

高等学校化学学报 ›› 2023, Vol. 44 ›› Issue (10): 20230196.doi: 10.7503/cjcu20230196
收稿日期:2023-04-20
出版日期:2023-10-10
发布日期:2023-07-29
通讯作者:
王祖民,于然波
E-mail:wangzm@ipe.ac.cn;ranboyu@ustb.edu.cn
基金资助:
LI Mengdie1, WANG Zumin1,2( ), QI Jian2, YU Ranbo1(
), QI Jian2, YU Ranbo1( )
)
Received:2023-04-20
Online:2023-10-10
Published:2023-07-29
Contact:
WANG Zumin, YU Ranbo
E-mail:wangzm@ipe.ac.cn;ranboyu@ustb.edu.cn
Supported by:摘要:
我国提出2030年“碳达峰”和2060年“碳中和”战略目标. 将CO2转化为高附加值的化学产品和液态燃料, 在实现碳减排的同时, 还可减轻对煤、 石油等传统资源的依赖, 具有重要意义. 光催化CO2还原是一种非常重要的途径, 设计和制备高效的CO2还原光催化剂是关键. 通过半导体和金属或具有匹配的电子带结构的半导体之间形成良好的异质结结构可以有效地促进电荷转移并抑制光生电子和空穴的重新复合, 从而提高光催化性能. 本综合评述重点讨论了氧化物异质结构用于光催化CO2还原的最新进展, 系统总结了形成异质结构的类型、 组成等因素, 对提高CO2光催化性能的内在机理进行了深入阐述, 并对该领域研究发展的方向进行了展望.
中图分类号:
TrendMD:
李孟蝶, 王祖民, 齐健, 于然波. 金属氧化物异质结的构建及在光催化CO2还原反应中应用的研究进展. 高等学校化学学报, 2023, 44(10): 20230196.
LI Mengdie, WANG Zumin, QI Jian, YU Ranbo. Progress in the Construction of Metal Oxide Heterojunctions and Their Application in Photocatalytic CO2 Reduction. Chem. J. Chinese Universities, 2023, 44(10): 20230196.
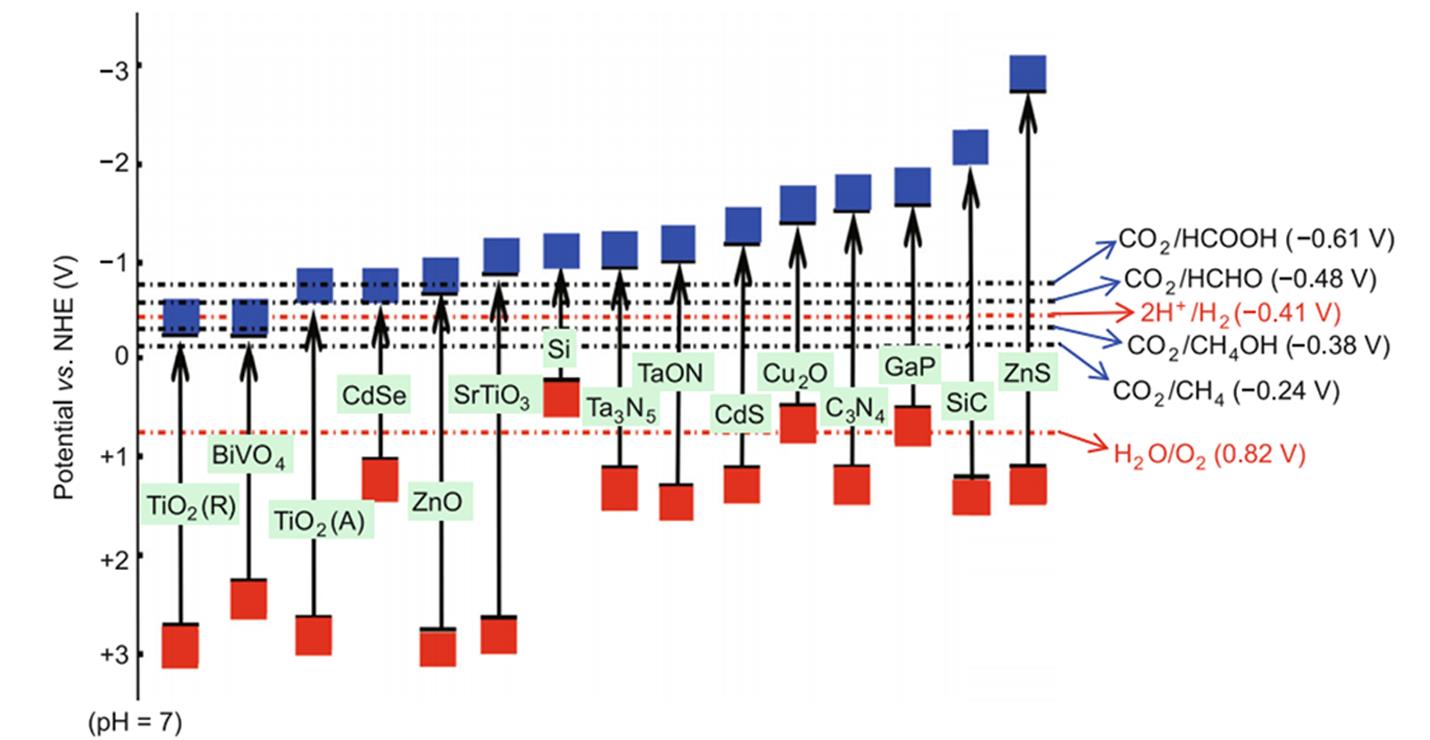
Fig.2 Reduction potentials of different products and corresponding reduction/oxidation potentials of several photocatalysts[13]Copyright 2014, Springer Nature.
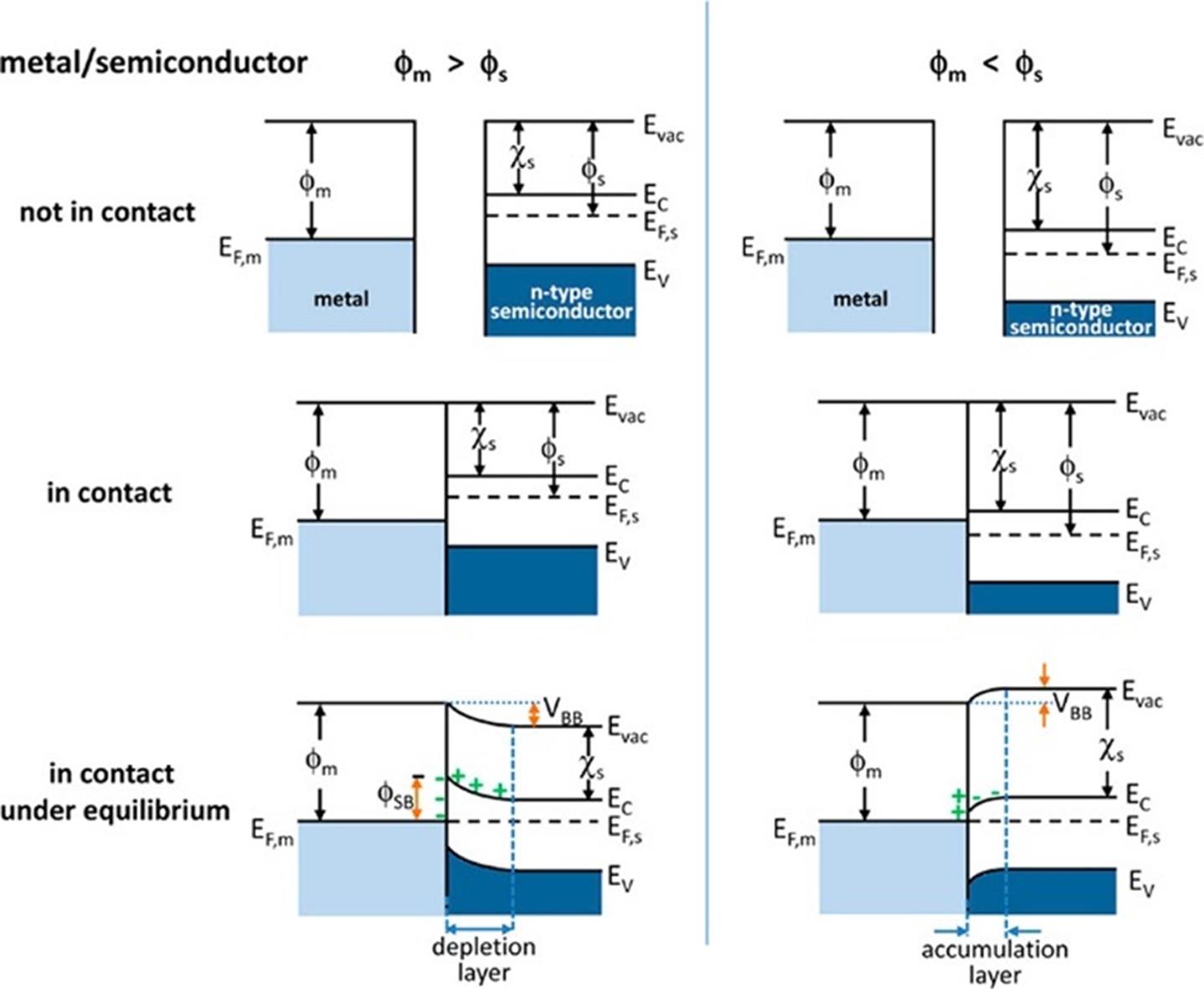
Fig.4 Energy band diagrams of metal and n⁃type semiconductor contacts[25]Evac, Vacuum energy; Ec, energy of conduction band minimum; Ev, energy of valence band maximum; ϕm, metal work function; ϕs, semiconductor work function; χs, electron affinity of the semiconductor. Copyright 2019, American Chemical Society.
