

高等学校化学学报 ›› 2024, Vol. 45 ›› Issue (2): 20230447.doi: 10.7503/cjcu20230447
周生冉1, 彭超1, 高思宇1, 于迪2, 张春雷2, 王斓懿1, 范晓强1, 于学华1( ), 赵震1,2(
), 赵震1,2( )
)
收稿日期:2023-10-21
出版日期:2024-02-10
发布日期:2023-12-15
通讯作者:
于学华,赵震
E-mail:yuxuehua1986@163.com;zhenzhao@cup.edu.cn
基金资助:
ZHOU Shengran1, PENG Chao1, GAO Siyu1, YU Di2, ZHANG Chunlei2, WANG Lanyi1, FAN Xiaoqiang1, YU Xuehua1( ), ZHAO Zhen1,2(
), ZHAO Zhen1,2( )
)
Received:2023-10-21
Online:2024-02-10
Published:2023-12-15
Contact:
YU Xuehua, ZHAO Zhen
E-mail:yuxuehua1986@163.com;zhenzhao@cup.edu.cn
Supported by:摘要:
基于氧化锰(MnO x )催化剂优异的氧化还原性能, 采用水热法制备了一系列具有层状结构的MnO δ 催化剂, 并对其物化性能进行了表征. 研究了水热反应温度、 煅烧温度以及原料组成对催化剂晶体结构、 形貌和氧化还原性能的影响, 并将MnO δ 催化剂应用于柴油机尾气炭烟颗粒的催化燃烧. 结果表明, 当水热反应时间为12 h, 煅烧温度为550 ℃, 反应原料中KOH和K2CO3同时存在时, 所制备的MnO δ -t12催化剂具有最佳的催化燃烧炭烟颗粒的活性, T10, T50和T90值分别为274, 321和354 ℃.
中图分类号:
TrendMD:
周生冉, 彭超, 高思宇, 于迪, 张春雷, 王斓懿, 范晓强, 于学华, 赵震. MnOδ 催化剂的制备及对柴油机尾气炭烟颗粒燃烧的催化性能. 高等学校化学学报, 2024, 45(2): 20230447.
ZHOU Shengran, PENG Chao, GAO Siyu, YU Di, ZHANG Chunlei, WANG Lanyi, FAN Xiaoqiang, YU Xuehua, ZHAO Zhen. Preparation of MnOδ Catalysts and Their Catalytic Performance for Combustion of Diesel Exhaust Soot Particles. Chem. J. Chinese Universities, 2024, 45(2): 20230447.
| Catalyst | mKMnO4/g | mM* n(NO3)2/g | mK2CO3/g | mKOH/g | Hydrothermal time/h | Calcination temperature/℃ |
|---|---|---|---|---|---|---|
| MnO δ ⁃t6 | 2.37 | 3.58 | 0.75 | 2.50 | 6 | 550 |
| MnO δ ⁃t12 | 2.37 | 3.58 | 0.75 | 2.50 | 12 | 550 |
| MnO δ ⁃t18 | 2.37 | 3.58 | 0.75 | 2.50 | 18 | 550 |
| MnO δ ⁃t24 | 2.37 | 3.58 | 0.75 | 2.50 | 24 | 550 |
| MnO δ ⁃t48 | 2.37 | 3.58 | 0.75 | 2.50 | 48 | 550 |
| MnO δ ⁃no all | 2.37 | 3.58 | 0 | 0 | 12 | 550 |
| MnO δ ⁃only K2CO3 | 2.37 | 3.58 | 0.75 | 0 | 12 | 550 |
| MnO δ ⁃only KOH | 2.37 | 3.58 | 0 | 2.50 | 12 | 550 |
| MnO δ ⁃T450 | 2.37 | 3.58 | 0.75 | 2.50 | 12 | 450 |
| MnO δ ⁃T650 | 2.37 | 3.58 | 0.75 | 2.50 | 12 | 650 |
| MnO δ ⁃T750 | 2.37 | 3.58 | 0.75 | 2.50 | 12 | 750 |
| NnO δ ⁃T850 | 2.37 | 3.58 | 0.75 | 2.50 | 12 | 850 |
