellow solid | 88 | 159.2—159.8 | 410.1246(410.1245) |
| 5b | Yellow solid | 86 | 102.6—103.2 | 438.1561(438.1558) |
| 5c | Yellow solid | 83 | 183.3—184.2 | 466.1873(466.1871) |
| 5d | Yellow solid | 92 | 169.8—170.4 | 494.2178(494.2184) |
| 5e | Yellow solid | 74 | 132.5—133.4 | 562.1865(562.1871) |
| 5f | Yellow solid | 85 | 153.9—154.8 | 698.0313(698.0312) |
| 5g | Yellow solid | 81 | 229.9—230.7 | 630.1085(630.1092) |
| 5h | Yellow solid | 83 | 181.3—182.2 | 598.1677(598.1683) |
| 5i | Yellow solid | 80 | 227.1—227.9 | 598.1675(598.1677) |
| 5j | Yellow solid | 67 | 109.4—110.3 | 646.2802(646.2810) |
| 5k | Yellow solid | 93 | 79.1—80.0 | 590.2174(590.2184) |
| 5l | Yellow solid | 93 | 127.1—128.4 | 618.2491(618.2497) |
| Compd. | 1H NMR(400 MHz), δa | 13C NMR(100 MHz), δb |
|---|---|---|
| 5a | 8.92(d, J=8.0 Hz, 1H), 8.19(s, 1H), 7.88(s, 1H), 7.77(t, J=7.6 Hz, 1H), 7.70(d, J=7.2 Hz, 1H), 7.55(d, J=8.0 Hz, 1H), 4.34(t, J=7.6 Hz, 1H), 4.00(s, 3H), 3.93(s, 3H), 2.20—2.28(m, 1H), 1.00(t, J=6.0 Hz, 6H) | 182.9, 180.9, 173.1, 165.2, 158.7, 158.6, 138.9, 134.4, 134.1, 125.1, 123.4, 119.0, 118.2, 117.3, 117.2, 58.9, 56.5, 56.3, 29.7, 19.4, 18.9 |
| 5b | 12.73(s, 1H), 8.97(d, J=8.4 Hz, 1H), 8.21(d, J=1.2 Hz, 1H), 7.88(d, J=0.8 Hz, 1H), 7.75(t, J=7.6 Hz, 1H), 7.72(dd, J=1.2, 7.6 Hz, 1H), 7.55(dd, J=1.2, 8.0 Hz, 1H), 4.34(t, J=7.6 Hz, 1H), 4.30(q, J=6.8 Hz, 2H), 4.23(q, J=6.8 Hz, 2H), 2.19—2.27(m, 1H), 1.40—1.46(m, 6H), 1.00(t, J=6.8 Hz, 6H) | 183.0, 180.7, 172.9, 165.3, 158.1, 158.0, 138.7, 134.4, 134.2, 125.2, 123.5, 120.1, 118.4, 118.2, 117.4, 65.0, 64.7, 58.7, 29.5, 19.4, 19.0, 14.6 |
| 5c | 8.91(d, J=8.4 Hz, 1H), 8.18(d, J=1.2 Hz, 1H ), 7.87(d, J=1.2 Hz, 1H), 7.75(t, J=7.6 Hz, 1H), 7.71(dd, J=1.2, 7.6 Hz, 1H), 7.54(dd, J=1.2, 8.0 Hz, 1H), 4.33(t, J=7.6 Hz, 1H), 4.19(t, J=6.4 Hz, 2H), 4.11(t, J=6.4 Hz, 2H), 2.19—2.27(m, 1H), 1.76—1.87(m, 4H), 1.08(q, J=7.6 Hz, 6H), 1.00(d, J=5.6 Hz, 3H), 0.98(d, J=5.2 Hz, 3H) | 183.1, 180.6, 173.0, 165.2, 158.3, 158.2, 138.8, 134.3, 134.2, 134.1, 125.4, 123.7, 120.1, 118.4, 118.1, 117.2, 70.6, 70.4, 58.8, 29.6, 22.1, 19.4, 18.9, 10.4, 10.3 |