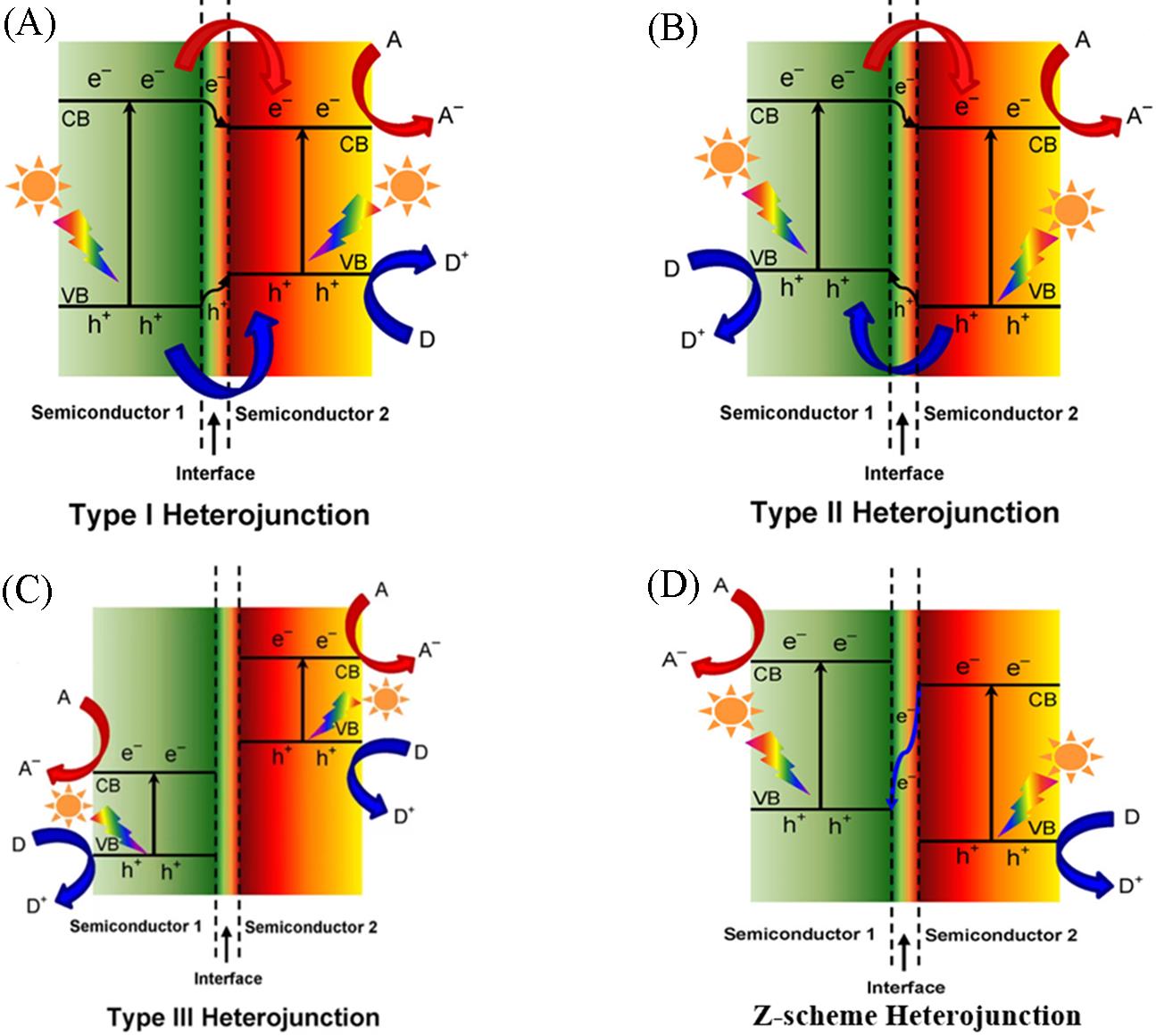
Fig.5 Schematic energy band diagrams of three different types of heterojunctions of type I(A), type II(B), type III(C) and Z⁃scheme(D) in a typical semiconductor hybrid nanocomposite[26]Copyright 2016, American Chemical Society.
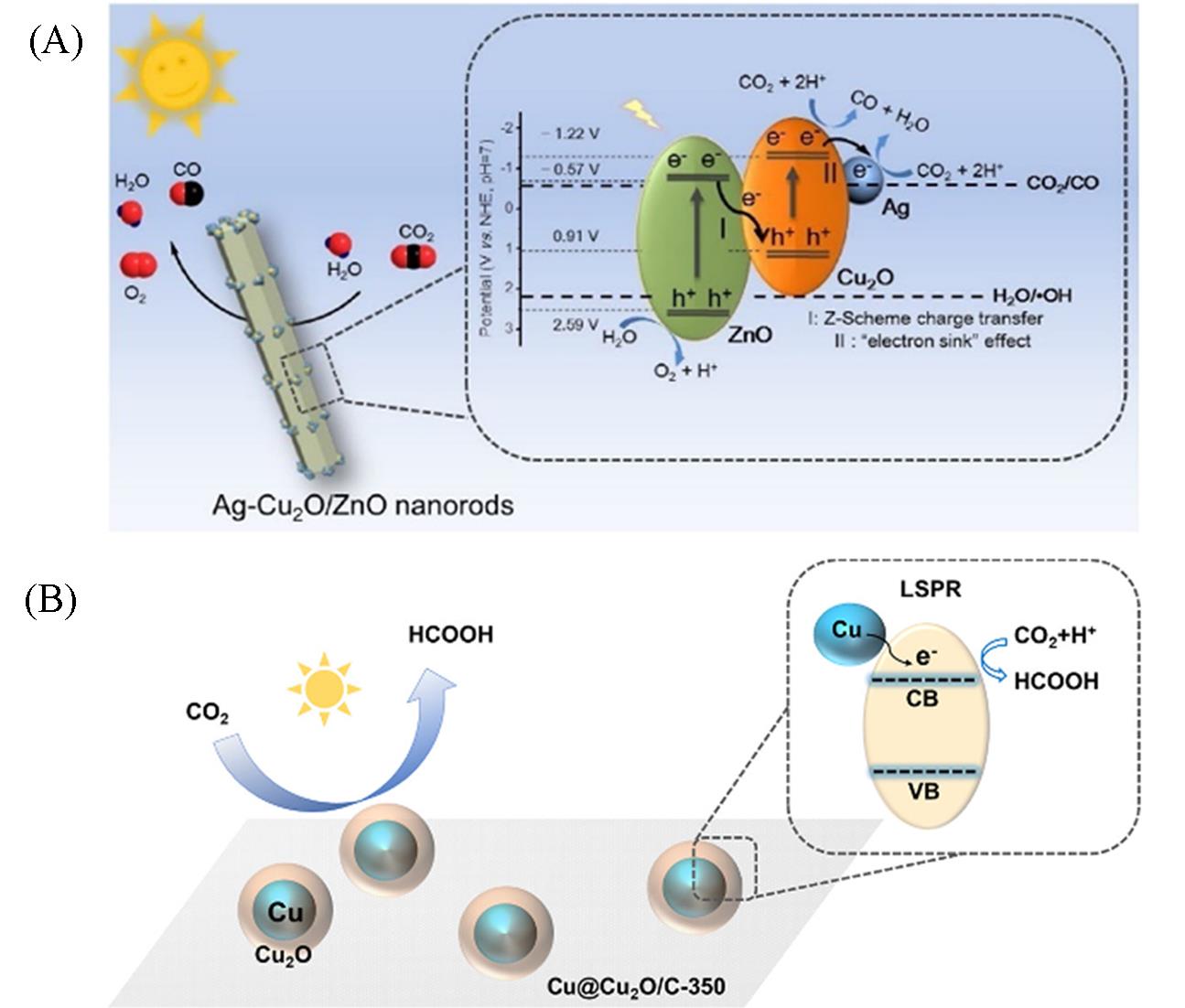
Fig.7 Possible mechanism of Ag⁃Cu2O/ZnO NRs photocatalyst in CO2 reduction process(A)[33] and possible mechanism of Cu@Cu2O/C⁃350 photocatalyst in CO2 reduction process(B)[34](A) Copyright 2019, Elsevier; (B) Copyright 2022, Elsevier.