Table 1 Expression ways and recipes of raw materials for the preparation of MnO δ catalysts
| Catalyst | mKMnO4/g | mM* n(NO3)2/g | mK2CO3/g | mKOH/g | Hydrothermal time/h | Calcination temperature/℃ |
|---|---|---|---|---|---|---|
| MnO δ ⁃t6 | 2.37 | 3.58 | 0.75 | 2.50 | 6 | 550 |
| MnO δ ⁃t12 | 2.37 | 3.58 | 0.75 | 2.50 | 12 | 550 |
| MnO δ ⁃t18 | 2.37 | 3.58 | 0.75 | 2.50 | 18 | 550 |
| MnO δ ⁃t24 | 2.37 | 3.58 | 0.75 | 2.50 | 24 | 550 |
| MnO δ ⁃t48 | 2.37 | 3.58 | 0.75 | 2.50 | 48 | 550 |
| MnO δ ⁃no all | 2.37 | 3.58 | 0 | 0 | 12 | 550 |
| MnO δ ⁃only K2CO3 | 2.37 | 3.58 | 0.75 | 0 | 12 | 550 |
| MnO δ ⁃only KOH | 2.37 | 3.58 | 0 | 2.50 | 12 | 550 |
| MnO δ ⁃T450 | 2.37 | 3.58 | 0.75 | 2.50 | 12 | 450 |
| MnO δ ⁃T650 | 2.37 | 3.58 | 0.75 | 2.50 | 12 | 650 |
| MnO δ ⁃T750 | 2.37 | 3.58 | 0.75 | 2.50 | 12 | 750 |
| NnO δ ⁃T850 | 2.37 | 3.58 | 0.75 | 2.50 | 12 | 850 |
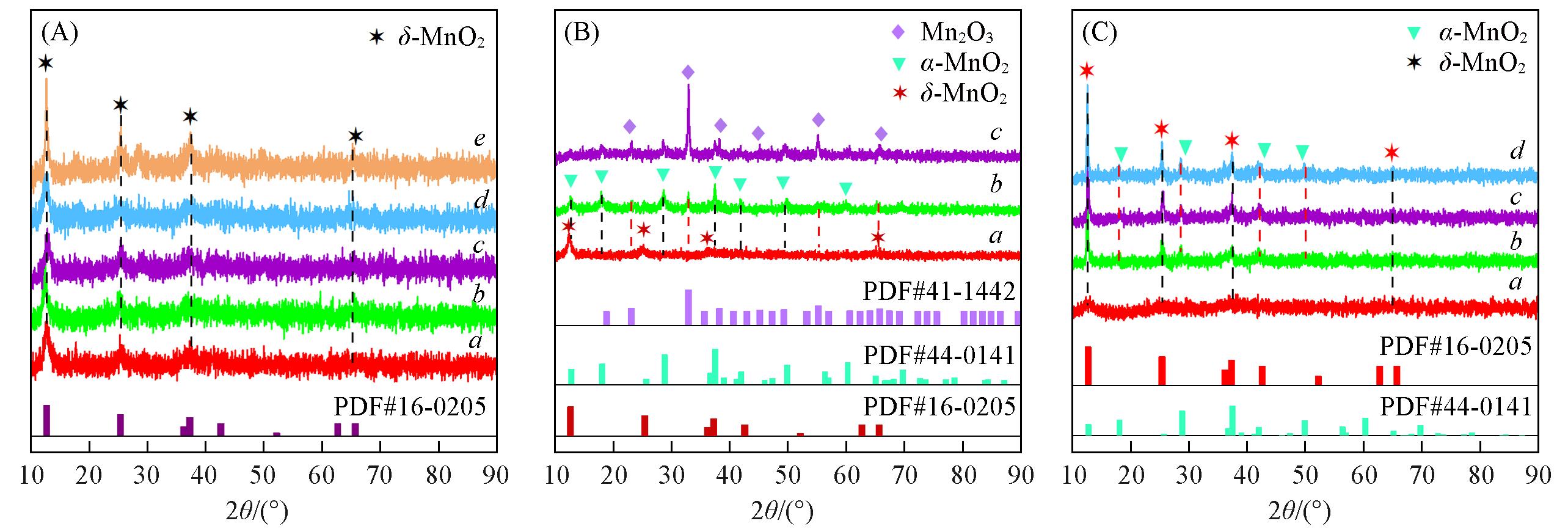
Fig.1 XRD patterns of synthetic catalysts at different hydrothermal reaction time(A), raw material(B) and calcination temperature(C)(A) a. MnO δ -t6; b. MnO δ -t12; c. MnO δ -t18; d. MnO δ -t24; e. MnO δ -t48;(B) a. MnO δ -only KOH; b. MnO δ -only K2CO3; c. MnO δ -no all;(C) a. MnO δ -T450; b. MnO δ -T650; c. MnO δ -T750; d. MnO δ -T850.
| Catalyst | Crystalline phase primary(secondary) | SBETa / (m²‧g-1) | Smeso b / (m²‧g-1) | Pore volume c /(cm³‧g-1) | Pore volume d /(cm³‧g-1) | Pore size e /nm | Crystalline sizes f /nm |
|---|---|---|---|---|---|---|---|
| MnO δ ⁃t6 | δ⁃MnO2 | 10.9 | 7.615 | 0.030 | 0.028 | 15.7 | 9.3 |
| MnO δ ⁃t12 | δ⁃MnO2 | 12.4 | 9.228 | 0.041 | 0.040 | 16.4 | 9.8 |
| MnO δ ⁃t18 | δ⁃MnO2 | 16.7 | 13.766 | 0.052 | 0.050 | 14.6 | 10.3 |
| MnO δ ⁃t24 | δ⁃MnO2 | 18.6 | 15.135 | 0.065 | 0.064 | 17.0 | 11.2 |
| MnO δ ⁃t48 | δ⁃MnO2 | 22.7 | 20.394 | 0.068 | 0.067 | 14.9 | 13.5 |
| MnO δ ⁃no all | Mn2O3(α⁃MnO2) | 32.6 | 31.416 | 0.073 | 0.073 | 9.3 | 25.7 |
| MnO δ ⁃only K2CO3 | α⁃MnO2(Mn2O3) | 29.9 | 28.197 | 0.077 | 0.078 | 11.1 | 17.9 |
| MnO δ ⁃only KOH | δ⁃MnO2 | 13.2 | 10.210 | 0.047 | 0.046 | 18.0 | 12.2 |
| MnO δ ⁃T450 | δ⁃MnO2 | 14.1 | 11.309 | 0.050 | 0.049 | 17.3 | 6.9 |
| MnO δ ⁃T650 | δ⁃MnO2(α⁃MnO2) | 6.9 | 4.009 | 0.020 | 0.019 | 18.9 | 23.4 |
| MnO δ ⁃T750 | δ⁃MnO2(α⁃MnO2) | 5.9 | 3.681 | 0.019 | 0.018 | 19.0 | 24.8 |
| NnO δ ⁃T850 | δ⁃MnO2(α⁃MnO2) | 5.1 | 3.059 | 0.015 | 0.014 | 18.9 | 33.9 |
Table 2 Texture properties of MnO δ catalysts prepared under different experimental conditions
| Catalyst | Crystalline phase primary(secondary) | SBETa / (m²‧g-1) | Smeso b / (m²‧g-1) | Pore volume c /(cm³‧g-1) | Pore volume d /(cm³‧g-1) | Pore size e /nm | Crystalline sizes f /nm |
|---|---|---|---|---|---|---|---|
| MnO δ ⁃t6 | δ⁃MnO2 | 10.9 | 7.615 | 0.030 | 0.028 | 15.7 | 9.3 |
| MnO δ ⁃t12 | δ⁃MnO2 | 12.4 | 9.228 | 0.041 | 0.040 | 16.4 | 9.8 |
| MnO δ ⁃t18 | δ⁃MnO2 | 16.7 | 13.766 | 0.052 | 0.050 | 14.6 | 10.3 |
| MnO δ ⁃t24 | δ⁃MnO2 | 18.6 | 15.135 | 0.065 | 0.064 | 17.0 | 11.2 |
| MnO δ ⁃t48 | δ⁃MnO2 | 22.7 | 20.394 | 0.068 | 0.067 | 14.9 | 13.5 |
| MnO δ ⁃no all | Mn2O3(α⁃MnO2) | 32.6 | 31.416 | 0.073 | 0.073 | 9.3 | 25.7 |
| MnO δ ⁃only K2CO3 | α⁃MnO2(Mn2O3) | 29.9 | 28.197 | 0.077 | 0.078 | 11.1 | 17.9 |
| MnO δ ⁃only KOH | δ⁃MnO2 | 13.2 | 10.210 | 0.047 | 0.046 | 18.0 | 12.2 |
| MnO δ ⁃T450 | δ⁃MnO2 | 14.1 | 11.309 | 0.050 | 0.049 | 17.3 | 6.9 |
| MnO δ ⁃T650 | δ⁃MnO2(α⁃MnO2) | 6.9 | 4.009 | 0.020 | 0.019 | 18.9 | 23.4 |
| MnO δ ⁃T750 | δ⁃MnO2(α⁃MnO2) | 5.9 | 3.681 | 0.019 | 0.018 | 19.0 | 24.8 |
| NnO δ ⁃T850 | δ⁃MnO2(α⁃MnO2) | 5.1 | 3.059 | 0.015 | 0.014 | 18.9 | 33.9 |
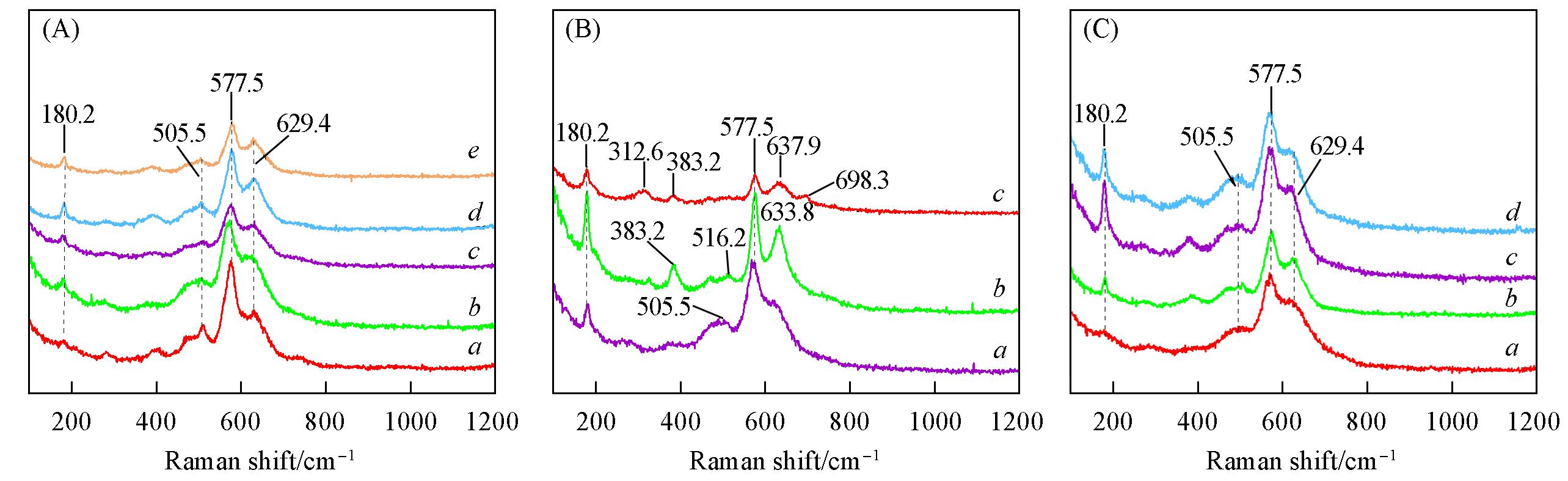
Fig.2 Raman spectra of synthetic catalysts at different hydrothermal reaction time(A), raw material(B) and calcination temperature(C)(A) a. MnO δ -t6; b. MnO δ -t12; c. MnO δ -t18; d. MnO δ -t24; e. MnO δ -t48;(B) a. MnO δ -only KOH; b. MnO δ -only K2CO3; c. MnO δ -no all;(C) a. MnO δ -T450; b. MnO δ -T650; c. MnO δ -T750; d. MnO δ -T850.
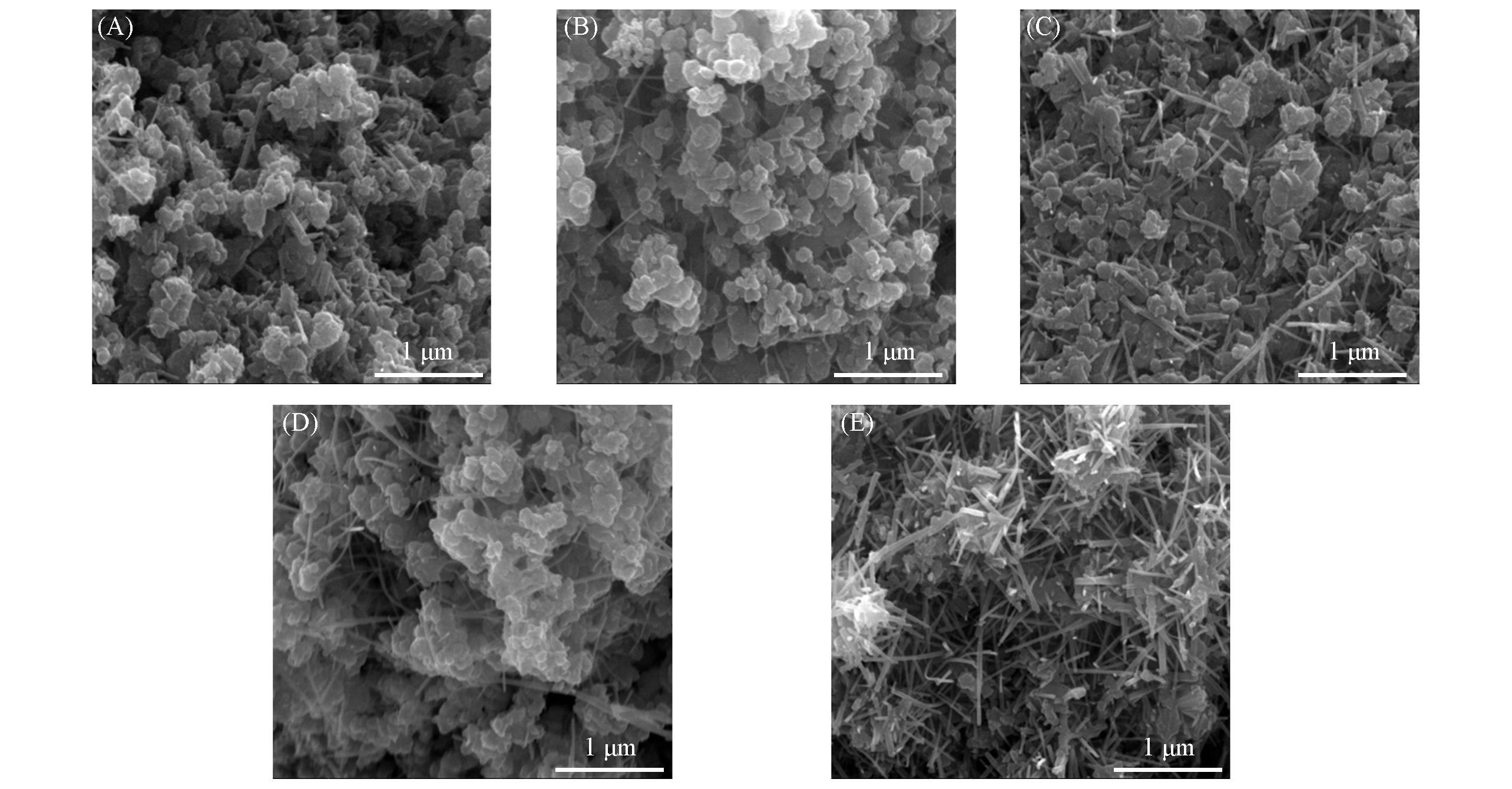
Fig.3 SEM images of catalysts prepared under different hydrothermal reaction time(A) MnO δ -t6;(B) MnO δ -t12;(C) MnO δ -t18;(D) MnO δ -t24;(E) MnO δ -t48.
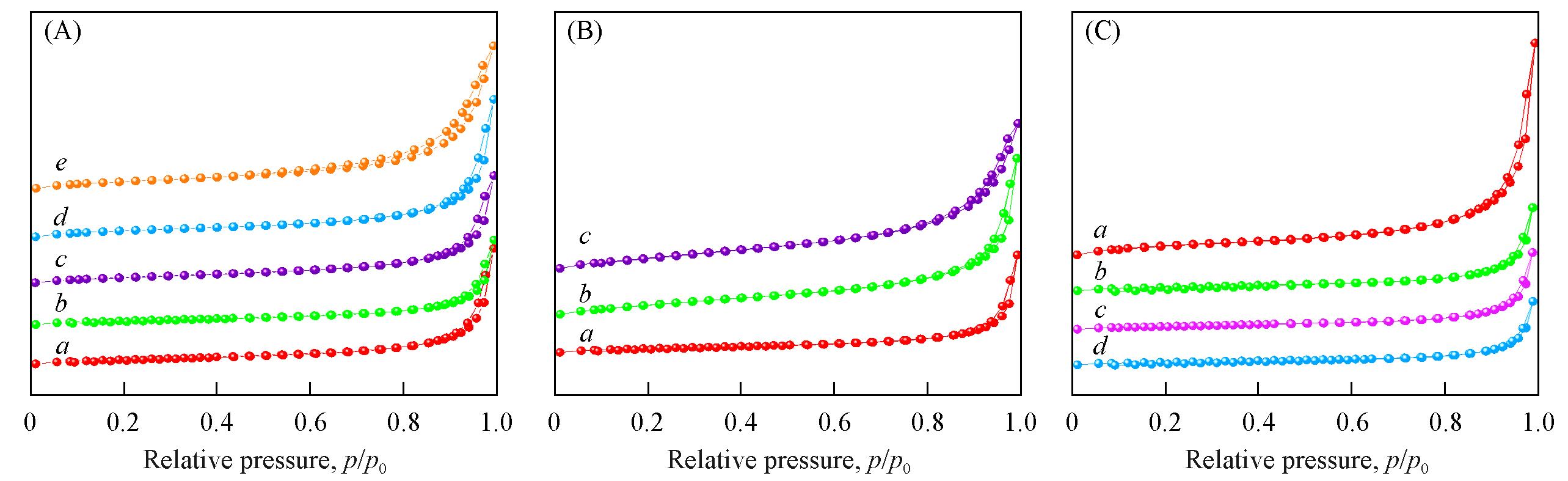
Fig.6 N2 adsorption⁃desorption isotherms of synthetic catalysts under different hydrothermal reaction time(A), raw materials(B) and calcination temperature(C)(A) a. MnO δ -t6; b. MnO δ -t12; c. MnO δ -t18; d. MnO δ -t24; e. MnO δ -t48;(B) a. MnO δ -only KOH; b. MnO δ -only K2CO3; c. MnO δ -no all;(C) a. MnO δ -T450; b. MnO δ -T650; c. MnO δ -T750; d. MnO δ -T850.

Fig.7 H2⁃TPR curves of synthetic catalysts at different hydrothermal reaction time(A), raw material(B) and calcination temperature(C)(A) a. MnO δ -t6; b. MnO δ -t12; c. MnO δ -t18; d. MnO δ -t24; e. MnO δ -t48;(B) a. MnO δ -only KOH; b. MnO δ -only K2CO3; c. MnO δ -no all;(C) a. MnO δ -T450; b. MnO δ -T650; c. MnO δ -T750; d. MnO δ -T850.
| Catalyst | Contact mode | T10/℃ | T50/℃ | T90/℃ | ΔT10a /℃ | ΔT50b /℃ | ΔT90c /℃ | |
|---|---|---|---|---|---|---|---|---|
| Pure soot | Loose | 461 | 552 | 594 | 38.5 | |||
| MnO δ ⁃t6 | Loose | 287 | 325 | 359 | 174 | 227 | 235 | 97.6 |
| MnO δ ⁃t12 | Loose | 274 | 321 | 354 | 187 | 231 | 240 | 97.5 |
| MnO δ ⁃t18 | Loose | 289 | 332 | 366 | 172 | 220 | 228 | 98.3 |
| MnO δ ⁃t24 | Loose | 290 | 334 | 370 | 171 | 218 | 224 | 98.2 |
| MnO δ ⁃t48 | Loose | 289 | 334 | 366 | 172 | 218 | 228 | 98.1 |
| MnO δ ⁃no all | Loose | 293 | 352 | 389 | 168 | 200 | 205 | 99.4 |
| MnO δ ⁃only K2CO3 | Loose | 285 | 342 | 379 | 176 | 210 | 215 | 99.4 |
| MnO δ ⁃only KOH | Loose | 288 | 331 | 366 | 173 | 221 | 228 | 97.1 |
| MnO δ ⁃T450 | Loose | 272 | 323 | 357 | 189 | 229 | 237 | 96.5 |
| MnO δ ⁃T650 | Loose | 286 | 337 | 372 | 175 | 215 | 221 | 97.2 |
| MnO δ ⁃T750 | Loose | 288 | 337 | 373 | 173 | 215 | 222 | 97.7 |
| NnO δ ⁃T850 | Loose | 291 | 341 | 374 | 170 | 211 | 220 | 97.7 |
Table 3 Catalytic activities for soot combustion of MnO δ catalysts prepared under different experimental conditions
| Catalyst | Contact mode | T10/℃ | T50/℃ | T90/℃ | ΔT10a /℃ | ΔT50b /℃ | ΔT90c /℃ | |
|---|---|---|---|---|---|---|---|---|
| Pure soot | Loose | 461 | 552 | 594 | 38.5 | |||
| MnO δ ⁃t6 | Loose | 287 | 325 | 359 | 174 | 227 | 235 | 97.6 |
| MnO δ ⁃t12 | Loose | 274 | 321 | 354 | 187 | 231 | 240 | 97.5 |
| MnO δ ⁃t18 | Loose | 289 | 332 | 366 | 172 | 220 | 228 | 98.3 |
| MnO δ ⁃t24 | Loose | 290 | 334 | 370 | 171 | 218 | 224 | 98.2 |
| MnO δ ⁃t48 | Loose | 289 | 334 | 366 | 172 | 218 | 228 | 98.1 |
| MnO δ ⁃no all | Loose | 293 | 352 | 389 | 168 | 200 | 205 | 99.4 |
| MnO δ ⁃only K2CO3 | Loose | 285 | 342 | 379 | 176 | 210 | 215 | 99.4 |
| MnO δ ⁃only KOH | Loose | 288 | 331 | 366 | 173 | 221 | 228 | 97.1 |
| MnO δ ⁃T450 | Loose | 272 | 323 | 357 | 189 | 229 | 237 | 96.5 |
| MnO δ ⁃T650 | Loose | 286 | 337 | 372 | 175 | 215 | 221 | 97.2 |
| MnO δ ⁃T750 | Loose | 288 | 337 | 373 | 173 | 215 | 222 | 97.7 |
| NnO δ ⁃T850 | Loose | 291 | 341 | 374 | 170 | 211 | 220 | 97.7 |
| Catalyst | Reaction condition | mSoot/mCatalyst | T10/Ti /(℃) | T50/Tm/(℃) | T90/Tf/(℃) | Ref. |
|---|---|---|---|---|---|---|
| CsMnO x /3DOM⁃m TSO⁃0.7 | 0.2%NO+10%O2, Ar balance | 1∶10 | 284 | 341 | 376 | [ |
| PtPd/3DOM TiO2 | 0.2%NO+5%O2, Ar balance | 1∶10 | 262 | 338 | 386 | [ |
| K⁃OMS⁃2/3DOMm Ti0.7Si0.3O | 0.2%NO+5%O2, Ar balance | 1∶10 | 273 | 330 | 385 | [ |
| 3DOM Mn0.5Ce0.5O δ | 0.2%NO+10%O2, Ar balance | 1∶10 | 297 | 358 | 396 | [ |
| CeO2@MnO2 | 500 ppm NO+5%O2, N2 balance | 1∶9 | 312 | 373 | 423 | [ |
| MnO x ⁃CeO2⁃Al2O3 | 1000 ppm NO+10%O2, N2 balance | 1∶10 | 455 | [ | ||
| La0.9Ce0.05K0.05CoO3 | 0.2%NO+10%O2, Ar balance | 1∶10 | 269 | 309 | 342 | [ |
| Mn0.09Ce0.91O2 | 0.05%NO+5%O2, N2 balance | 1∶10 | 481 | [ | ||
| MnCe⁃1∶4 | 2000 ppm NO+10%O2, Ar balance | 1∶10 | 289 | 340 | 373 | [ |
| MnO δ ⁃t12 | 2000 ppm NO+10%O2, Ar balance | 1∶10 | 274 | 321 | 354 | This work |
Table 4 Catalytic activities of as-prepared catalysts and reported catalysts for soot combustion under loose contact conditions*
| Catalyst | Reaction condition | mSoot/mCatalyst | T10/Ti /(℃) | T50/Tm/(℃) | T90/Tf/(℃) | Ref. |
|---|---|---|---|---|---|---|
| CsMnO x /3DOM⁃m TSO⁃0.7 | 0.2%NO+10%O2, Ar balance | 1∶10 | 284 | 341 | 376 | [ |
| PtPd/3DOM TiO2 | 0.2%NO+5%O2, Ar balance | 1∶10 | 262 | 338 | 386 | [ |
| K⁃OMS⁃2/3DOMm Ti0.7Si0.3O | 0.2%NO+5%O2, Ar balance | 1∶10 | 273 | 330 | 385 | [ |
| 3DOM Mn0.5Ce0.5O δ | 0.2%NO+10%O2, Ar balance | 1∶10 | 297 | 358 | 396 | [ |
| CeO2@MnO2 | 500 ppm NO+5%O2, N2 balance | 1∶9 | 312 | 373 | 423 | [ |
| MnO x ⁃CeO2⁃Al2O3 | 1000 ppm NO+10%O2, N2 balance | 1∶10 | 455 | [ | ||
| La0.9Ce0.05K0.05CoO3 | 0.2%NO+10%O2, Ar balance | 1∶10 | 269 | 309 | 342 | [ |
| Mn0.09Ce0.91O2 | 0.05%NO+5%O2, N2 balance | 1∶10 | 481 | [ | ||
| MnCe⁃1∶4 | 2000 ppm NO+10%O2, Ar balance | 1∶10 | 289 | 340 | 373 | [ |
| MnO δ ⁃t12 | 2000 ppm NO+10%O2, Ar balance | 1∶10 | 274 | 321 | 354 | This work |
| Cycle times | T10/℃ | T50/℃ | T90/℃ | |
|---|---|---|---|---|
| Cycle⁃1 | 274 | 321 | 354 | 97.5 |
| Cycle⁃2 | 284 | 328 | 357 | 97.5 |
| Cycle⁃3 | 286 | 328 | 358 | 97.6 |
| Cycle⁃4 | 286 | 331 | 361 | 97.1 |
| Cycle⁃5 | 286 | 332 | 362 | 97.6 |
Table 5 Stability of MnO δ ⁃t12 catalyst for soot combustion
| Cycle times | T10/℃ | T50/℃ | T90/℃ | |
|---|---|---|---|---|
| Cycle⁃1 | 274 | 321 | 354 | 97.5 |
| Cycle⁃2 | 284 | 328 | 357 | 97.5 |
| Cycle⁃3 | 286 | 328 | 358 | 97.6 |
| Cycle⁃4 | 286 | 331 | 361 | 97.1 |
| Cycle⁃5 | 286 | 332 | 362 | 97.6 |
| 1 | Mei X. Y., Zhu X. B., Zhang Y. X., Zhang Z. L., Zhong Z. H., Xin Y., Zhang J., Nat. Catal., 2021, 4(12), 1002—1011 |
| 2 | Luo T. Y., Liu S. R., Li M., Liu W., Wu X. D., Liu S., J. Catal., 2022, 408, 56—63 |
| 3 | Gao S. Y., Yu D., Zhou S. R. Zhang C. L., Wang L. Y., Fan X. Q., Yu X. H., Zhao Z., J. Mater. Chem. A, 2023, 11, 19210 |
| 4 | Yu X. H., Yu D., Wang L. Y., Ren Y., Chen M. Z., Fan X. Q., Zhao Z., Sojka Z., Kotarba A., Wei Y. C., Liu J., Catal. Sci. Technol., 2023, 13, 1208 |
| 5 | Dhinesh B., Bharathi R. N., Lalvani J. I. J., Parthasarathy M., Annamalai K., J. Energy Inst., 2017, 90(4), 634—645 |
| 6 | Dhinesh B., Lalvani J. I. J., Parthasarathy M., Annamalai K., Energy Convers. Manag., 2016, 117, 466—474 |
| 7 | Zhang Z. H., Balasubramanian R., Appl. Energy, 2014, 119, 530—536 |
| 8 | Foo K. K., Sun Z. W., Medwell P. R., Alwahabi Z. T., Nathan G. J., Dally B. B., Combust Flame, 2018, 194, 376—386 |
| 9 | Agarwal A. K., Dhar A., Gupta J. G., Kim W. I., Lee C. S., Park S., Appl. Energy, 2014, 130, 212—221 |
| 10 | Cao C. M., Xing L. L., Yang Y. X., Tian Y., Ding T., Zhang J., Hu T. D., Zheng L. R., Li X. G., Appl. Catal. B, 2017, 218, 32—45 |
| 11 | Zhao M. J., Deng J. L., Liu J., Li Y. H., Liu J. X., Duan Z. C., Xiong J., Zhao Z., Wei Y. C., Song W. Y., Sun Y. Q., ACS Catal., 2019, 9(8), 7548—7567 |
| 12 | Kim M. J., Lee E. J., Lee E., Kim D. H., Lee D. W., Kim C. H., Lee K. Y., Appl. Surf. Sci., 2021, 569, 151041 |
| 13 | Wei Y. C., Zhao Z., Liu J., Xu C. M., Jiang G. Y., Duan A. J., Small, 2013, 9(23), 3957—3963 |
| 14 | Mukherjee D., Reddy B. M., Catalysis Today, 2018, 309, 227—235 |
| 15 | Liu T. Z., Li Q., Xin Y., Zhang Z. L., Tang X. F., Zheng L. R.. Gao P. X., Appl. Catal. B, 2018, 232, 108—116 |
| 16 | Hernández⁃Giménez A. M., Xavier L. P. D., Bueno⁃López X., Appl. Catal. Gen., 2013, 462, 100—106 |
| 17 | Liao Y. X., Liu P., Zhang J., Wang C., Chen L. W., Yan D. F., Ren Q. M., Liang X. L., Fu M. G., Suib Steven L., Ye D. Q., Chemosphere, 2023, 334, 138995 |
| 18 | Singer C., Kureti S., Appl. Catal. B, 2020, 272, 118961 |
| 19 | Chen H., Wang Y., Lv Y. K., RSC Adv., 2016, 6(59), 54032—54040 |
| 20 | Chen B. B., Wu B., Yu L. M., Crocker M., Shi C., ACS Catal., 2020, 10(11), 6176—6187 |
| 21 | Wang J. L., Li J., Zhang P. Y., Zhang G. K., Appl. Catal. B, 2018, 224, 863—870 |
| 22 | Xu F., Huang Z. W., Hu P. P., Chen Y. X., Zheng L., Gao J. Y., Tang X. F., ChemComm, 2015, 51(48), 9888—9891 |
| 23 | Zhang J. H., Li Y. B., Wang L., Zhang C. B., He H., Catal. Sci. Technol., 2015, 5(4), 2305—2313 |
| 24 | Liang S. H., Bulgan F. T. G., Zong R. L., Zhu Y. F., J. Phys. Chem. C, 2008, 112(14), 5307—5315 |
| 25 | Cheng L., Men Y., Wang J. G., Wang H., An W., Wang Y. Q., Duan Z. C., Liu J., Appl. Catal. B, 2017, 204, 374—384 |
| 26 | Yu D., Ren Y., Yu X. H., Fan X. Q., Wang L. Y., Wang R. D., Zhao Z., Cheng K., Chen Y. S., Sojka Z., Kotarba A., Wei Y. C., Liu J., Appl. Catal. B, 2021, 285, 119779 |
| 27 | Yu X. H., Li J. M., Wei Y. C., Zhao Z., Liu, J., Jin B. F., Duan A. J., Jiang G. Y., Ind. Eng. Chem. Res., 2014, 53(23), 9653— 9664 |
| 28 | Liu Y., Yang W. J., Zhang P. Y., Zhang J. Y., Appl. Surf. Sci., 2018, 442, 640—649 |
| 29 | Senthilkumar N., Kumar G. G., Manthiram A., Adv. Energy Mater., 2018, 8(6), 1702207 |
| 30 | Li Q. Q., Huang X. C., Su G. J., Zheng M. H., Huang C. H., Wang M. J., Ma C. Y., Wei D., Environ. Sci. Technol., 2018, 52(22), 13351—13360 |
| 31 | Cui D. Y., Gao K., Lu P., Yang H., Liu Y. N., Xue D. F., Funct. Mater. Lett., 2011, 4(1), 57—60 |
| 32 | Thackeray M. M. Prog. Solid. State Ch., 1997, 25(1/2), 1—71 |
| 33 | Liu J. T., Ge X., Ye X. X., Wang G. Z., Zhang H. M., Zhou H. J., Zhang Y. X., Zhao H. J., J. Mater. Chem. A, 2016, 4(5), 1970—1979 |
| 34 | Chan Z. M., Kitchaev D. A., Weker J. N., Schnedermann C., Lim K., Ceder G., Tumas W., Toney M. F., Nocera D. G., Proc. Natl. Acad. Sci. USA, 2018, 115(23), e5261—e5268 |
| 35 | Cabello G., Davoglio R. A., Appl. Catal. B, 2017, 218, 192—198 |
| 36 | Santos V. P., Pereira M. F. R., Orfao J. J. M., Figueiredo J. L., Appl. Catal. B, 2010, 99(1/2), 353—363 |
| 37 | Zheng Y. L., Wang W. Z., Jiang D., Zhang L., Chem. Eng. J., 2016, 284, 21—27 |
| 38 | Peng C., Yu D., Zhang C. L., Chen M. Z., Wang L. Y., Yu X. H., Fan X. Q., Zhao Z., Cheng K., Chen Y. S., Wei Y. C., Liu J., J. Environ Sci.(China), 2023, 125, 82—94 |
| 39 | Wu Q. Q., Xiong J., Li J. M., Liu J., Zhao Z., Hao S. J., Catal. Today, 2019, 327, 143—153 |
| 40 | Xiong J., Wei Y. C., Zhang Y. L., Zhang P., Yu Q., Mei X. L., Liu X., Zhao Z., Jian L., ACS Catal., 2020, 10(13), 7123—7135 |
| 41 | Yu X. H., Li J. M., Wei Y. C., Zhao Z., Liu J., Jin B. F., Duan A. J., Jiang G. Y., Ind. Eng. Chem. Res., 2014, 53(23), 9653—9664 |
| 42 | Feng N. J., Zhu Z. J., Zhao P., Wang L., Wan H., Guan G. F., Appl. Surf. Sci., 2020, 515(15), 146013 |
| 43 | Wu X. D., Liu S., Weng D., Lin F., Ran R., J. Hazard. Mater., 2021, 187, 283—290 |
| 44 | Yu D., Wang L. Y., Zhang C. L., Peng C., Yu X. H., Fan X. Q., Liu B., Li K. X., Li Z. G., Wei Y. C., Jian L., Zhao Z., ACS Catal., 2022, 12, 15056—15075 |
| 45 | Wang J. G., Yang S. F., Sun H. H., Qiu J. Q., Men Y., J. Colloid Interface Sci., 2020, 577, 355—367 |
| 46 | Gao S. Y., Yu D., Zhou S. R., Zhang C. L., Wang L. Y., Fan X. Q., Yu X. H., Zhao Z., Processes, 2023, 11, 2902 |
| [1] | 徐德义, 丁超俊, 李芳, 刘月明, 何鸣元. 磷改性失活TS-1高效催化C5=裂解制备C2=/C3=反应的研究[J]. 高等学校化学学报, 2023, 44(8): 20230094. |
| [2] | 冯瑞沁, 方云, 樊晔, 夏咏梅. 金纳米花的简易合成及催化硼氢化钠还原对硝基酚性能[J]. 高等学校化学学报, 2023, 44(8): 20230027. |
| [3] | 姚依璇, 鲁长波, 张洪伟, 张杜鑫, 商红岩, 田原宇. [Fe]在环氧化物选择性开环反应中的催化性能[J]. 高等学校化学学报, 2023, 44(8): 20230137. |
| [4] | 黄鼎旻, 孙浩田, 王振炜, 刘豪, 苏贤斌. 室温下3-喹啉硼酸催化的羧酸酰胺化反应[J]. 高等学校化学学报, 2023, 44(6): 20230004. |
| [5] | 朱影影, 闫晓雨, 于静, 满文鑫, 刘凤, 周颖, 马献涛. 无催化剂下二硫醚的高效合成[J]. 高等学校化学学报, 2023, 44(6): 20230045. |
| [6] | 黄金, 郭丽君, 李锋, 李杨, 李翠勤. 树状PAMAM改性纳米二氧化硅负载镍催化剂的制备及催化乙烯齐聚性能[J]. 高等学校化学学报, 2023, 44(6): 20220778. |
| [7] | 赵倩, 李赏, 程矿伟, 文智勇, 张晓宇, 易少杰, 潘牧. 氮掺杂PtCo/C合金催化剂的研究[J]. 高等学校化学学报, 2023, 44(6): 20230016. |
| [8] | 侯俊英, 侯传源, 郝建军, 李建昌, 王雅雅. 铜掺杂多孔氧化镍非均相催化材料的制备及催化氧化性能[J]. 高等学校化学学报, 2023, 44(6): 20230042. |
| [9] | 卞宣昂, 周超, 赵运宣, 张铁锐. 基于水滑石衍生钌催化剂光驱动合成氨的研究[J]. 高等学校化学学报, 2023, 44(6): 20230095. |
| [10] | 杜磊, 刘兆清. 非贵金属催化剂在羟甲基糠醛电氧化增值中的应用[J]. 高等学校化学学报, 2023, 44(5): 20220710. |
| [11] | 王军, 杜石谦, 陶李. 高温聚合物电解质膜燃料电池催化剂的研究进展[J]. 高等学校化学学报, 2023, 44(5): 20220722. |
| [12] | 张小玉, 曲干, 薛冬萍, 闫文付, 张佳楠. 碳基催化剂用于电催化氧还原生产H2O2的研究进展: 策略、 计算及实际应用[J]. 高等学校化学学报, 2023, 44(5): 20220775. |
| [13] | 李瑞松, 苗政培, 李静, 田新龙. 中空贵金属纳米材料氧还原催化的研究进展[J]. 高等学校化学学报, 2023, 44(5): 20220730. |
| [14] | 夏傲, 曾啸雄, 李佳萌, 陈军, 谈国强. Al3+离子掺杂 δ-MnO2的制备和电化学性能[J]. 高等学校化学学报, 2023, 44(4): 20220658. |
| [15] | 徐若韬, 王强, 鲍庆嘉, 王伟宇, 张志, 刘朝阳, 徐君, 邓风. γ -Al2O3颗粒溶液浸润过程的原位磁共振成像[J]. 高等学校化学学报, 2023, 44(4): 20220587. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||