| 5d | 8.87(d, J=8.4 Hz, 1H), 8.18(d, J=1.6 Hz, 1H ), 7.87(d, J=1.6 Hz, 1H), 7.74(t, J=8.0 Hz, 1H), 7.71(dd, J=1.6, 7.6 Hz, 1H), 7.54(dd, J=1.6, 8.0 Hz, 1H), 4.35(t, J=7.6 Hz, 1H), 4.22(t, J=6.0 Hz, 2H), 4.15(t, J=6.0 Hz, 2H), 2.19—2.27(m, 1H), 1.74—1.83(m, 4H), 1.54—1.61(m, 4H), 0.96—1.01(m, 12H) | 183.0, 180.7,172.9, 165.3, 158.2, 158.1, 138.7, 134.3, 134.2, 134.1, 125.5, 123.8, 120.1, 118.4, 118.1, 117.3, 68.9, 68.7, 58.7, 30.8, 30.7, 29.6, 19.4, 18.9, 18.7, 13.7 |
| 5e | 8.94(d, J=8.4 Hz, 1H), 8.23(s, 1H), 8.00(s, 1H), 7.74—7.80(m, 2H), 7.63—7.68(m, 4H), 7.36—7.43(m, 7H), 5.42(s, 2H), 5.34(s, 2H), 4.34(t, J=7.2 Hz, 1H), 2.20—2.27(m, 1H), 0.99(t, J=7.6 Hz, 6H) | 182.9, 181.0, 173.0, 165.1, 157.7, 157.6, 138.9, 136.8, 136.7, 134.4, 134.3, 134.2, 128.5, 128.3, 128.2, 127.7, 127.6, 127.0, 126.9, 126.8, 125.6, 123.9, 120.5, 118.9, 118.6, 117.7, 70.4, 70.1, 58.8, 29.7, 19.4, 18.9 |
| 5f | 8.69(d, J=6.8 Hz, 1H), 8.21(d, J=1.2 Hz, 1H), 8.03(d, J=1.6 Hz, 1H), 7.79(d, J=1.2 Hz, 1H), 7.78(s, 1H), 7.69(t, J=4.8 Hz, 1H), 7.42—7.50(m, 6H), 5.41(s, 2H), 5.33(s, 2H), 4.29(q, J=6.4 Hz, 1H), 2.19—2.27(m, 1H), 0.99(d, J=2.4 Hz, 3H), 0.97(d, J=2.4 Hz, 3H) | 182.6, 180.4, 164.8, 157.8, 157.7, 139.2, 136.2, 134.3, 134.2, 134.1, 131.4, 131.3, 131.2, 128.7, 126.2, 124.7, 121.6, 119.6, 119.4, 118.3, 67.1, 67.0, 59.2, 40.2, 30.0, 19.5, 18.8 |
| 5g | 8.75(d, J=7.6 Hz, 1H), 8.21(d, J=1.2 Hz, 1H), 7.97(d, J=1.2 Hz, 1H), 7.73—7.80(m, 2H), 7.60—7.67(m, 5H), 7.44(s, 2H), 7.42(s, 2H), 5.37(s, 2H), 5.30(s, 2H), 4.32(q, J=6.4 Hz, 1H), 2.20—2.28(m, 1H), 1.00(d, J=5.2 Hz, 3H), 0.98(t, J=5.2 Hz, 3H) | 182.8, 181.0, 165.0, 157.5, 157.4, 139.0, 135.9, 135.8, 134.5, 134.2, 134.1, 132.2, 132.1, 128.9, 128.8, 128.3, 128.2, 125.6, 123.9, 120.4, 118.8, 118.7, 117.8, 69.7, 69.4, 58.9, 29.7, 19.4, 18.9 |
| 5h | 8.46(s, 1H), 8.10(s, 1H), 7.93(s, 1H), 7.55—7.71(m, 7H), 7.18(q, J=8.0 Hz, 4H), 5.31(s, 2H), 5.24(s, 2H), 4.25(t, J=6.8 Hz, 1H), 2.19—2.28(m, 1H), 0.94(s, 3H), 0.93(s, 3H) | 182.7, 180.8, 164.5, 162.8, 160.4, 157.6, 139.5, 134.3, 134.0, 133.0, 129.0, 125.2, 123.7, 120.4, 118.7, 117.4, 115.1, 114.9, 69.8, 69.5, 59.7, 30.5, 19.7, 18.7 |
| 5i | 12.75(s, 1H), 8.99(d, J=8.4 Hz, 1H), 8.28(d, J=1.6 Hz, 1H), 8.01(d, J=1.6 Hz, 1H), 7.78—7.84(m, 2H), 7.67(dd, J=2.0, 8.0 Hz, 1H), 7.42—7.56(m, 6H), 7.16—7.21(m, 2H), 5.45(s, 2H), 5.38(s, 2H), 4.36(q, J=7.2 Hz, 1H), 2.20—2.28(m, 1H), 1.01(d, J=6.8 Hz, 3H), 0.99(d, J=6.8 Hz, 3H) | 182.8, 181.0, 172.9, 165.2, 163.5, 161.1, 157.6, 157.5, 139.8, 139.7, 139.6, 139.6, 138.9, 134.6, 134.2, 134.3, 130.3, 130.2, 125.3, 123.6, 122.5, 120.4, 118.8, 117.9, 114.4, 114.2, 113.6, 113.3, 69.6, 69.4, 58.7, 29.6, 19.4, 18.9 |
| 5j | 8.90(d, J=7.2 Hz, 1H), 8.23(s, 1H), 8.01(s, 1H), 7.74—7.80(m, 2H), 7.65(d, J=8.0 Hz, 1H), 7.58(t, J=8.8 Hz, 4H), 7.29(d, J=7.6 Hz, 4H), 5.36(s, 2H), 5.29(s, 2H), 4.34(t, J=7.6 Hz, 1H), 2.89—2.96(m, 2H), 2.20—2.29(m, 1H), 1.24(s, 6H), 1.23(s, 6H), 0.99(t, J=7.6 Hz, 6H) | 182.9, 180.9, 165.1, 157.8, 157.7, 147.8, 147.7, 138.9, 134.4, 134.2, 134.1, 134.0, 127.1, 127.0, 126.2, 126.1, 125.5, 123.8, 120.5, 118.8, 118.6, 117.6, 70.4, 70.1, 58.9, 33.3, 29.7, 23.9, 23.8, 19.4, 18.9 |
| 5k | 8.26(d, J=1.2 Hz, 1H), 7.91(s, 1H), 7.79(d, J=7.6 Hz, 1H), 7.58(t, J=8.0 Hz, 1H), 7.41—7.45(m, 4H), 7.27—7.38(m, 6H), 7.20—7.24(m, 2H), 4.90(q, J=5.2 Hz, 1H), 4.41(t, J=7.2 Hz, 2H), 4.34(t, J=6.8 Hz, 2H), 3.26—3.30(m, 4H), 2.35—2.41(m, 1H), 1.09(d, J=3.6 Hz, 3H), 1.07(d, J=3.2 Hz, 3H) | 184.6, 181.6, 174.9, 165.7, 159.2, 158.8, 138.3, 138.1, 138.0, 134.7, 134.6, 134.1, 129.5, 128.7, 128.6, 126.8, 126.7, 126.6, 124.5, 120.1, 119.5, 119.3, 116.5, 100.1, 71.0, 70.9, 57.9, 36.0, 35.9, 31.8, 19.2, 18.3 |
| 5l | 8.30(d, J=7.6 Hz, 1H), 8.10(s, 1H), 7.83(s, 1H), 7.67—7.73(m, 2H), 7.49(dd, J=1.2, 7.2 Hz, 1H), 7.12—7.26(m, 10H), 4.21—4.27(m, 1H), 4.18(t, J=6.0 Hz, 2H), 4.11(t, J=6.0 Hz, 2H), 2.90(t, J=7.6 Hz, 4H), 2.17—2.24(m, 1H), 2.04—2.10(m, 4H), 0.93(s, 3H), 0.92(s, 3H) | 182.9, 180.7, 164.4, 158.2, 141.6, 141.5, 139.6, 134.2, 134.1, 134.0, 128.4, 128.3, 128.2, 125.7, 125.1, 123.6, 119.9, 118.2, 118.0, 116.9, 68.0, 67.8, 59.7, 31.3, 30.6, 30.5, 19.7, 18.7 |
Table 6 1H NMR and 13C NMR data of target compounds 5a—5l
| Compd. | 1H NMR(400 MHz), δa | 13C NMR(100 MHz), δb |
|---|---|---|
| 5a | 8.92(d, J=8.0 Hz, 1H), 8.19(s, 1H), 7.88(s, 1H), 7.77(t, J=7.6 Hz, 1H), 7.70(d, J=7.2 Hz, 1H), 7.55(d, J=8.0 Hz, 1H), 4.34(t, J=7.6 Hz, 1H), 4.00(s, 3H), 3.93(s, 3H), 2.20—2.28(m, 1H), 1.00(t, J=6.0 Hz, 6H) | 182.9, 180.9, 173.1, 165.2, 158.7, 158.6, 138.9, 134.4, 134.1, 125.1, 123.4, 119.0, 118.2, 117.3, 117.2, 58.9, 56.5, 56.3, 29.7, 19.4, 18.9 |
| 5b | 12.73(s, 1H), 8.97(d, J=8.4 Hz, 1H), 8.21(d, J=1.2 Hz, 1H), 7.88(d, J=0.8 Hz, 1H), 7.75(t, J=7.6 Hz, 1H), 7.72(dd, J=1.2, 7.6 Hz, 1H), 7.55(dd, J=1.2, 8.0 Hz, 1H), 4.34(t, J=7.6 Hz, 1H), 4.30(q, J=6.8 Hz, 2H), 4.23(q, J=6.8 Hz, 2H), 2.19—2.27(m, 1H), 1.40—1.46(m, 6H), 1.00(t, J=6.8 Hz, 6H) | 183.0, 180.7, 172.9, 165.3, 158.1, 158.0, 138.7, 134.4, 134.2, 125.2, 123.5, 120.1, 118.4, 118.2, 117.4, 65.0, 64.7, 58.7, 29.5, 19.4, 19.0, 14.6 |
| 5c | 8.91(d, J=8.4 Hz, 1H), 8.18(d, J=1.2 Hz, 1H ), 7.87(d, J=1.2 Hz, 1H), 7.75(t, J=7.6 Hz, 1H), 7.71(dd, J=1.2, 7.6 Hz, 1H), 7.54(dd, J=1.2, 8.0 Hz, 1H), 4.33(t, J=7.6 Hz, 1H), 4.19(t, J=6.4 Hz, 2H), 4.11(t, J=6.4 Hz, 2H), 2.19—2.27(m, 1H), 1.76—1.87(m, 4H), 1.08(q, J=7.6 Hz, 6H), 1.00(d, J=5.6 Hz, 3H), 0.98(d, J=5.2 Hz, 3H) | 183.1, 180.6, 173.0, 165.2, 158.3, 158.2, 138.8, 134.3, 134.2, 134.1, 125.4, 123.7, 120.1, 118.4, 118.1, 117.2, 70.6, 70.4, 58.8, 29.6, 22.1, 19.4, 18.9, 10.4, 10.3 |
| 5d | 8.87(d, J=8.4 Hz, 1H), 8.18(d, J=1.6 Hz, 1H ), 7.87(d, J=1.6 Hz, 1H), 7.74(t, J=8.0 Hz, 1H), 7.71(dd, J=1.6, 7.6 Hz, 1H), 7.54(dd, J=1.6, 8.0 Hz, 1H), 4.35(t, J=7.6 Hz, 1H), 4.22(t, J=6.0 Hz, 2H), 4.15(t, J=6.0 Hz, 2H), 2.19—2.27(m, 1H), 1.74—1.83(m, 4H), 1.54—1.61(m, 4H), 0.96—1.01(m, 12H) | 183.0, 180.7,172.9, 165.3, 158.2, 158.1, 138.7, 134.3, 134.2, 134.1, 125.5, 123.8, 120.1, 118.4, 118.1, 117.3, 68.9, 68.7, 58.7, 30.8, 30.7, 29.6, 19.4, 18.9, 18.7, 13.7 |
| 5e | 8.94(d, J=8.4 Hz, 1H), 8.23(s, 1H), 8.00(s, 1H), 7.74—7.80(m, 2H), 7.63—7.68(m, 4H), 7.36—7.43(m, 7H), 5.42(s, 2H), 5.34(s, 2H), 4.34(t, J=7.2 Hz, 1H), 2.20—2.27(m, 1H), 0.99(t, J=7.6 Hz, 6H) | 182.9, 181.0, 173.0, 165.1, 157.7, 157.6, 138.9, 136.8, 136.7, 134.4, 134.3, 134.2, 128.5, 128.3, 128.2, 127.7, 127.6, 127.0, 126.9, 126.8, 125.6, 123.9, 120.5, 118.9, 118.6, 117.7, 70.4, 70.1, 58.8, 29.7, 19.4, 18.9 |
| 5f | 8.69(d, J=6.8 Hz, 1H), 8.21(d, J=1.2 Hz, 1H), 8.03(d, J=1.6 Hz, 1H), 7.79(d, J=1.2 Hz, 1H), 7.78(s, 1H), 7.69(t, J=4.8 Hz, 1H), 7.42—7.50(m, 6H), 5.41(s, 2H), 5.33(s, 2H), 4.29(q, J=6.4 Hz, 1H), 2.19—2.27(m, 1H), 0.99(d, J=2.4 Hz, 3H), 0.97(d, J=2.4 Hz, 3H) | 182.6, 180.4, 164.8, 157.8, 157.7, 139.2, 136.2, 134.3, 134.2, 134.1, 131.4, 131.3, 131.2, 128.7, 126.2, 124.7, 121.6, 119.6, 119.4, 118.3, 67.1, 67.0, 59.2, 40.2, 30.0, 19.5, 18.8 |
| 5g | 8.75(d, J=7.6 Hz, 1H), 8.21(d, J=1.2 Hz, 1H), 7.97(d, J=1.2 Hz, 1H), 7.73—7.80(m, 2H), 7.60—7.67(m, 5H), 7.44(s, 2H), 7.42(s, 2H), 5.37(s, 2H), 5.30(s, 2H), 4.32(q, J=6.4 Hz, 1H), 2.20—2.28(m, 1H), 1.00(d, J=5.2 Hz, 3H), 0.98(t, J=5.2 Hz, 3H) | 182.8, 181.0, 165.0, 157.5, 157.4, 139.0, 135.9, 135.8, 134.5, 134.2, 134.1, 132.2, 132.1, 128.9, 128.8, 128.3, 128.2, 125.6, 123.9, 120.4, 118.8, 118.7, 117.8, 69.7, 69.4, 58.9, 29.7, 19.4, 18.9 |
| 5h | 8.46(s, 1H), 8.10(s, 1H), 7.93(s, 1H), 7.55—7.71(m, 7H), 7.18(q, J=8.0 Hz, 4H), 5.31(s, 2H), 5.24(s, 2H), 4.25(t, J=6.8 Hz, 1H), 2.19—2.28(m, 1H), 0.94(s, 3H), 0.93(s, 3H) | 182.7, 180.8, 164.5, 162.8, 160.4, 157.6, 139.5, 134.3, 134.0, 133.0, 129.0, 125.2, 123.7, 120.4, 118.7, 117.4, 115.1, 114.9, 69.8, 69.5, 59.7, 30.5, 19.7, 18.7 |
| 5i | 12.75(s, 1H), 8.99(d, J=8.4 Hz, 1H), 8.28(d, J=1.6 Hz, 1H), 8.01(d, J=1.6 Hz, 1H), 7.78—7.84(m, 2H), 7.67(dd, J=2.0, 8.0 Hz, 1H), 7.42—7.56(m, 6H), 7.16—7.21(m, 2H), 5.45(s, 2H), 5.38(s, 2H), 4.36(q, J=7.2 Hz, 1H), 2.20—2.28(m, 1H), 1.01(d, J=6.8 Hz, 3H), 0.99(d, J=6.8 Hz, 3H) | 182.8, 181.0, 172.9, 165.2, 163.5, 161.1, 157.6, 157.5, 139.8, 139.7, 139.6, 139.6, 138.9, 134.6, 134.2, 134.3, 130.3, 130.2, 125.3, 123.6, 122.5, 120.4, 118.8, 117.9, 114.4, 114.2, 113.6, 113.3, 69.6, 69.4, 58.7, 29.6, 19.4, 18.9 |
| 5j | 8.90(d, J=7.2 Hz, 1H), 8.23(s, 1H), 8.01(s, 1H), 7.74—7.80(m, 2H), 7.65(d, J=8.0 Hz, 1H), 7.58(t, J=8.8 Hz, 4H), 7.29(d, J=7.6 Hz, 4H), 5.36(s, 2H), 5.29(s, 2H), 4.34(t, J=7.6 Hz, 1H), 2.89—2.96(m, 2H), 2.20—2.29(m, 1H), 1.24(s, 6H), 1.23(s, 6H), 0.99(t, J=7.6 Hz, 6H) | 182.9, 180.9, 165.1, 157.8, 157.7, 147.8, 147.7, 138.9, 134.4, 134.2, 134.1, 134.0, 127.1, 127.0, 126.2, 126.1, 125.5, 123.8, 120.5, 118.8, 118.6, 117.6, 70.4, 70.1, 58.9, 33.3, 29.7, 23.9, 23.8, 19.4, 18.9 |
| 5k | 8.26(d, J=1.2 Hz, 1H), 7.91(s, 1H), 7.79(d, J=7.6 Hz, 1H), 7.58(t, J=8.0 Hz, 1H), 7.41—7.45(m, 4H), 7.27—7.38(m, 6H), 7.20—7.24(m, 2H), 4.90(q, J=5.2 Hz, 1H), 4.41(t, J=7.2 Hz, 2H), 4.34(t, J=6.8 Hz, 2H), 3.26—3.30(m, 4H), 2.35—2.41(m, 1H), 1.09(d, J=3.6 Hz, 3H), 1.07(d, J=3.2 Hz, 3H) | 184.6, 181.6, 174.9, 165.7, 159.2, 158.8, 138.3, 138.1, 138.0, 134.7, 134.6, 134.1, 129.5, 128.7, 128.6, 126.8, 126.7, 126.6, 124.5, 120.1, 119.5, 119.3, 116.5, 100.1, 71.0, 70.9, 57.9, 36.0, 35.9, 31.8, 19.2, 18.3 |
| 5l | 8.30(d, J=7.6 Hz, 1H), 8.10(s, 1H), 7.83(s, 1H), 7.67—7.73(m, 2H), 7.49(dd, J=1.2, 7.2 Hz, 1H), 7.12—7.26(m, 10H), 4.21—4.27(m, 1H), 4.18(t, J=6.0 Hz, 2H), 4.11(t, J=6.0 Hz, 2H), 2.90(t, J=7.6 Hz, 4H), 2.17—2.24(m, 1H), 2.04—2.10(m, 4H), 0.93(s, 3H), 0.92(s, 3H) | 182.9, 180.7, 164.4, 158.2, 141.6, 141.5, 139.6, 134.2, 134.1, 134.0, 128.4, 128.3, 128.2, 125.7, 125.1, 123.6, 119.9, 118.2, 118.0, 116.9, 68.0, 67.8, 59.7, 31.3, 30.6, 30.5, 19.7, 18.7 |
| Compd. | IC50/(μmol·L-1) | ||||
|---|---|---|---|---|---|
| Hela | MCF7 | HepG2 | KB | HEK293T | |
| Rhein-Na | >100 | >100 | >100 | >100 | >100 |
| 5a | >100 | >100 | >100 | >100 | >100 |
| 5b | >100 | >100 | >100 | >100 | >100 |
| 5c | >100 | >100 | >100 | >100 | >100 |
| 5d | 67.8 | >100 | 54.4 | 28.6 | 43.1 |
| 5e | 44.9 | 36.5 | 36.1 | 15.6 | 21.5 |
| 5f | >100 | >100 | >100 | >100 | >100 |
| 5g | >100 | >100 | >100 | >100 | >100 |
| 5h | >100 | >100 | >100 | >100 | >100 |
| 5i | 35.4 | 38.6 | 29.6 | 17.8 | 12.9 |
| 5j | 37.5 | 13.9 | 5.8 | 9.4 | 1.9 |
| 5k | >100 | >100 | 56.5 | >100 | >100 |
| 5l | 9.4 | 4.5 | 3.6 | 4.9 | 1.6 |
| DDP | 5.5 | 15.8 | 14.3 | 6.1 | 3.3 |
| Doxorubicin | 0.99 | 2.8 | 4.7 | 3.7 | 0.7 |
Table 7 Antitumor activity of target compoundsin vitro*
| Compd. | IC50/(μmol·L-1) | ||||
|---|---|---|---|---|---|
| Hela | MCF7 | HepG2 | KB | HEK293T | |
| Rhein-Na | >100 | >100 | >100 | >100 | >100 |
| 5a | >100 | >100 | >100 | >100 | >100 |
| 5b | >100 | >100 | >100 | >100 | >100 |
| 5c | >100 | >100 | >100 | >100 | >100 |
| 5d | 67.8 | >100 | 54.4 | 28.6 | 43.1 |
| 5e | 44.9 | 36.5 | 36.1 | 15.6 | 21.5 |
| 5f | >100 | >100 | >100 | >100 | >100 |
| 5g | >100 | >100 | >100 | >100 | >100 |
| 5h | >100 | >100 | >100 | >100 | >100 |
| 5i | 35.4 | 38.6 | 29.6 | 17.8 | 12.9 |
| 5j | 37.5 | 13.9 | 5.8 | 9.4 | 1.9 |
| 5k | >100 | >100 | 56.5 | >100 | >100 |
| 5l | 9.4 | 4.5 | 3.6 | 4.9 | 1.6 |
| DDP | 5.5 | 15.8 | 14.3 | 6.1 | 3.3 |
| Doxorubicin | 0.99 | 2.8 | 4.7 | 3.7 | 0.7 |
| [1] | Xu X., Li B. P., Zhang H. F., Shanghai J. Tradit. Chin. Med., 2003, 37(4), 56—59 |
| (徐翔, 郦柏平, 张慧芬. 上海中医药杂志,2003, 37(4), 56—59) | |
| [2] | Fu X. S., Chen F., Liu X. H., Xu H., Zhou Y. Z., Chin. J. New Drugs, 2011, 20(16), 1534—1538 |
| (傅兴圣, 陈菲, 刘渊红, 许虎, 周逸芝. 中国新药杂志,2011, 20(16), 1534—1538) | |
| [3] | Sheng X. Y., Wang M., Lu M., Xi B. L., Sheng H. G., Zang Y. Q., Am. J. Physiol. Endocrinol. Metab., 2011, 300(5), E886—E893 |
| [4] | Tang J. C., Yang H., Song X. Y., Song X. H., Yan S. L., Shao J. Q., Zhang T. L., Zhang J. N., Phytother. Res., 2009, 23(2), 159—164 |
| [5] | Badria F. A., Ibrahim A. S., Drug Discov. Ther., 2013, 7(2), 84—89 |
| [6] | Fernand V. E., Losso J. N., Truax R. E., Villar E. E., Bwambok D. K., Fakayode S. O., Lowry M., Warner I. M., Chem. Biol. Interact., 2011, 192(3), 220—232 |
| [7] | Ip S. W., Weng Y. S., Lin S. Y., Yang M. D., Tang N. Y., Su C. C., Chung J. G., Anticancer Res., 2007, 27(1A), 379—389 |
| [8] | Wang Q., Zhang N. N., Li H. Y., Jiang M., Gao J., Bai G., Acta Pharm. Sin., 2012, 47(12), 1618—1622 |
| (王倩, 张楠楠, 李红艳, 姜民, 高洁, 白钢. 药学学报,2012, 47(12), 1618—1622) | |
| [9] | Hsia T. C., Yang J. S., Chen G. W.,Chiu T. H., Lu H. F., Yang M. D., Yu F. S., Liu K. C., Lai K. C., Lin C. C., Chung J. G., Anticancer Res., 2009, 29(1), 309—318 |
| [10] | Peng L. L., Yang J. Y., Ning C., Zhang J., Xiao X. C., He D., Wang X. Y., Li Z. P., Fu S. S., Ning J. P., Biol. Pharm. Bull., 2012, 35(10), 1676—1685 |
| [11] | Liu X., Cheng J., Zheng X. C., Chen Y. G., Wu C., Li B., Fu J. F., Cao H. W., Lu Y. L., Li J., Zheng J., Zhou H., Int. Immuno-pharmacol., 2009, 9(9), 1021—1031 |
| [12] | Yu L., Xiang H., Fan J. W., Wang D. C., Yang F., Guo N., Jin Q., Deng X. M., J. Biotechnol., 2008, 135(3), 304—308 |
| [13] | Chung J. G., Tsou M. F., Wang H. H., Lo H. H., Hsieh S. E., Yen Y. S., Wu L. T., Chang S. H., Ho C. C., Hung C. F., J. Appl. Toxicol., 1998, 18(2), 117—123 |
| [14] | Hao K., Qi Q., Wan P., Zhang J. W., Hao H. P., Liang Y., Xie L., Wang G. J., Sun J. G., Basic Clin. Pharmacol. Toxicol., 2014, 114(2), 160—167 |
| [15] | Gao Q., Qin W. S., Jia Z. H., Zheng J. M., Zeng C. H., Li L. S., Liu Z. H., Planta Med., 2010, 76(1), 27—33 |
| [16] | Ye M. Y., Yao G. Y., Wei J. C., Pan Y. M., Liao Z. X., Wang H. S., Int. J. Mol. Sci., 2013, 14(5), 9424—9439 |
| [17] | Liang Y. K., Yue Z. Z., Li J. X., Tan C., Miao Z. H., Tan W. F., Yang C. H., Eur. J. Med. Chem., 2014, 84, 505—515 |
| [18] | Ji C. M., Zhen Y. Z., Fan X. Y., Wang Z. J., Jiang S. F., Zhu L. H., Zhang G. L., Basic Clin. Med., 2014, 34(2), 155—159 |
| (纪春梅, 甄永占, 范晓禹, 王志军, 蒋守芳, 朱丽华, 章广玲. 基础医学与临床,2014, 34(2), 155—159) | |
| [19] | Guo L., Cao R. H., Fan W. X., Ma Q., Chem. J. Chinese Universities, 2014, 35(3), 518—523 |
| (郭亮, 曹日晖, 范文玺, 马芹. 高等学校化学学报,2014, 35(3), 518—523) | |
| [20] | Guo L., Cao R. H., Fan W. X., Gan Z. Y., Ma Q., Chem. J. Chinese Universities, 2016, 37(6), 1093—1099 |
| (郭亮, 曹日晖, 范文玺, 甘紫云, 马芹. 高等学校化学学报,2016, 37(6), 1093—1099) | |
| [21] | Zhang S. B., Qian X., Zhang D. H., Zhu J. M., Wu Y., Guo Y., Xu L., Chem. Res. Chinese Universities, 2016, 32(1), 149—154 |
| [22] | Ma J. J., Hu G., Xie L. J., Chen L., Xu B. X., Gong P., Chem. Res. Chinese Universities, 2015, 31(6), 958—963 |
| [23] | Long E. C., Barton J. K., Acc. Chem. Res., 1990, 23(9), 271—273 |
| [24] | Sirajuddin M., Ali S., Badshah A., J. Photochem. Photobiol., B: Biol., 2013, 124, 1—19 |
| [1] | 赵盈喆, 张建玲. 金属-有机框架基材料在二氧化碳光催化转化中的应用[J]. 高等学校化学学报, 2022, 43(7): 20220223. |
| [2] | 杨丹, 刘旭, 戴翼虎, 祝艳, 杨艳辉. 金团簇电催化二氧化碳还原反应的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220198. |
| [3] | 孙海珠, 杨国夺, 杨柏. 碳点的设计合成、 结构调控及应用[J]. 高等学校化学学报, 2021, 42(2): 349. |
| [4] | 侯华, 王宝山. 六氟化硫替代气体绝缘强度的官能团加和理论方法[J]. 高等学校化学学报, 2021, 42(12): 3709. |
| [5] | 叶晓栋, 齐国栋, 徐君, 邓风. Au负载SBA-15分子筛上葡萄糖氧化反应[J]. 高等学校化学学报, 2020, 41(5): 960. |
| [6] | 肖艳华, 张广杰, 宗良, 刘国宏, 任丽君, 董俊兴. 开口箭化学成分及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(9): 1897. |
| [7] | 常俊朋, 赵佳瑞, 陈思佳, 孟凯, 石微妮, 李瑞芳. 抗菌肽SAMP1及其类似肽的构效关系[J]. 高等学校化学学报, 2019, 40(4): 705. |
| [8] | 吕明君, 李雯, 杨新颖, 方浩. N9位芳基取代嘌呤-8-酮类衍生物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(2): 254. |
| [9] | 方芳,薛良敏,丛婧,田超,王孝伟,刘俊义,张志丽. 2-位或4-位取代吡啶并嘧啶类非经典叶酸拮抗剂的合成及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(10): 2111. |
| [10] | 张培全,杨倩倩,龙惠丹,陈鑫. 金诺芬衍生物的合成及抗肿瘤活性[J]. 高等学校化学学报, 2019, 40(10): 2097. |
| [11] | 刘莉, 马洋洋, 王宽, 贾云静, 李婉, 朱华结. β-咔啉衍生物的抗肿瘤及抗菌活性[J]. 高等学校化学学报, 2018, 39(4): 674. |
| [12] | 余敏, 黄晶晶, 马敏, 付瑞燕, 鄢嫣, 张福生, 殷俊峰, 谢宁宁. 三肽的锌螯合活性及定量构效关系分析[J]. 高等学校化学学报, 2018, 39(2): 234. |
| [13] | 侯华, 余小娟, 周文俊, 罗运柏, 王宝山. 绝缘气体介电强度的构效关系[J]. 高等学校化学学报, 2018, 39(11): 2477. |
| [14] | 王磊, 郑国钧, 季奇, 陈博, 巩龙龙, 高聪敏, 杜镇建, 张兴民. PI3K/mTOR抑制剂的合成及生物活性[J]. 高等学校化学学报, 2017, 38(9): 1590. |
| [15] | 刘玉明, 田丽珺, 胡栋, 聂建兵. 4-N-苯胺基喹啉衍生物的合成及胆碱酯酶抑制活性[J]. 高等学校化学学报, 2017, 38(3): 392. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||