| Photocatalyst b | Formation rate/(µmol·g-1·h-1) | R(e-)/ (µmol·g-1·h-1) | Selectivity for CO2 reduction(%) | Transient photocurrent responses/(μA·cm-2) c | ||
|---|---|---|---|---|---|---|
| CO | CH4 | H2 | ||||
| TiO2 | 1.2 | 0.38 | 2.1 | 10 | 56 | ca. 10 |
| Pt⁃TiO2 | 1.1 | 5.2 | 33 | 110 | 40 | ca. 158 |
| Pd⁃TiO2 | 1.1 | 4.3 | 25 | 85 | 42 | ca. 140 |
| Rh⁃TiO2 | 0.62 | 3.5 | 18 | 66 | 45 | ca. 63 |
| Au⁃TiO2 | 1.5 | 3.1 | 20 | 67 | 41 | ca. 99 |
| Ag⁃TiO2 | 1.7 | 2.1 | 16 | 51 | 39 | ca. 43 |
Table 1 Catalytic behaviors of TiO2 promoted by noble metal cocatalysts for photocatalytic reduction of CO2 in the presence of H2O vapor a
| Photocatalyst b | Formation rate/(µmol·g-1·h-1) | R(e-)/ (µmol·g-1·h-1) | Selectivity for CO2 reduction(%) | Transient photocurrent responses/(μA·cm-2) c | ||
|---|---|---|---|---|---|---|
| CO | CH4 | H2 | ||||
| TiO2 | 1.2 | 0.38 | 2.1 | 10 | 56 | ca. 10 |
| Pt⁃TiO2 | 1.1 | 5.2 | 33 | 110 | 40 | ca. 158 |
| Pd⁃TiO2 | 1.1 | 4.3 | 25 | 85 | 42 | ca. 140 |
| Rh⁃TiO2 | 0.62 | 3.5 | 18 | 66 | 45 | ca. 63 |
| Au⁃TiO2 | 1.5 | 3.1 | 20 | 67 | 41 | ca. 99 |
| Ag⁃TiO2 | 1.7 | 2.1 | 16 | 51 | 39 | ca. 43 |
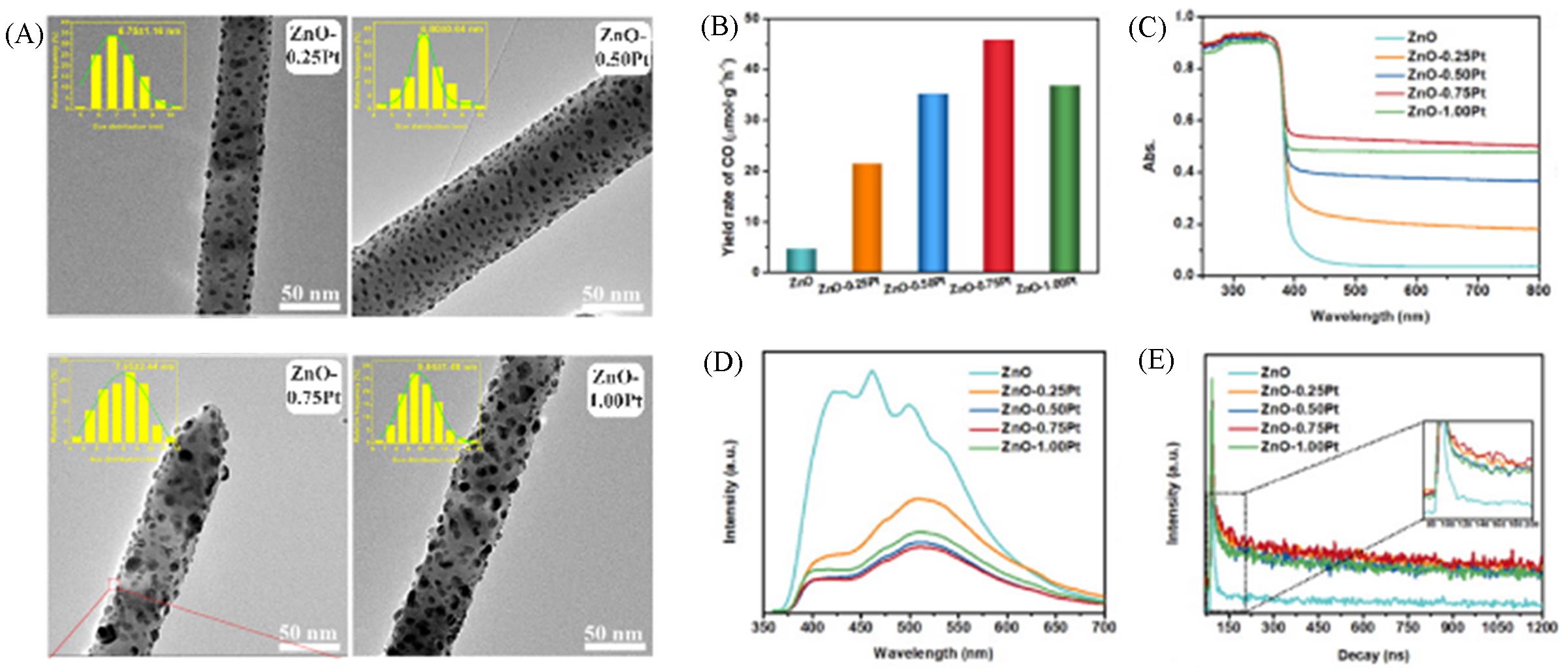
Fig.8 TEM images of Pt/ZnO with different Pt loadings(A), comparison of CO yields of bare ZnO fibers and Pt/ZnO nanocomposites(B), UV⁃Vis spectra(C), steady⁃state(D) and time⁃resolved PL spectra(E)[36]Copyright 2022, Elsevier.
| Photocatalyst | Light source | Plasmonic NPs | Main product | Side product | Highest rate/(μmol·g-1·h-1) | AQE(%) | Ref. |
|---|---|---|---|---|---|---|---|
| Cu@Cu2O/C⁃350 | Visible light irradiation (420 nm<λ<780 nm) | Cu | HCOOH | — | 31 μg/h | 0.12(560 nm) | [ |
| ZnO⁃Cu⁃CdS | 300 W Xe lamp | Cu | CH4 | CO | 890 | 8.8(420 nm) | [ |
| Au⁃3DOM TiO2 | 300 W Xe lamp λ>420 nm | Au | CH4 | — | 23.1 | — | [ |
| Ag⁃TiO2 hollow sphere | 300 W Xe lamp λ>420 nm | Ag | CH4 | — | 1.5 | — | [ |
| Pd⁃Au/Al2O3 | Xe lampλ>420 nm | Au | CH4 | — | 930.3 | — | [ |
| Pt⁃Au/SiO2 | Visible light 300 nm<λ<800 nm | Au | CO | H2 | 68.6 | 2.83(520 nm) | [ |
Table 2 Photocatalysts developed using the SPR effect of Au NPs, Ag NPs
| Photocatalyst | Light source | Plasmonic NPs | Main product | Side product | Highest rate/(μmol·g-1·h-1) | AQE(%) | Ref. |
|---|---|---|---|---|---|---|---|
| Cu@Cu2O/C⁃350 | Visible light irradiation (420 nm<λ<780 nm) | Cu | HCOOH | — | 31 μg/h | 0.12(560 nm) | [ |
| ZnO⁃Cu⁃CdS | 300 W Xe lamp | Cu | CH4 | CO | 890 | 8.8(420 nm) | [ |
| Au⁃3DOM TiO2 | 300 W Xe lamp λ>420 nm | Au | CH4 | — | 23.1 | — | [ |
| Ag⁃TiO2 hollow sphere | 300 W Xe lamp λ>420 nm | Ag | CH4 | — | 1.5 | — | [ |
| Pd⁃Au/Al2O3 | Xe lampλ>420 nm | Au | CH4 | — | 930.3 | — | [ |
| Pt⁃Au/SiO2 | Visible light 300 nm<λ<800 nm | Au | CO | H2 | 68.6 | 2.83(520 nm) | [ |
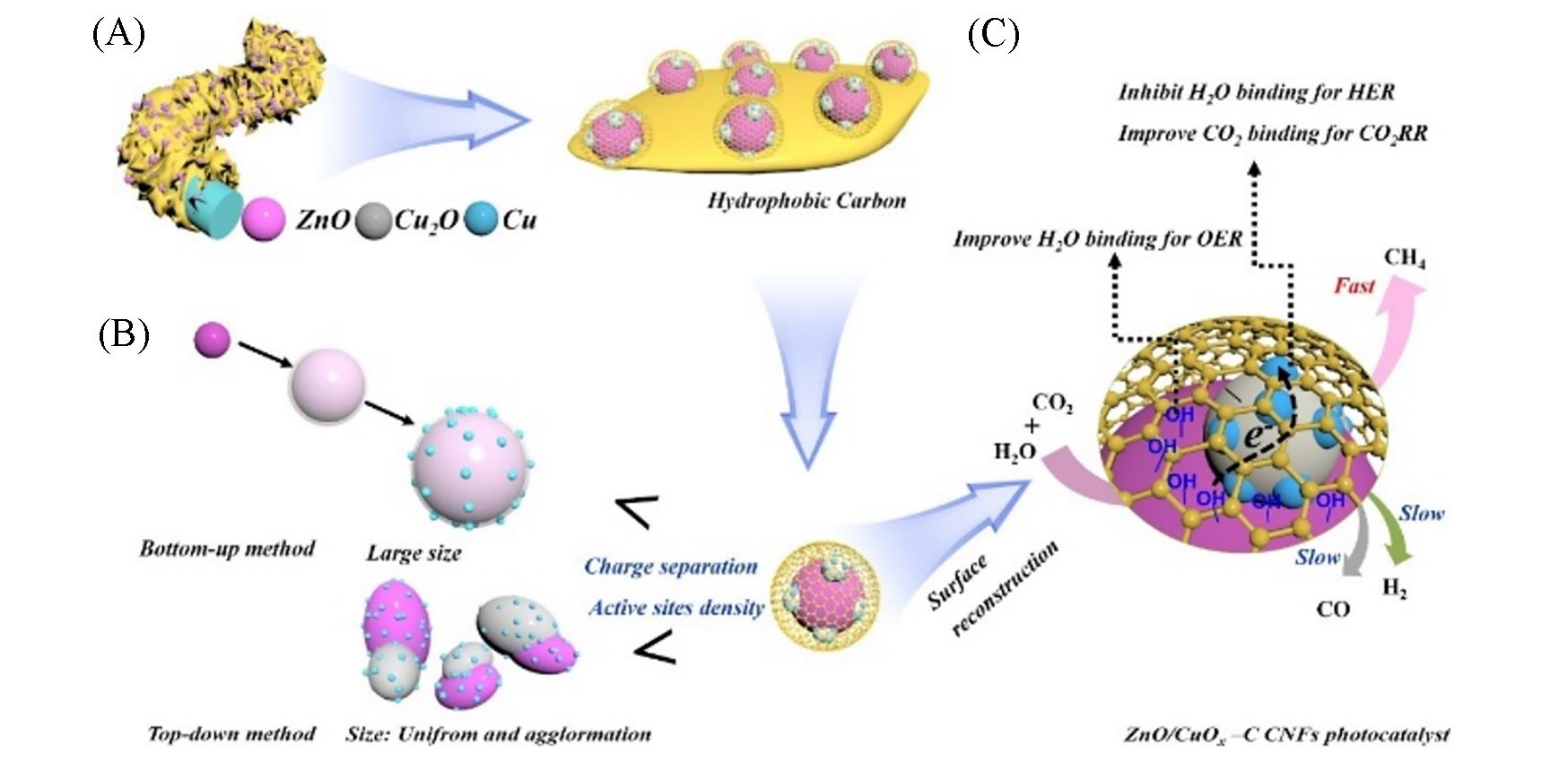
Fig.11 Hierarchical structure of ZnO/CuO x heterojunction embedded in porous carbon nanosheet array supported by CNFs(A), the particle size of ZnO/CuO x smaller than previously reported heterojunction photocatalysts synthesized by top⁃down and bottom⁃up methods(B), the surface reconstruction induced by solar light irradiation leading to rich accumulated electrons for CO2RR towards CH4, and inhibiting the H2 and CO generation(C)[47]Copyright 2021, Elsevier.
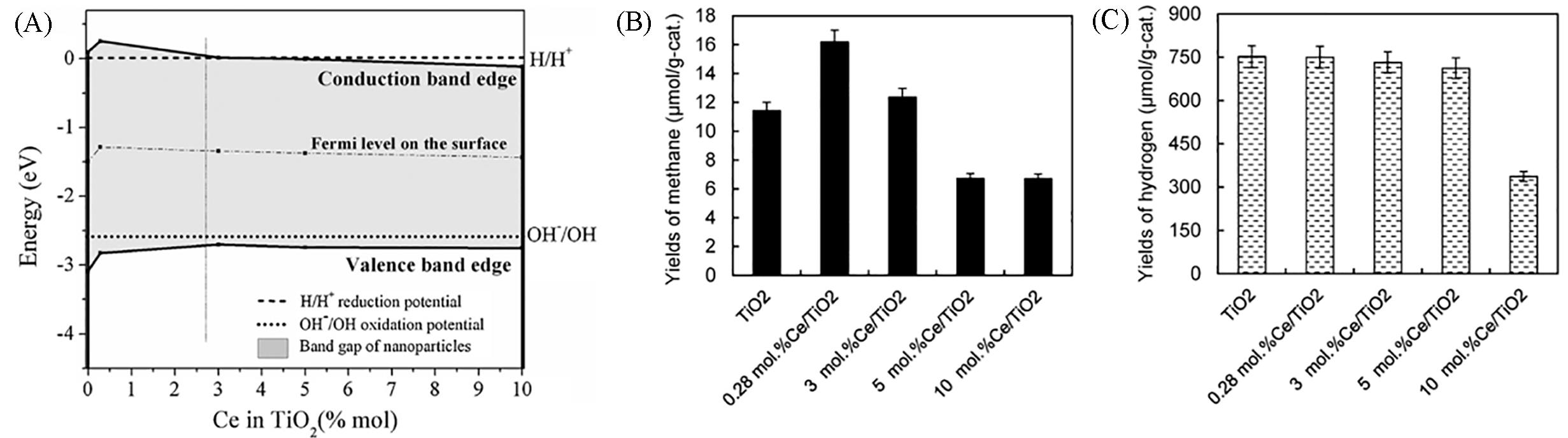
Fig.12 Illustration of the shifts of energies of electrons and holes in conduction and valence bands in dependence on the Ce loading(A), yields of methane(B) and hydrogen(C) over individual investigated photocatalysts in the CO2 photocatalytic reduction[48]Copyright 2014, Elsevier.
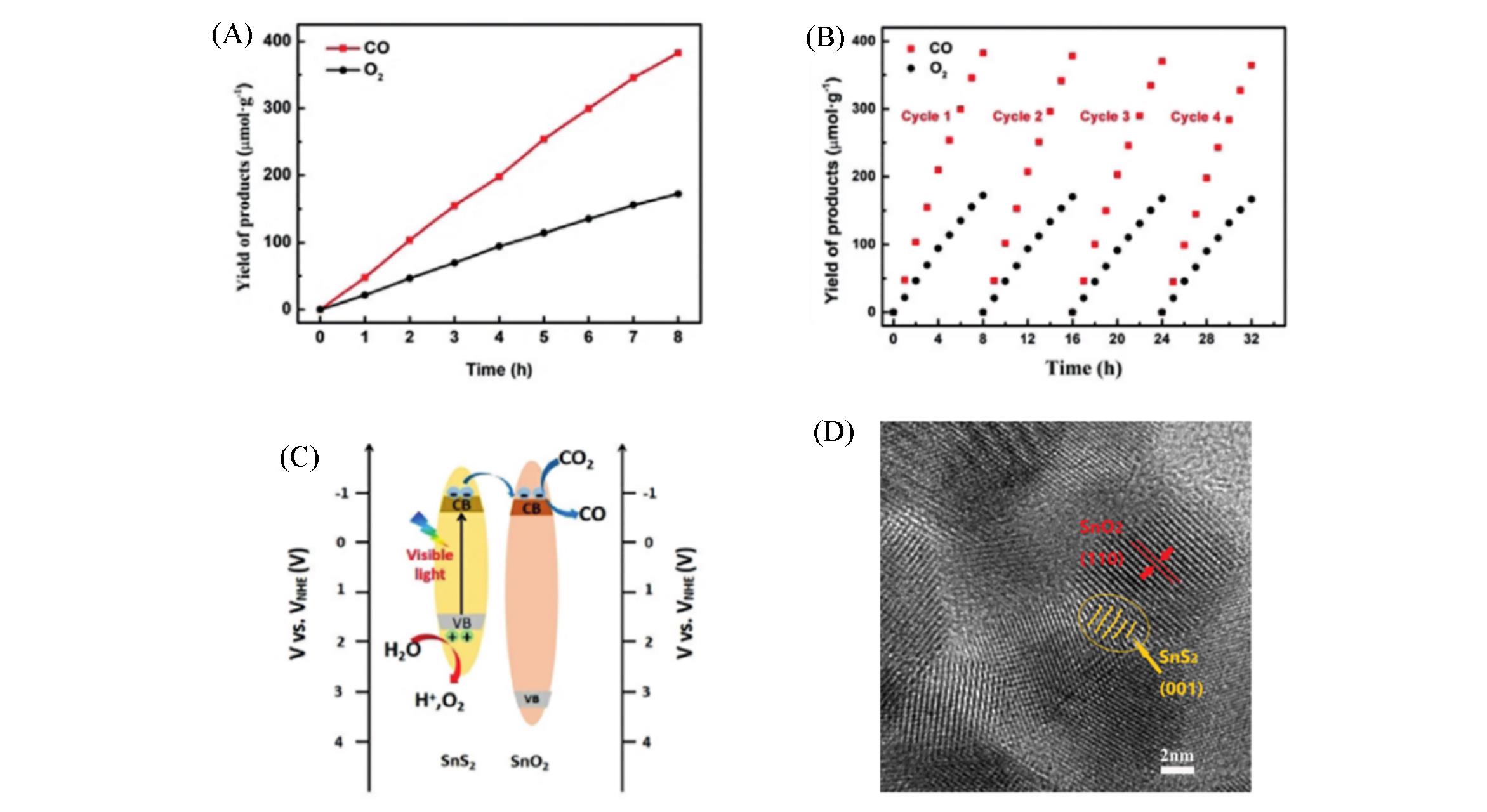
Fig.13 Yield(A) and stability(B) of CO2 photoreduction of CO and O2 under visible light irradiation, diagram of possible energy band structure and main CO2 reduction mechanism(C) and high⁃resolution transmission electron microscopy image(D)[52]Copyright 2020, Wiley-VCH.
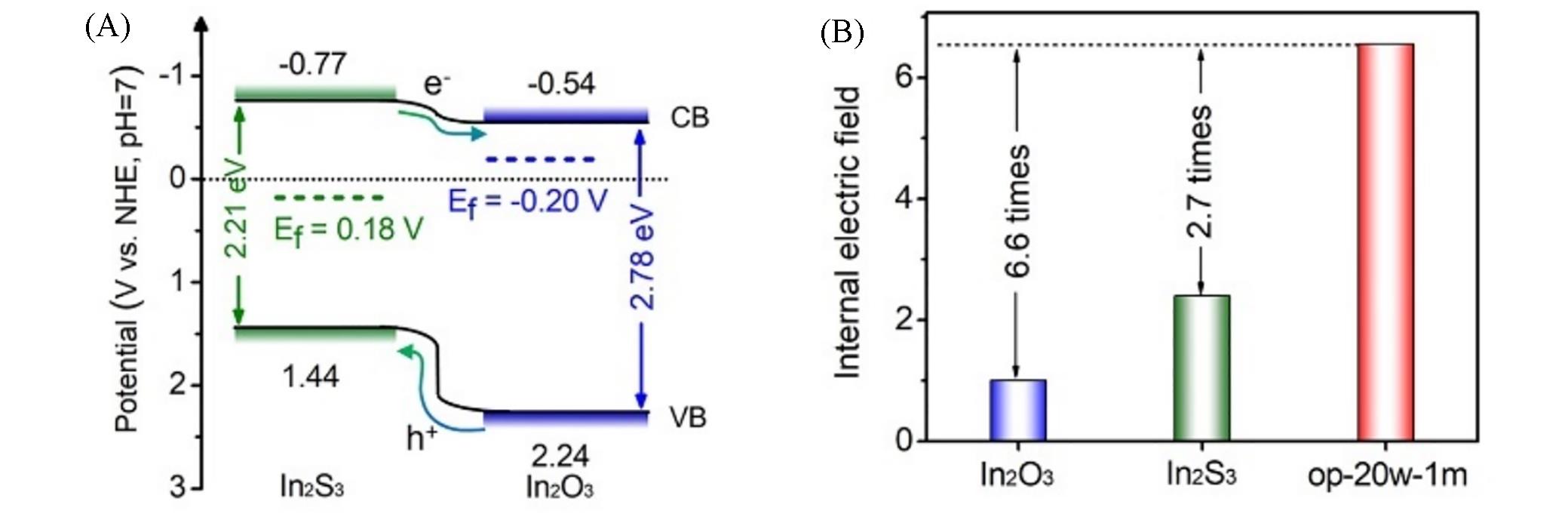
Fig.14 Band gap structure and charge migration(A) and internal electric field strength(B) of In2O3/In2S3 in⁃plane heterojunction[57]Copyright 2021, Elsevier.
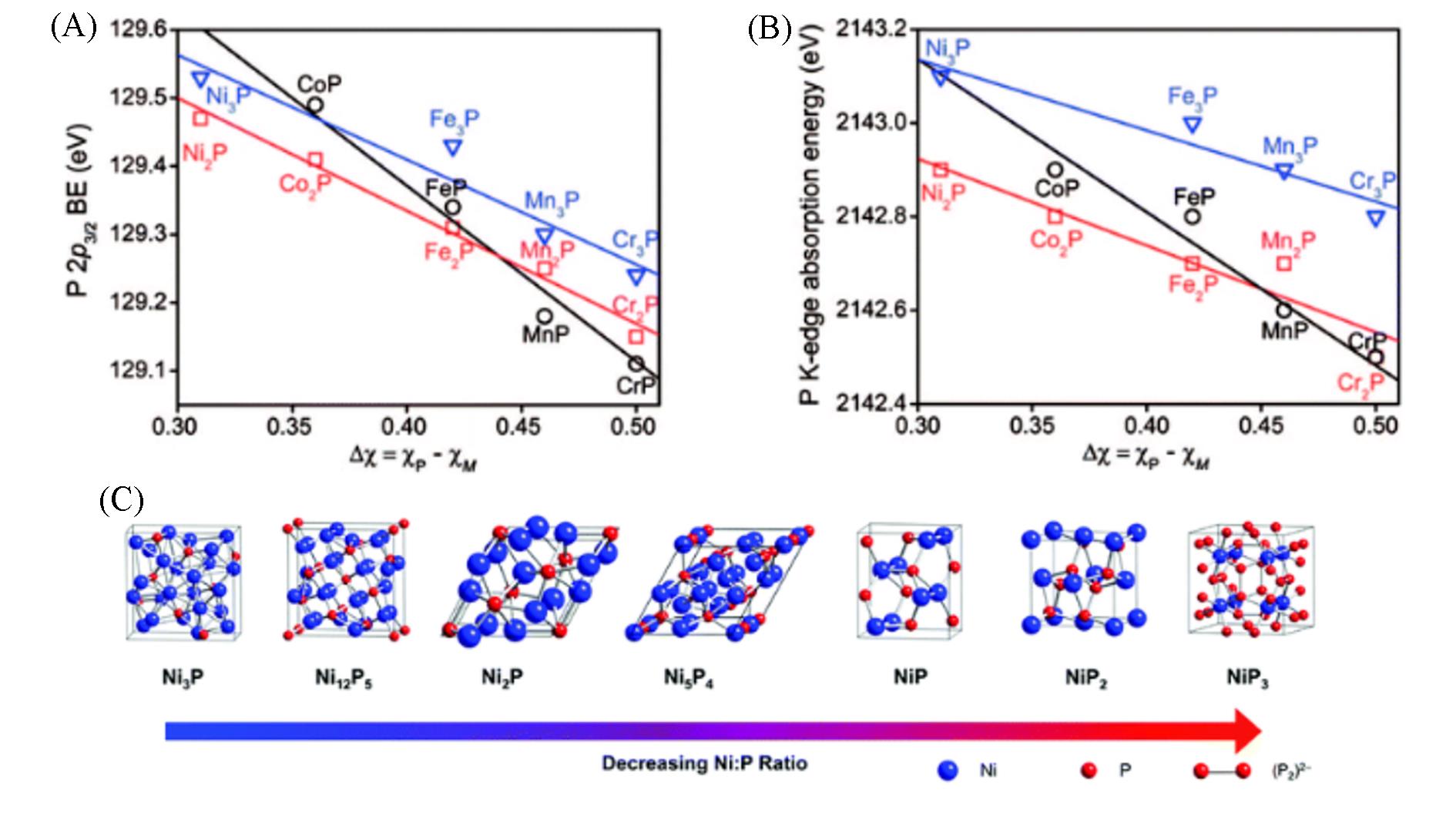
Fig.15 Dependence of P2p3/2 binding energy(A) and K⁃edge absorption energy on electronegativity differences for MP, M2P and M3P(B)[61], crystal structures of various nickel phosphides spanning a range of Ni∶P ratios(C)[62](C) The (P2)2- dimer, which appears in the NiP2 and NiP3 polyphosphides, is also shown. (A, B) Copyright 2008, American Chemical Society; (C) Copyright 2016, American Chemical Society.
| Sample | Ni12P5 TEM mean particle size/nm | CO rate/(mmol·g | CO selectivity(%) |
|---|---|---|---|
| Ni12P5 | 86±30 | 156±3 | 99.5±0.1 |
| 10.4% Ni12P5/SiO2 | 13±7 | 960±12 | 99.7±0.1 |
| 5.2% Ni12P5/SiO2 | 9±3 | 678±13 | 99.7±0.1 |
| 3.1% Ni12P5/SiO2 | 8±4 | 334±10 | 99.7±0.1 |
Table 3 Summary of the properties and catalytic performance of representative Ni12P5 samples
| Sample | Ni12P5 TEM mean particle size/nm | CO rate/(mmol·g | CO selectivity(%) |
|---|---|---|---|
| Ni12P5 | 86±30 | 156±3 | 99.5±0.1 |
| 10.4% Ni12P5/SiO2 | 13±7 | 960±12 | 99.7±0.1 |
| 5.2% Ni12P5/SiO2 | 9±3 | 678±13 | 99.7±0.1 |
| 3.1% Ni12P5/SiO2 | 8±4 | 334±10 | 99.7±0.1 |
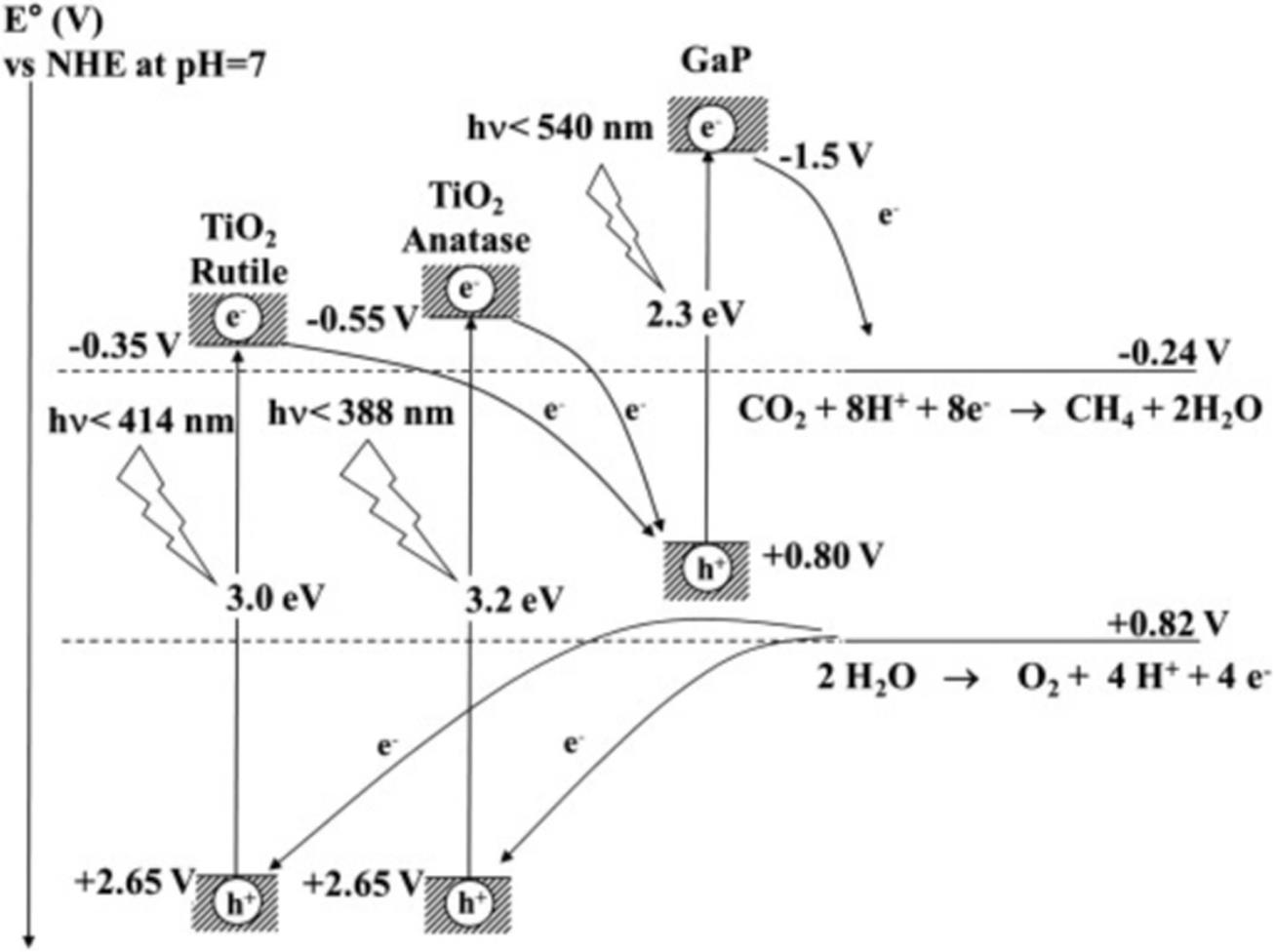
Fig.16 Relationship between the band structures of TiO2 and GaP and the reduction potentials(versus NHE at pH=7) for the most favorite processes of oxidation and reduction[65]Copyright 2014, Elsevier.
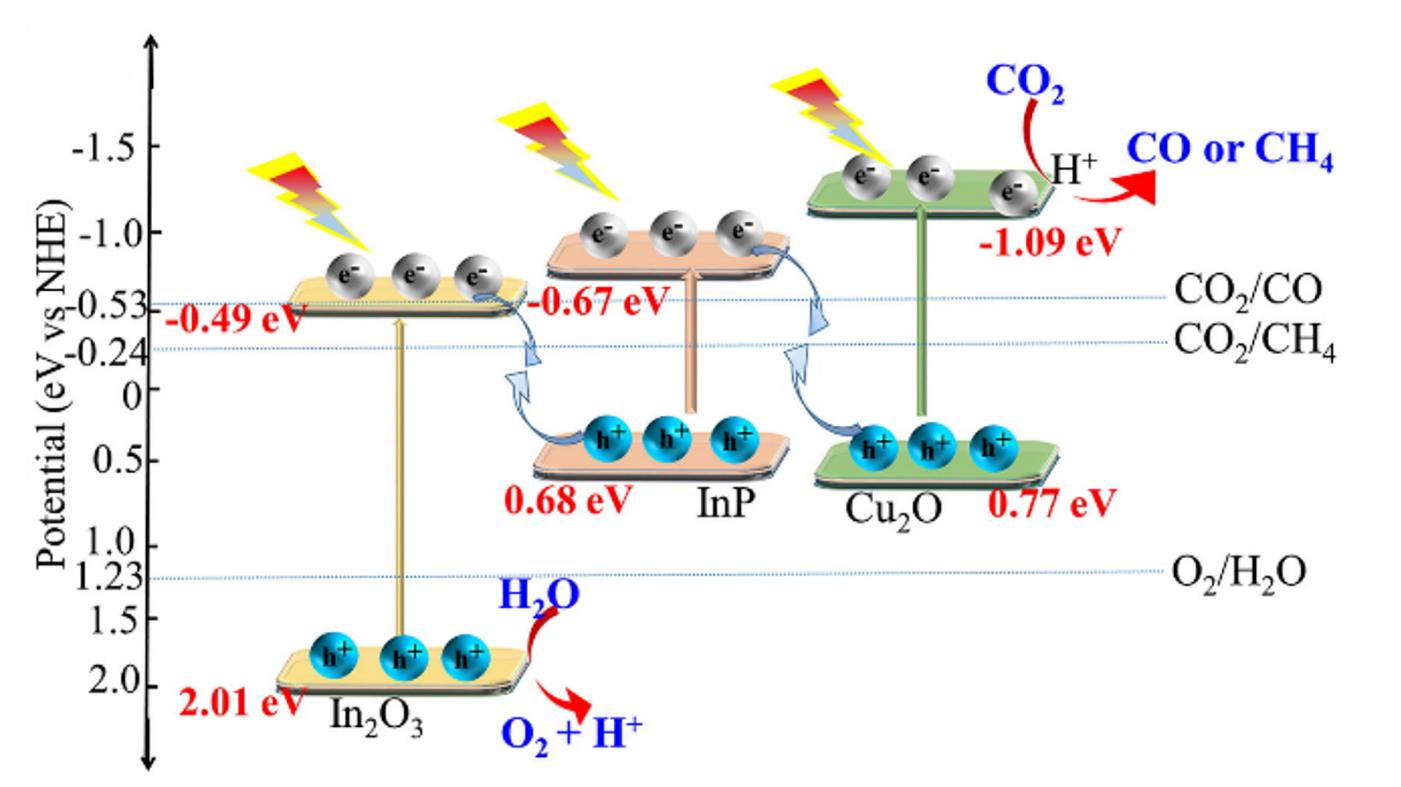
Fig.17 Double Z⁃type charge transfer mode and proposed mechanism of photocatalytic CO2 reduction with water as reducing agent[66]Copyright 2022, Elsevier.
| Photocatalyst | Light source | Main product | Side product | Rate of main product/(μmol·g-1·h-1) | Ref. |
|---|---|---|---|---|---|
| 1∶10⁃GaP/TiO2 | 1500 W high pressure Xe lamp | CH4 | — | 11.818 | [ |
| In2O3@InP60/Cu2O⁃1 | 300 W Xe lamp | CH4 | CO | 7.76 | [ |
| Ni2P/NiO/CN(0.25) | Visible⁃light from a Xe lamp(PLS⁃SXE300UV) λ>420 nm | CO | CH4 | 1.506 | [ |
Table 4 Typical photocatalytic CO2 reduction systems using transition metal phosphides as co-catalysts
| Photocatalyst | Light source | Main product | Side product | Rate of main product/(μmol·g-1·h-1) | Ref. |
|---|---|---|---|---|---|
| 1∶10⁃GaP/TiO2 | 1500 W high pressure Xe lamp | CH4 | — | 11.818 | [ |
| In2O3@InP60/Cu2O⁃1 | 300 W Xe lamp | CH4 | CO | 7.76 | [ |
| Ni2P/NiO/CN(0.25) | Visible⁃light from a Xe lamp(PLS⁃SXE300UV) λ>420 nm | CO | CH4 | 1.506 | [ |
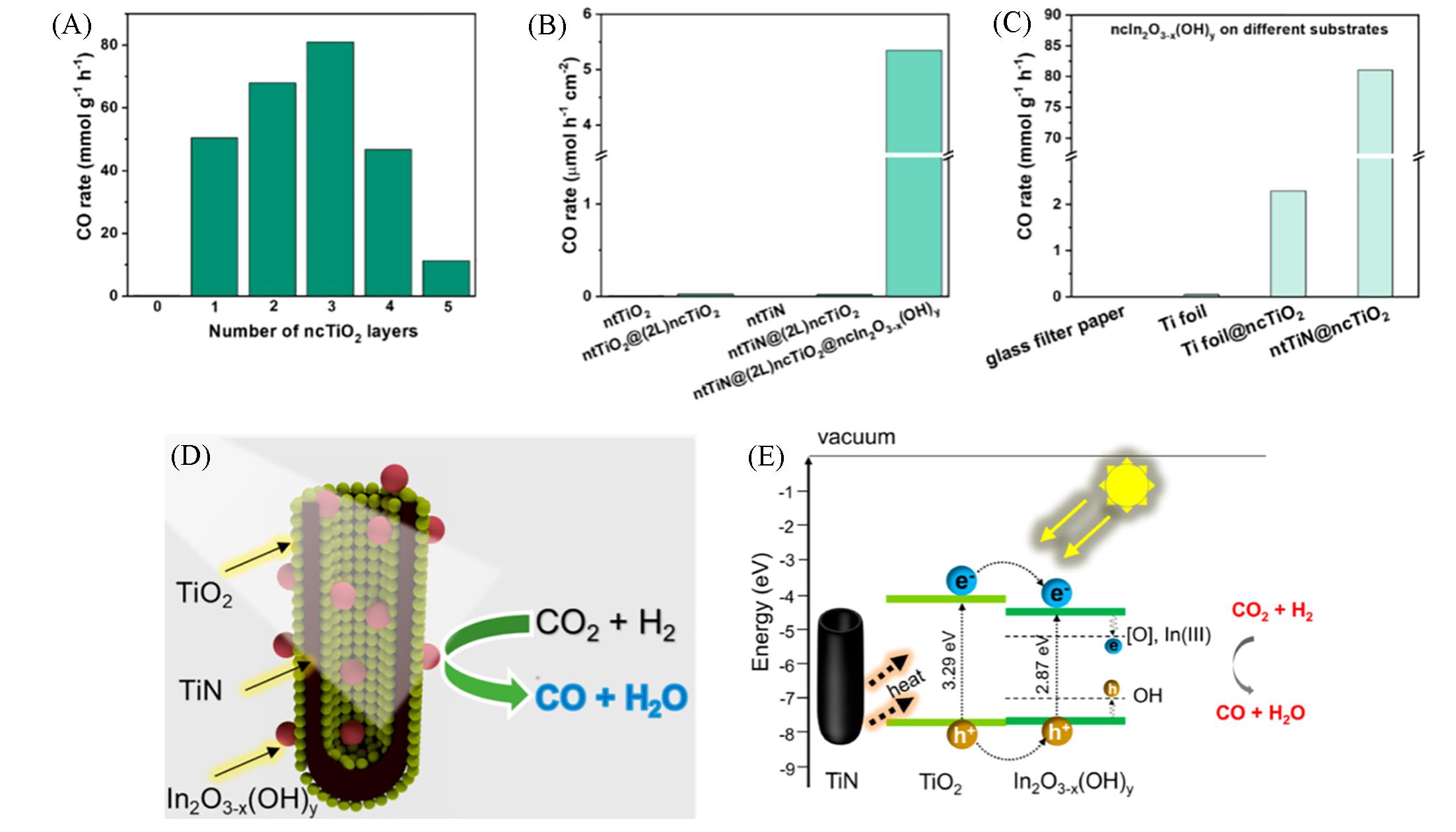
Fig.18 CO production rates in the reactor for ntTiN@ncTiO2@ncIn2O3-x (OH) y with different numbers of ncTiO2 layers(A), different combinations of constituent phases(B) and ncIn2O3-x (OH) y supported on different substrates(C), proposed activation mechanism for the photocatalytic reaction(D, E)[69]Rates are normalized with respect to either the mass of In2O3-x (OH) y used (A, C) or the area of samples exposed to light irradiation (B). Copyright 2021, American Chemical Society.
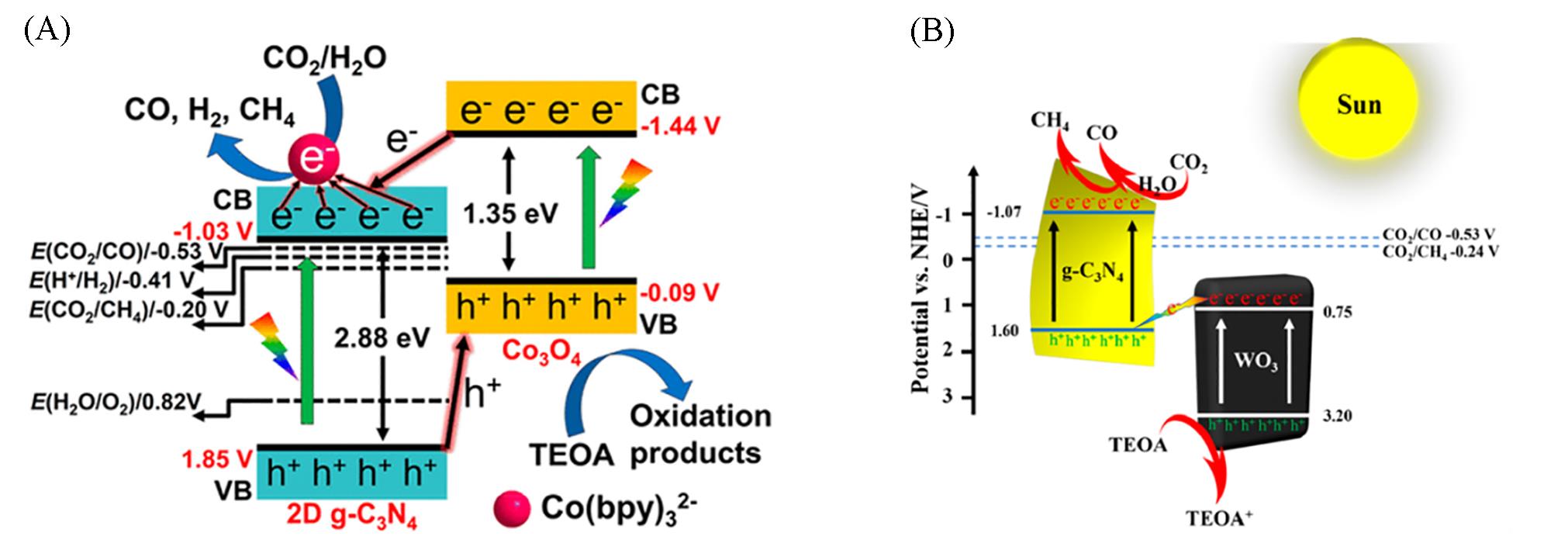
Fig.20 Schematic representation of charge transfer behavior and CO2 photoreduction sites(A)[79] and proposed photocatalytic mechanism of the WO3/CN photocatalyst(B)[80](A) Copyright 2018, American Chemical Society; (B) Copyright 2020, American Chemical Society.
| 1 | Gao S., Lin Y., Jiao X., Sun Y., Luo Q., Zhang W., Li D., Yang J., Xie Y., Nature, 2016, 529, 68—71 |
| 2 | Schwartz S. E., Energy Environ. Sci., 2008, 1, 430—453 |
| 3 | Ross M. B., De Luna P., Li Y., Dinh C. T., Kim D., Yang P., Sargent E. H., Nat. Catal., 2019, 2, 648—658 |
| 4 | Chang X., Wang T., Gong J., Energy Environ. Sci., 2016, 9, 2177—2196 |
| 5 | Butburee T., Sun Z., Centeno A., Xie F., Zhao Z., Wu D., Peerakiatkhajohn P., Thaweesak S., Wang H., Wang L., Nano Energy, 2019, 62, 426—433 |
| 6 | Liu X., Chen T., Xue Y., Fan J., Shen S., Hossain M. S. A. A., Amin M. A., Pan L., Xu X., Yamauchi Y., Coord. Chem. Rev., 2022, 459, 214440 |
| 7 | Wang L., Wan J., Zhao Y., Yang N., Wang D., J. Am. Chem. Soc., 2019, 141, 2238—2241 |
| 8 | Jiang M. P., Huang K. K., Liu J. H., Wang D., Wang Y., Wang X., Li Z. D., Wang X.Y., Geng Z. B., Hou X. Y., Feng S. H., Chem, 2020, 6, 2335—2346 |
| 9 | Qi M. Y., Lin Q., Tang Z. R., Xu Y. J., Appl. Catal. B, 2022, 307, 121158 |
| 10 | Yang M., Wang P., Li Y., Tang S., Lin X., Zhang H., Zhu Z., Chen F., Appl. Catal. B, 2022, 306, 121065 |
| 11 | Zhao K., Zhao S., Gao C., Qi J., Yin H., Wei D., Mideksa M. F., Wang X., Gao Y., Tang Z., Yu R., Small, 2018, 14, 1800762 |
| 12 | Li D., Kassymova M., Cai X., Zang S. Q., Jiang H. L., Coord. Chem. Rev., 2020, 412, 213262 |
| 13 | Li X., