

高等学校化学学报 ›› 2024, Vol. 45 ›› Issue (8): 20240177.doi: 10.7503/cjcu20240177
班志勇1,2, 杨曹雨2,3, 冯清2,3, 殷国俊1( ), 李国栋2,3(
), 李国栋2,3( )
)
收稿日期:2024-04-10
出版日期:2024-08-10
发布日期:2024-07-08
通讯作者:
殷国俊,李国栋
E-mail:gjyin@126.com;liguodong@nanoctr.cn
作者简介:第一联系人:共同第一作者.
基金资助:
BAN Zhiyong1,2, YANG Caoyu2,3, FENG Qing2,3, YIN Guojun1( ), LI Guodong2,3(
), LI Guodong2,3( )
)
Received:2024-04-10
Online:2024-08-10
Published:2024-07-08
Contact:
YIN Guojun, LI Guodong
E-mail:gjyin@126.com;liguodong@nanoctr.cn
Supported by:摘要:
偶氮苯和氧化偶氮苯类化合物在颜料、 光学材料、 荧光探针和光电器件等领域具有广泛的应用前景. 目前, 已发展了不同的催化剂和氧化剂用于苯胺氧化偶联制备偶氮苯及氧化偶氮苯类化合物, 其中开发绿色环保的氧化体系一直是该研究领域的热点和难点问题. 本文综合评述了分别以双氧水和氧气作为氧化剂时, 催化苯胺选择性氧化制备偶氮苯及氧化偶氮苯类化合物的研究进展, 同时探讨了苯胺氧化的机理, 主要包括亚硝基苯中间体机理与自由基偶联机理. 最后, 总结了催化剂合成和催化机制方面存在的潜在问题和挑战, 并对未来的研究方向进行了展望, 从而为相关领域的发展提供借鉴.
中图分类号:
TrendMD:
班志勇, 杨曹雨, 冯清, 殷国俊, 李国栋. 绿色氧化剂催化苯胺氧化偶联反应的研究进展. 高等学校化学学报, 2024, 45(8): 20240177.
BAN Zhiyong, YANG Caoyu, FENG Qing, YIN Guojun, LI Guodong. Research Progress on Catalytic Oxidative Coupling Reaction of Aniline with Green Oxidants. Chem. J. Chinese Universities, 2024, 45(8): 20240177.
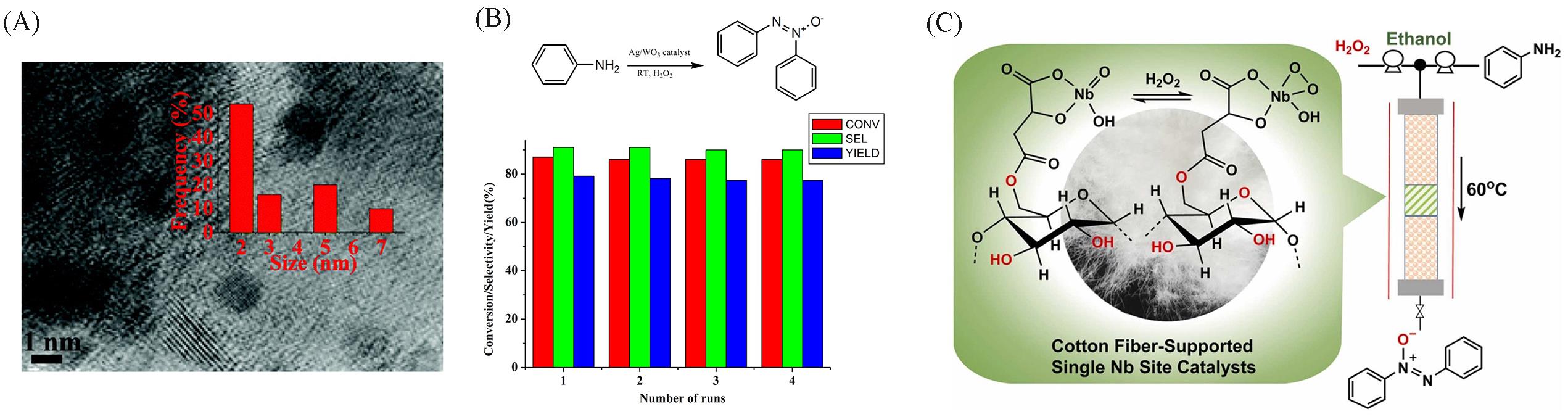
Fig.2 Transmission electron microscope image of 2.5%(mass fraction) Ag/WO3 and particle size distribution of Ag nanoparticles(A), recyclability test of 2.5%(mass fraction) Ag/WO3 nanostructure catalyst for the oxidation of aniline to azoxybenzene(B)[62], schematic diagram of Cot⁃MA⁃12⁃Nb catalyst in continuous⁃flow catalysis(C)[64](A, B) Copyright 2015, the Royal Society of Chemistry; (C) Copyright 2023, Elsevier Inc.
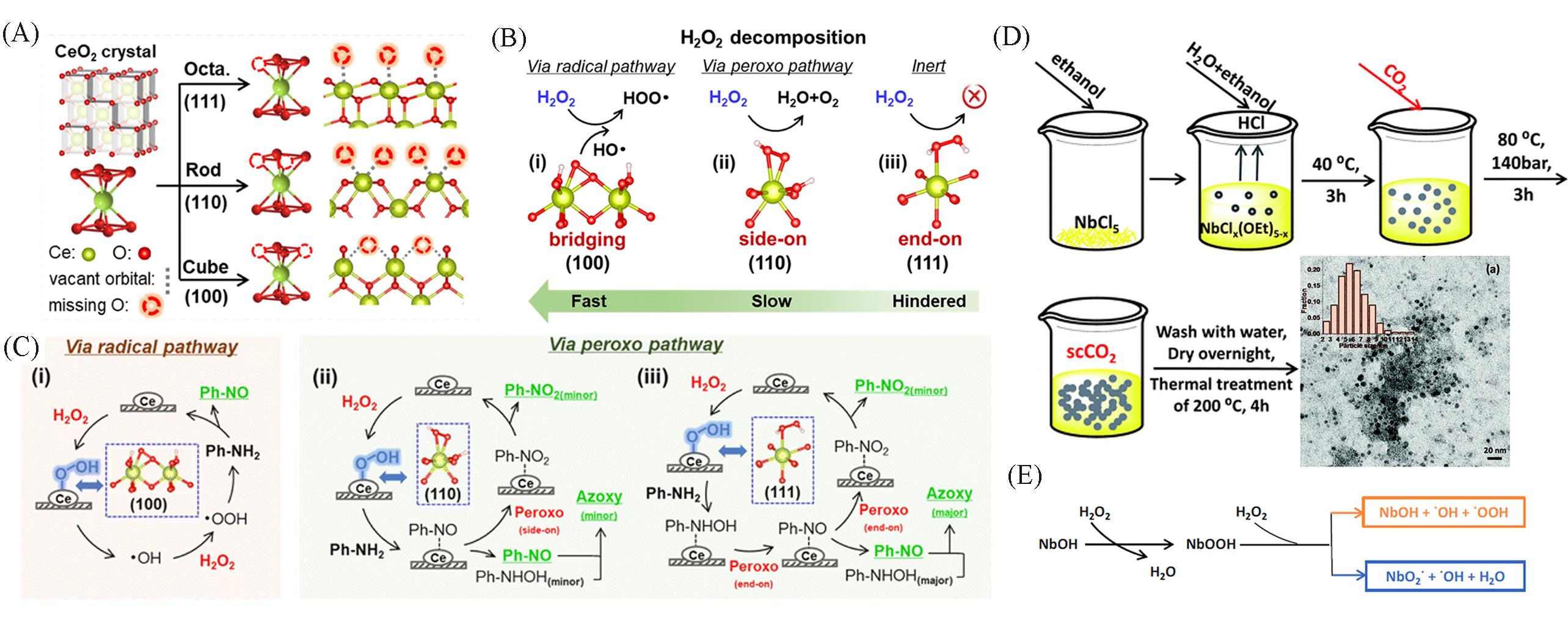
Fig.3 Schematic diagram of the coordination mode of CeO2 in the (111), (110), and (100) crystal facets(A), the configuration of peroxide species formed by H2O2 on the CeO2 (100), (110) and (111) crystal facets and their activation of H2O2(B), the aniline oxidation process on the CeO2(100), (110) and (111) crystal facets(C)[73], schematic diagram of the supercritical CO2⁃assisted Nb2O5 synthesis process(D), schematic diagram of the activation process of H2O2 by NbOH(E)[80](A—C) Copyright 2023, the Royal Society of Chemistry; (D—E) Copyright 2019, the Royal Society of Chemistry.
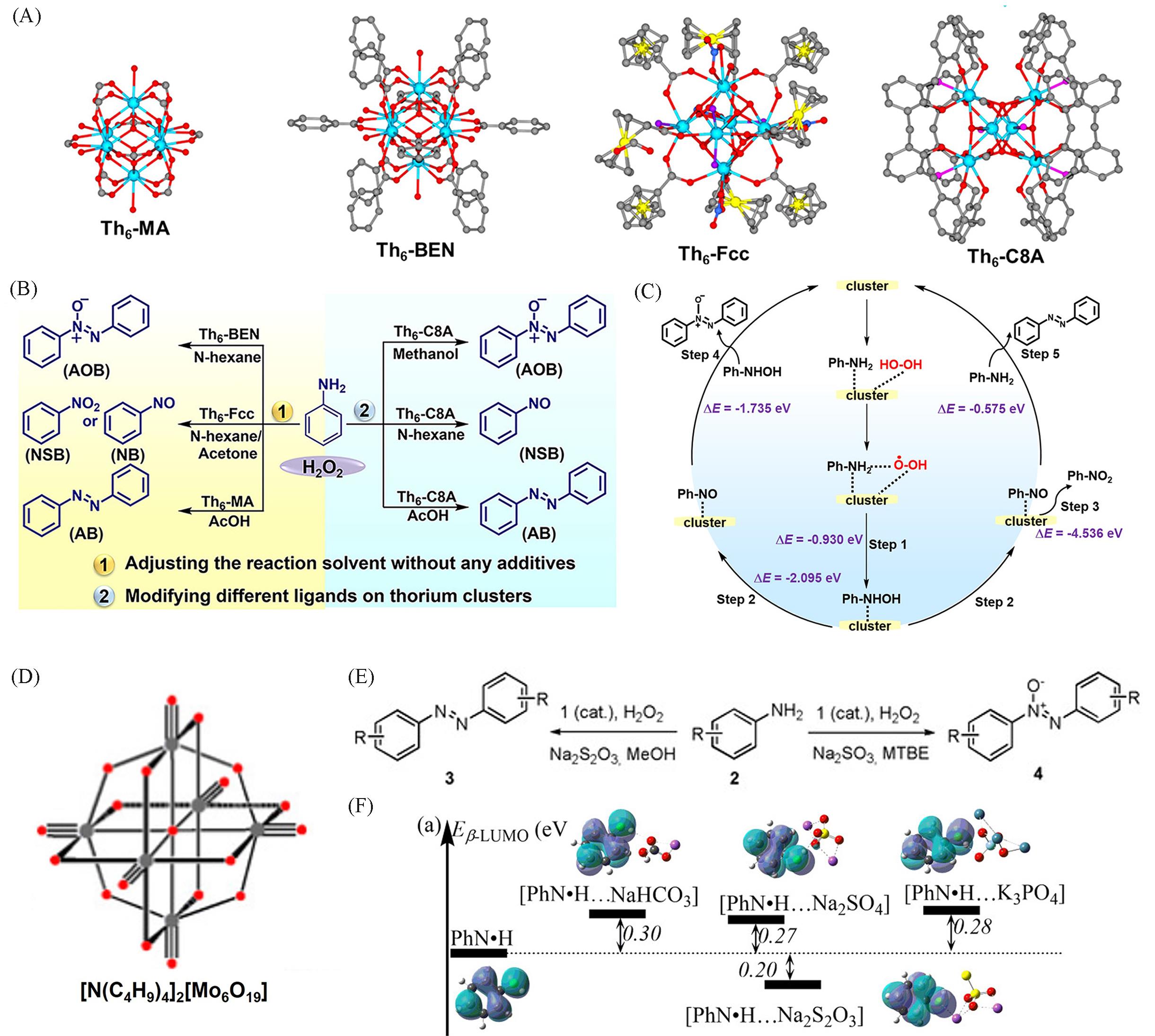
Fig.4 Structures of Th6⁃MA, Th6⁃BEN, Th6⁃Fcc and Th6⁃C8A clusters(A), selectivity of aniline oxidation products by using ligands and reaction solvents for regulating Th clusters(B), reaction mechanism of aniline oxidation through nitrosobenzene intermediates(C)[93], [Mo6O19]2- cluster structure(D), selective oxidation of aniline to different products by solvents and additives modulating the reaction(E)[94], energy changes of β⁃LUMO of aniline radical intermediates after binding to different additive ions(F)[95](A—C) Copyright 2022, American Chemical Society; (D—E) Copyright 2020, Wiley-VCH GmbH; (F) Copyright 2023, American Chemical Society.
| Catalyst | Oxidant | Temp./oC | Conv.(%) | Selectivity(%) | Generation rate/ (mmol·g-1·h-1) | Ref. | |
|---|---|---|---|---|---|---|---|
| Azobenzene | Azoxybenzene | ||||||
| CuCr2O4 | H2O2 | 70 | 78 | — | 92 | 7.700 | [ |
| [N(C4H9)4]2[Mo6O19] | H2O2 | 60 | — | — | 99%(Yield) | 1.517 | [ |
| [N(C4H9)4]2[Mo6O19] | H2O2 | 50 | — | — | 93%(Yield) | 1.900 | [ |
| Zr(OH)4 | H2O2 | r. t. | 98 | — | 98 | 25.82 | [ |
| Zr(OH)4 | H2O2 | 40 | 95 | — | 97 | 2.064 | [ |
| {Ni6}POM | H2O2 | 70 | 99.4 | — | 86 | 0.301 | [ |
| Th6⁃C8A | H2O2 | r. t. | 98 | — | 98 | 0.100 | [ |
| TBA6⁃Nb | H2O2 | 30 | 99 | — | 96 | 594.0 | [ |
| Ru⁃POM catalysts | H2O2 | 40 | 99 | — | 97 | 10.12 | [ |
| Cot⁃MA⁃12⁃Nb | H2O2 | 60 | 98 | — | 99 | 38.81 | [ |
| Cu⁃CeO2 | H2O2 | 50 | 95 | — | 92 | 7.283 | [ |
| CeO2 | H2O2 | r. t. | 84.4 | — | 62.4 | 6.583 | [ |
| 1.8% Ag/Fe2O3 | H2O2 | 50 | 92 | — | 94 | 5.405 | [ |
| Nb2O5⁃scCO2 | H2O2 | r. t. | 86 | — | 92 | 1055 | [ |
| USHT⁃200⁃2/56 | H2O2 | 27 | 54 | — | 100 | 1.808 | [ |
| 2.5% Ag/WO3 | H2O2 | r. t. | 87 | — | 91 | 1.649 | [ |
| Nb⁃peroxo@Fe2O3 | H2O2 | r. t. | 99.6 | — | 83.7 | 14.87 | [ |
| sub⁃15 nm CeO2 nanowire | H2O2 | r. t. | 70 | — | 60 | 1.873 | [ |
| UiO⁃66 | H2O2 | 60 | 99 | — | 99 | 22.07 | [ |
Table 1 Summary of research progress on aniline oxidation coupling reactions using hydrogen peroxide as oxidant*
| Catalyst | Oxidant | Temp./oC | Conv.(%) | Selectivity(%) | Generation rate/ (mmol·g-1·h-1) | Ref. | |
|---|---|---|---|---|---|---|---|
| Azobenzene | Azoxybenzene | ||||||
| CuCr2O4 | H2O2 | 70 | 78 | — | 92 | 7.700 | [ |
| [N(C4H9)4]2[Mo6O19] | H2O2 | 60 | — | — | 99%(Yield) | 1.517 | [ |
| [N(C4H9)4]2[Mo6O19] | H2O2 | 50 | — | — | 93%(Yield) | 1.900 | [ |
| Zr(OH)4 | H2O2 | r. t. | 98 | — | 98 | 25.82 | [ |
| Zr(OH)4 | H2O2 | 40 | 95 | — | 97 | 2.064 | [ |
| {Ni6}POM | H2O2 | 70 | 99.4 | — | 86 | 0.301 | [ |
| Th6⁃C8A | H2O2 | r. t. | 98 | — | 98 | 0.100 | [ |
| TBA6⁃Nb | H2O2 | 30 | 99 | — | 96 | 594.0 | [ |
| Ru⁃POM catalysts | H2O2 | 40 | 99 | — | 97 | 10.12 | [ |
| Cot⁃MA⁃12⁃Nb | H2O2 | 60 | 98 | — | 99 | 38.81 | [ |
| Cu⁃CeO2 | H2O2 | 50 | 95 | — | 92 | 7.283 | [ |
| CeO2 | H2O2 | r. t. | 84.4 | — | 62.4 | 6.583 | [ |
| 1.8% Ag/Fe2O3 | H2O2 | 50 | 92 | — | 94 | 5.405 | [ |
| Nb2O5⁃scCO2 | H2O2 | r. t. | 86 | — | 92 | 1055 | [ |
| USHT⁃200⁃2/56 | H2O2 | 27 | 54 | — | 100 | 1.808 | [ |
| 2.5% Ag/WO3 | H2O2 | r. t. | 87 | — | 91 | 1.649 | [ |
| Nb⁃peroxo@Fe2O3 | H2O2 | r. t. | 99.6 | — | 83.7 | 14.87 | [ |
| sub⁃15 nm CeO2 nanowire | H2O2 | r. t. | 70 | — | 60 | 1.873 | [ |
| UiO⁃66 | H2O2 | 60 | 99 | — | 99 | 22.07 | [ |
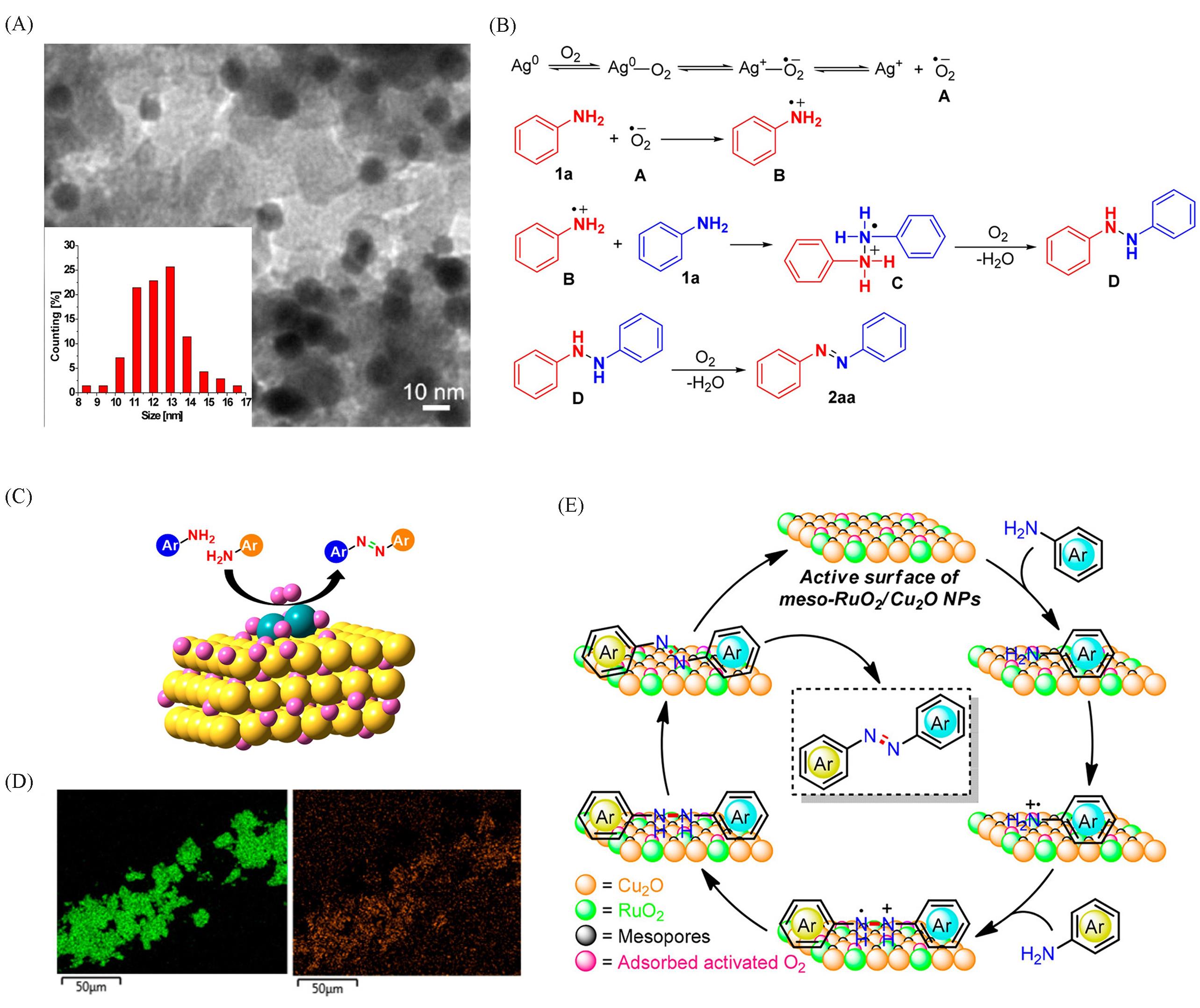
Fig.5 TEM image of 3.2%(mass fraction) Ag/C catalyst and particle size distribution of Ag nanoparticles(A), mechanism of Ag nanoparticles catalyzing aniline oxidative coupling reaction(B)[106], schematic diagram of selective oxidation of aniline to azobenzene by RuO2/Cu2O catalyst(C), element mapping images of Cu(green) and Ru(orange) in RuO2/Cu2O catalyst(D), mechanism of aniline oxidative coupling reactions catalyzed by RuO2/Cu2O(E)[110](A, B) Copyright 2013, American Chemical Society; (C—E) Copyright 2018, American Chemical Society.
| Catalyst | Oxidant | Temp./℃ | Conv.(%) | Selectivity(%) | Generation rate/ (mmol·g-1·h-1) | Ref. | |
|---|---|---|---|---|---|---|---|
| Azobenzene | Azoxybenzene | ||||||
| 1.5% Au/TiO2 | O2 | 100 | 100 | 92 | — | 26.50 | [ |
| 5% Au1Pd3@C | O2 | 60 | 98 | 99 | — | 43.26 | [ |
| 0.5% Au/CeO2 | O2 | 100 | 100 | 93 | — | 50.00 | [ |
| Zr(OH)4 | O2 | 110 | 97 | — | 90 | 0.782 | [ |
| Zr(OH)4 | O2 | 100 | 95 | 94 | — | 4.001 | [ |
| CuBr | Air | 60 | — | 96%(Yield) | — | 5.556 | [ |
| Meso⁃Mn2O3 | Air | 110 | 99 | 99 | — | 1.225 | [ |
| 3.2% Ag/C | Air | 60 | — | 97%(Yield) | — | 31.17 | [ |
Table 2 Summary of research progress on aniline oxidation coupling reactions using oxygen as oxidant *
| Catalyst | Oxidant | Temp./℃ | Conv.(%) | Selectivity(%) | Generation rate/ (mmol·g-1·h-1) | Ref. | |
|---|---|---|---|---|---|---|---|
| Azobenzene | Azoxybenzene | ||||||
| 1.5% Au/TiO2 | O2 | 100 | 100 | 92 | — | 26.50 | [ |
| 5% Au1Pd3@C | O2 | 60 | 98 | 99 | — | 43.26 | [ |
| 0.5% Au/CeO2 | O2 | 100 | 100 | 93 | — | 50.00 | [ |
| Zr(OH)4 | O2 | 110 | 97 | — | 90 | 0.782 | [ |
| Zr(OH)4 | O2 | 100 | 95 | 94 | — | 4.001 | [ |
| CuBr | Air | 60 | — | 96%(Yield) | — | 5.556 | [ |
| Meso⁃Mn2O3 | Air | 110 | 99 | 99 | — | 1.225 | [ |
| 3.2% Ag/C | Air | 60 | — | 97%(Yield) | — | 31.17 | [ |
| 47 | 鲁新环, 陶佩佩, 黄锋锋, 张香归, 林志成, 潘海军, 张海福, 周丹, 夏清华. 高等学校化学学报, 2019, 40(3), 528—535 |
| 48 | Zhao W. S., Shi Y. N., Jiang Y. H., Zhang X. F., Long C., An P. F., Zhu Y. F., Shao S. X., Yan Z., Li G. D., Tang Z. Y., Angew. Chem. Int. Ed., 2021, 60(11), 5811—5815 |
| 49 | De Almeida L. D., Wang H. L., Junge K., Cui X. J., Beller M., Angew. Chem. Int. Ed., 2021, 60(2), 550—565 |
| 50 | Zupanc A., Install J., Jereb M., Repo T., Angew. Chem. Int. Ed., 2023, 62(5), e202214453 |
| 51 | Meemken F., Baiker A., Chem. Rev., 2017, 117(17), 11522—11569 |
| 52 | Shi Y. F., Lyu Z. H., Zhao M., Chen R. H., Nguyen Q. N., Xia Y. N., Chem. Rev., 2021, 121(2), 649—735 |
| 53 | Miao X., Chen W. X., Lv S. N., Li A. R., Li Y. H., Zhang Q. H., Yue Y. H., Zhao H. W., Liu L. M., Guo S. J., Guo L., Adv. Mater., 2023, 35(14), 2211790 |
| 54 | Jin H. Q., Zhao R. Q., Cui P. X., Liu X. L., Yan J., Yu X. H., Ma D., Song W. G., Cao C. Y., J. Am. Chem. Soc., 2023, 145(22), 12023—12032 |
| 55 | Jin H. Q., Zhou K. X., Zhang R. X., Cui H. J., Yu Y., Cui P. X., Song W. G., Cao C. Y., Nat. Comm., 2023, 14(1), 2494 |
| 56 | Pei J. J., Shang H. S., Mao J. J., Chen Z., Sui R., Zhang X. J., Zhou D. N., Wang Y., Zhang F., Zhu W., Wang T., Chen W. X., Zhuang Z. B., Nat. Comm., 2024, 15(1), 416 |
| 57 | Duan H. H., Liu J. C., Xu M., Zhao Y. F., Ma X. L., Dong J. C., Zheng X. S., Zheng J. W., Allen C. S., Danaie M., Peng Y. K., Issariyakul T., Chen D. L., Kirkland A. I., Buffet J. C., Li J., Tsang S. C. E., O’Hare D., Nat. Catal., 2019, 2(12), 1078—1087 |
| 58 | Zhao Q., Zheng L. R., Gao Y. X., Li J. J., Wei J. J., Zhang M., Sun J. H., Ou Yang J., Na N., J. Am. Chem. Soc., 2023, 145(23), 12586—12600 |
| 59 | Krylova G., Dimitrijevic N. M., Talapin D. V., Guest J. R., Borchert H., Lobo A., Rajh T., Shevchenko E. V., J. Am. Chem. Soc., 2010, 132(26), 9102—9110 |
| 60 | He D., Jones A. M., Garg S., Pham A. N., Waite T. D., J. Phys. Chem. C, 2011, 115(13), 5461—5468 |
| 61 | Bardi G., Boselli L., Pompa P. P., Nanoscale, 2023, 15(35), 14284—14300 |
| 62 | Ghosh S., Acharyya S. S., Sasaki T., Bal R., Green Chem., 2015, 17(3), 1867—1876 |
| 63 | Paul B., Sharma S. K., Adak S., Khatun R., Singh G., Das D., Joshi V., Bhandari S., Dhar S. S., Bal R., New J. Chem., 2019, 43(23), 8911—8918 |
| 64 | Ding B. J., Jiang Y. J., Xu B. B., Dai S., Gong H. H., Zhao X. G., Yao Y. F., An P. F., Hou Z. S., Appl. Catal. A: Gen., 2023, 652, 119026 |
| 65 | Han L., Cai S., Gao M., Hasegawa J. Y., Wang P., Zhang J., Shi L., Zhang D., Chem. Rev., 2019, 119(19), 10916—10976 |
| 66 | Fan G. L., Li F., Evans D. G., Duan X., Chem Soc. Rev., 2014, 43(20), 7040—7066 |
| 67 | Fihri A., Bouhrara M., Nekoueishahraki B., Basset J. M., Polshettiwar V., Chem Soc. Rev., 2011, 40(10), 5181—5203 |
| 68 | Wachs I. E., Roberts C. A., Chem Soc. Rev., 2010, 39(12), 5002—5017 |
| 69 | Wang Z. J., Wei Y. Z., Qi J., Wan J. W., Wang Z. M., Yu R. B., Wang D., Adv. Funct. Mater., 2024, doi: 10.1002/adfm.202316547 |
| 70 | Wang J. Y., Wan J. W., Yang N. L., Li Q., Wang D., Nat. Rev. Chem., 2020, 4(3), 159—168 |
| 71 | Zhao Y. F., Waterhouse G. I. N., Chen G. B., Xiong X. Y., Wu L. Z., Tung C. H., Zhang T. R., Chem. Soc. Rev., 2019, 48(7), 1972—2010 |
| 72 | Da Silva A. G. M., Batalha D. C., Rodrigues T. S., Candido E. G., Luz S. C., de Freitas I. C., Fonseca F. C., de Oliveira D. C., Taylor J. G., Córdoba de Torresi S. I., Camargo P. H. C., Fajardo H. V., Catal. Sci. Technol., 2018, 8(7), 1828—1839 |
| 73 | Tian L. Y., Liao Y. S., Chou J. P., Tan Z. C., Chen J. L., Lee J. H., Benedict Lo T. W., Peng Y. K., J. Mater. Chem. A, 2023, 11(26), 14034—14042 |
| 74 | Passoni L. C., Siddiqui M. R. H., Steiner A., Kozhevnikov I. V., J. Mol. Catal. A: Chem., 2000, 153(1/2), 103—108 |
| 75 | Maksimchuk N. V., Maksimov G. M., Evtushok V. Y., Ivanchikova I. D., Chesalov Y. A., Maksimovskaya R. I., Kholdeeva O. A., Sole⁃Daura A., Poblet J. M., Carbo J. J., ACS Catal., 2018, 8(10), 9722—9737 |
| 76 | Maksimchuk N. V., Ivanchikova I. D., Maksimov G. M., Eltsov I. V., Eytushok V. Y., Kholdeeva O. A., Lebbie D., Errington R. J., Sole⁃Daura A., Poblet J. M., Carbo J. J., ACS Catal., 2019, 9(7), 6262—6275 |
| 77 | Kholdeeva O. A., Ivanchikova I. D., Maksimchuk N. V., Skobelev I. Y., Catal. Today, 2019, 333, 63—70 |
| 78 | Egami H., Oguma T., Katsuki T., J. Am. Chem. Soc., 2010, 132(16), 5886—5895 |
| 79 | Lima A. L. D., Fajardo H. V., Nogueira A. E., Pereira M. C., Oliveira L. C. A., de Mesquita J. P., Silva A. C., New J. Chem., 2020, 44(21), 8710—8717 |
| 80 | Tao Y. H., Singh B., Jindal V., Tang Z. C., Pescarmona P. P., Green Chem., 2019, 21(21), 5852—5864 |
| 81 | Qin J. H., Long Y., Sun F. K., Zhou P. P., Wang W. D., Luo N., Ma J. T., Angew. Chem. Int. Ed., 2021, 61(2), e202112907 |
| 82 | Hickman A. J., Sanford M. S., Nature, 2012, 484(7393), 177—185 |
| 83 | Dub P. A., Gordon J. C., Nat. Rev. Chem., 2018, 2(12), 396—408 |
| 84 | Li X. Z., Mitchell S., Fang Y. Y., Li J., Perez⁃Ramirez J., Lu J., Nat. Rev. Chem., 2023, 7(11), 754—767 |
| 85 | Obligacion J. V., Chirik P. J., Nat. Rev. Chem., 2018, 2(5), 15—34 |
| 86 | Wodrich M. D., Hu X. L., Nat. Rev. Chem., 2018, 2(1), 1—7 |
| 87 | Manssen M., Schafer L. L., Chem Soc. Rev., 2020, 49(19), 6947—6994 |
| 88 | Zheng F. B., Lin T., Wang K., Wang Y. L., Li G. D., Nano Res., 2023, 16(12), 12919—12935 |
| 89 | Zhang X. F., Yang C. Y., An P. F., Cui C. P., Ma Y. M., Liu H. T., Wang H., Yan X. Y., Li G. D., Tang Z. Y., Sci. Adv., 2022, 8, eadd5678 |
| 90 | Zhang X. F., Liu H. T., An P. F., Shi Y. N., Han J. Y., Yang Z. J., Long C., Guo J., Zhao S. L., Zhao K., Yin H. J., Zheng L. R., Zhang B. H., Liu X. P., Zhang L. J., Li G. D., Tang Z. Y., Sci. Adv., 2020, 6(17), eaaz4824 |
| 91 | Ding B. J., Xu B. B., Ding Z. J., Zhang T., Wang Y. J., Qiu H. W., He J. J., An P. F., Yao Y. F., Hou Z. S., Catal. Sci. Technol., 2022, 12(17), 5360—5371 |
| 92 | Cai S., Wu X. Y., Wu W. M., Wang S. S., Lu C. Z., Chin. Chem. Lett., 2024, 35(2), 108324 |
| 93 | Niu Q., Huang Q., Yu T. Y., Liu J., Shi J. W., Dong L. Z., Li S. L., Lan Y. Q., J. Am. Chem. Soc., 2022, 144(40), 18586—18594 |
| 94 | Han S., Cheng Y., Liu S. S., Tao C. F., Wang A. P., Wei W. G., Yu H., Wei Y. G., Angew. Chem. Int. Ed., 2021, 60(12), 6382—6385 |
| 95 | Su X. F., Wei Y. G., Ma N. N., Zhang H. C., Yan L. K., J. Phys. Chem. C, 2023, 127(8), 4124—4131 |
| 96 | Zheng J. H., Chen X., Yin X. X., Chen K., Liu A. L., Yu X. C., Wang S., Chen Z. W., Mol. Catal., 2023, 548, 113420 |
| 97 | Acharyya S. S., Ghosh S., Bal R., ACS Sustainable Chem. Eng., 2014, 2(4), 584—589 |
| 98 | Cai S., Wu X. Y., Wu W. M., Wang S. S., Lu C. Z., Chin. Chem. Lett., 2024, 35(2), 108324 |
| 99 | Li H. F., Yuan Z. L., Chen W. J., Yang M. N., Sun Y. H., Zhang S. H., Ma P. T., Wang J. P., Niu J. Y., J. Mater. Chem. A, 2023, 11(20), 10813—10822 |
| 100 | Shukla A., Singha R. K., Konathala L. N. S., Sasaki T., Bal R., RSC Adv., 2016, 6(27), 22812—22820 |
| 101 | Jagtap N., Ramaswamy V., Appl. Clay Sci., 2006, 33(2), 89—98 |
| 102 | Solomon E. L., Stahl S. S., Chem. Rev., 2018, 118(5), 2299—2301 |
| 103 | Qiu C. B., Jin L. Q., Huang Z. L., Tang Z. Q., Lei A. W., Shen Z. L., Sun N., Mo W. M., Hu B. X., Hu X. Q., ChemCatChem, 2012, 4(1), 76—80 |
| 104 | Cui Y., Shao X., Baldofski M., Sauer J., Nilius N., Freund H. J., Angew. Chem. Int. Ed., 2013, 52(43), 11385—11387 |
| 105 | Liu H. L., Liu W., Xue G. X., Tan T., Yang C. Y., An P. F., Chen W. X., Zhao W. S., Fan T., Cui C. Q., Tang Z. Y., Li G. D., J. Am. Chem. Soc., 2023, 145(20), 11085—11096 |
| 106 | Cai S. F., Rong H. P., Yu X. F., Liu X. W., Wang D. S., He W., Li Y. D., ACS Catal., 2013, 3(4), 478—486 |
| 107 | Grirrane A., Corma A., García H., Science, 2008, 322(5908), 1661—1664 |
| 108 | Perez Y., Aprile C., Corma A., Garcia H., Catal. Lett., 2009, 134(3), 204—209 |
| 1 | Jerca F. A., Jerca V. V., Hoogenboom R., Nat. Rev. Chem., 2021, 6(1), 51—69 |
| 2 | Cai B. G., Empel C., Yao W. Z., Koenigs R. M., Xuan J., Angew. Chem. Int. Ed., 2023, 62(48), e202312031 |
| 3 | Liu Y. F., Liu B., Dong Z. M., Jin S., Liang C. Y., Zhu R. T., Lu Y., Chem. J. Chinese Universities, 2010, 31(12), 2396—2399 |
| 刘宇芳, 刘博, 董振明, 金硕, 梁彩云, 朱瑞涛, 鲁云. 高等学校化学学报, 2010, 31(12), 2396—2399 | |
| 4 | Wibowo M., Ding L., J. Nat. Prod., 2020, 83(11), 3482—3491 |
| 5 | Benkhaya S., M'Rabet S., El Harfi A., Heliyon, 2020, 6(1), e03271 |
| 6 | Chung K. T., J. Environ. Sci. Health. C: Environ. Carcinog. Ecotoxicol. Rev., 2016, 34(4), 233—261 |
| 7 | Joseph J. M., Destaillats H., Hung H. M., Hoffmann M. R., J. Phys. Chem. A, 2000, 104(2), 301—307 |
| 8 | Scotter M. J., Food Addit. Contan. A, 2011, 28(5), 527—596 |
| 9 | Faisal S., Ali M., Naqvi S., Lin L., J. Nat. Fibers, 2022, 19(13), 6737—6747 |
| 10 | Russew M. M., Hecht S., Adv. Mater., 2010, 22(31), 3348—3360 |
| 11 | Jerca F. A., Jerca V. V., Hoogenboom R., Chem, 2017, 3(4), 533—536 |
| 12 | Beharry A. A., Woolley G. A., Chem. Soc. Rev., 2011, 40(8), 4422—4437 |
| 13 | Kumar G. S., Neckers D. C., Chem. Rev., 1989, 89(8), 1915—1925 |
| 14 | Feng X., Guo N., Chen H. P., Wang H. L., Yue L. Y., Chen X., Ng S. W., Liu X. F., Ma L. F., Wang L. Y., Dalton Trans., 2017, 46(41), 14192—14200 |
| 15 | He J., Kovach A., Chen D., Saris P. J. G., Yu R., Armani A. M., Opt. Express, 2020, 28(15), 22462—22477 |
| 16 | Li Y. R., Xue B., Yang J. H., Jiang J. L., Liu J., Zhou Y. Y., Zhang J. S., Wu M. J., Yuan Y., Zhu Z. S., Wang Z. J., Chen Y. l., Harabuchi Y., Nakajima T., Wang W., Maeda S., Gong J. P., Cao Y., Nat. Chem., 2023, 16(3), 446—455 |
| 17 | Yang J. H., Dai L. Y., Wang X. Z., Chen Y. Q., Chin. Chem. Lett., 2011, 22(9), 1047—1050 |
| 18 | Hou L., Shi Y. Y., Jiang G. X., Liu W., Han H. L., Feng Q. H., Ren J. X., Yuan Y. J., Wang Y. C., Shi J. J., Zhang Z. Z., Nanotechnology, 2016, 27(31), 315105 |
| 19 | Alizadeh S. R., Hashemi S. M., Med. Chem. Res., 2021, 30(4), 771—806 |
| 20 | Aoki Y., Yamamoto M., Hosseini⁃Mazinani S. M., Koshikawa N., Sugimoto K., Arisawa M., Antimicrob. Agents Chemother., 1996, 40(1), 127—132 |
| 21 | Mügge C., Heine T., Baraibar A. G., van Berkel W. J. H., Paul C. E., Tischler D., Appl. Microbiol. Biotechnol., 2020, 104(15), 6481—6499 |
| 22 | Garg R. P., Alemany L. B., Moran S., Parry R. J., J. Am. Chem. Soc., 2009, 131(28), 9608—9609 |
| 23 | Shimizu T., Tanifuji N., Yoshikawa H., Angew. Chem. Int. Ed., 2022, 61(36), e202206093 |
| 24 | Wang L. L., Zhai L. J., She W. Q., Wang M. C., Zhang J. L., Wang B. Z., Front. Chem., 2022, 10, 871684 |
| 25 | Han C. S., Lee K. W., Jaffe H. H., J. Am. Chem. Soc., 1967, 89(19), 4975—4981 |
| 26 | Mikheev Y. A., Russ. J. Phys. Chem., 2021, 95(9), 1803—1810 |
| 27 | Özen A. S., Erdem S. S., Aviyente V., Struct. Chem., 1998, 9(1), 15—25 |
| 28 | Szarmach M., Wagner⁃Wysiecka E., Luboch E., Tetrahedron, 2013, 69(51), 10893—10905 |
| 29 | Nguyen T. H. L., Gigant N., Joseph D., ACS Catal., 2018, 8(2), 1546—1579 |
| 30 | Merino E., Chem Soc. Rev., 2011, 40(7), 3835—3853 |
| 31 | Zhao M. Y., Tang Y. F., Han G. Z., Molecules, 2023, 28(18), 6741 |
| 32 | Firouzabadi H., Mostafavipoor Z., Bull. Chem. Soc. Jpn., 1983, 56(3), 914—917 |
| 33 | Takeda Y., Okumura S., Minakata S., Angew. Chem. Int. Ed., 2012, 51(31), 7804—7808 |
| 34 | Zhang M., Zhang R. L., Zhang A. Q., Li X. F., Liang H. H., Synth. Commun., 2009, 39(19), 3428—3435 |
| 35 | Bacon E. S., Richardson D. H., J. Chem. Soc., 1932, 884—888 |
| 36 | Podgorsek A., Zupan M., Iskra J., Angew. Chem. Int. Ed., 2009, 48(45), 8424—8450 |
| 37 | Guo Z., Liu B., Zhang Q. H., Deng W. P., Wang Y., Yang Y. H., Chem Soc. Rev., 2014, 43(10), 3480—3524 |
| 38 | Haynes W. M., CRC Handbook of Chemistry and Physics, Taylor & Francis Group, Boca Raton, FL, 2017, 5—79 |
| 39 | Pesterfield L., J. Chem. Educ., 2009, 86(10), 1182 |
| 40 | Agarwal N., Freakley S. J., McVicker R. U., Althahban S. M., Dimitratos N., He Q., Morgan D. J., Jenkins R. L., Willock D. J., Taylor S. H., Kiely C. J., Hutchings G. J., Science, 2017, 358(6360), 223—227 |
| 41 | Shah H. U. R., Ahmad K., Naseem H. A., Parveen S., Ashfaq M., Aziz T., Shaheen S., Babras A., Shahzad A., J. Mol. Struct., 2021, 1244, 131181 |
| 42 | Hage R., Lienke A., Angew. Chem. Int. Ed., 2006, 45(2), 206—222 |
| 109 | Oseghale C. O., Fapojuwo D. P., Alimi O. A., Akinnawo C. A., Mogudi B. M., Onisuru O. R., Meijboom R., Eur. J. Org. Chem., 2021, 2021(36), 5063—5073 |
| 110 | Fu F. Y., He S., Yang S., Wang C., Zhang X., Li P., Sheng H. T., Zhu M. Z., Sci. China Chem., 2015, 58(10), 1532—1536 |
| 111 | Saha A., Payra S., Selvaratnam B., Bhattacharya S., Pal S., Koodali R. T., Banerjee S., ACS Sustainable Chem. Eng., 2018, 6(9), 11345—11352 |
| 112 | Patel A. R., Patel G., Maity G., Patel S. P., Bhattacharya S., Putta A., Banerjee S., ACS Omega, 2020, 5(47), 30416—30424 |
| 113 | Zhang C., Jiao N., Angew. Chem. Int. Ed., 2010, 49(35), 6174—6177 |
| 114 | Dutta B., Biswas S., Sharma V., Savage N. O., Alpay S. P., Suib S. L., Angew. Chem. Int. Ed., 2016, 55(6), 2171—2175 |
| 115 | Zhang Y. F., Mellah M., ACS Catal., 2017, 7(12), 8480—8486 |
| 116 | Ma Y. F., Wu S. H., Jiang S. X., Xiao F. H., Deng G. J., Chin. J. Chem., 2021, 39(12), 3334—3338 |
| 117 | Luo L., Liu Y. G., Chen W. S., Xue X. M., Xu S. M., Li M., Zhou H., Ma L. A., Xu M., Kong X. G., Shao M. F., Li Z. H., Duan H. H., Chem Catal., 2023, 3(1), 100472 |
| 118 | Carreno N. L. V., Deon V. G., Silva R. M., Santana L. R., Pereira R. M., Orlandi M. O., Ventura W. M., Dias A., Taylor J. G., Fajardo H. V., Mesko M. F., ACS Sustainable Chem. Eng., 2018, 6(2), 1680—1691 |
| 43 | Brillas E., Sirés I., Oturan M. A., Chem. Rev., 2009, 109(12), 6570—6631 |
| 44 | Pan D. J., Chemical Engineering Design Communications, 2022, 48(12), 206—208 |
| 潘得驹. 化工设计通讯, 2022, 48(12), 206—208 | |
| 45 | Sun G., Li M. M. J., Nakagawa K., Li G. C., Wu T. S., Peng Y. K., Appl. Catal. B: Environ., 2022, 313, 121461 |
| 46 | Faccioli F., Bauer M., Pedron D., Sorarù A., Carraro M., Gross S., Eur. J. Inorg. Chem., 2014, 2015(2), 210—225 |
| 47 | Lu X. H., Tao P. P., Huang F. F., Zhang X. G., Lin Z. C., Pan H. J., Zhang H. F., Zhou D., Xia Q. H., Chem. J. Chinese Universities, 2019, 40(3), 528—535 |
| [1] | 张小龙, 张毅城, 李庆朝, 查飞, 常玥, 唐小华. SBA-15分子筛负载对甲苯磺酸催化合成过氧化二异丙苯的性能和反应过程模拟[J]. 高等学校化学学报, 2024, 45(6): 20240067. |
| [2] | 高永平, 刘柏, 康家宁, 吕杰琼, 于泽广, 张志会, 高文秀. 氮掺杂碳材料MG-T多相催化硝基苯加氢制苯胺[J]. 高等学校化学学报, 2024, 45(5): 20240040. |
| [3] | 陈贝怡, 观文娜, 靳钊, 刘婷婷, 周睿. 酰胺和咪唑基离子液体双极性基团共嵌入式亲水C18硅胶固定相的制备与应用[J]. 高等学校化学学报, 2024, 45(4): 20230503. |
| [4] | 陈荣, 温良英, 岳东, 杨仲卿. Cl2和O2在TiC(100)表面共吸附行为的密度泛函理论分析[J]. 高等学校化学学报, 2024, 45(4): 20230497. |
| [5] | 李婷婷, 岳彩凤, 霍媛青, 纪慧芳, 张瑞平. 无金属黑色素纳米酶用于肝纤维化治疗[J]. 高等学校化学学报, 2024, 45(1): 20230411. |
| [6] | 张小玉, 曲干, 薛冬萍, 闫文付, 张佳楠. 碳基催化剂用于电催化氧还原生产H2O2的研究进展: 策略、 计算及实际应用[J]. 高等学校化学学报, 2023, 44(5): 20220775. |
| [7] | 郭昊天, 鲁新环, 孙凡棋, 陶艺元, 段金贵, 张望, 周丹, 夏清华. 纳米球型Mo-MOF材料的调控合成及催化硫醚选择性氧化[J]. 高等学校化学学报, 2023, 44(12): 20230408. |
| [8] | 谢小兰, 刘湛, 吕佳敏, 余申, 李小云, 苏宝连, 陈丽华. Cu基等级孔ZSM-5分子筛单晶的制备及硝基苯加氢催化性能[J]. 高等学校化学学报, 2023, 44(10): 20230109. |
| [9] | 葛怡聪, 聂万丽, 孙国峰, 陈稼轩, 田冲. 银催化2-烯基苯胺与苯并异噁唑的[5+1]环化反应[J]. 高等学校化学学报, 2022, 43(8): 20220142. |
| [10] | 刘晓磊, 陆永强, 游淇, 刘国辉, 姚伟, 胡日茗, 闫纪宪, 崔玉, 杨小凤, 孙国新, 蒋绪川. 基于3-羟基沙利度胺的比率型荧光探针对过氧化氢的检测[J]. 高等学校化学学报, 2022, 43(6): 20220070. |
| [11] | 陈潇禄, 袁珍闫, 仲迎春, 任浩. 机械球磨制备三苯胺基PAF-106s及C2烃吸附性质[J]. 高等学校化学学报, 2022, 43(3): 20210771. |
| [12] | 朱浩天, 金美秀, 唐文思, 苏芳, 李阳光. 过渡金属-联咪唑-Dawson型钨磷酸盐杂化化合物的酶固定化性能[J]. 高等学校化学学报, 2022, 43(11): 20220328. |
| [13] | 徐梦祎, 黄雪雯, 李小杰, 魏玮, 刘晓亚. “串珠状”复合纳米组装体修饰丝网印刷电极构建的生物传感器[J]. 高等学校化学学报, 2021, 42(6): 1768. |
| [14] | 杨思娴, 钟文钰, 李超贤, 苏秋瑶, 许炳佳, 何谷平, 孙丰强. 聚苯胺纳米线/SnO2复合光催化材料的光化学制备与性能[J]. 高等学校化学学报, 2021, 42(6): 1942. |
| [15] | 李健, 于明明, 孙源, 冯文华, 冯兆池, 吴剑峰. 水溶液pH对甲烷低温氧化制备甲醇的影响[J]. 高等学校化学学报, 2021, 42(3): 776. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||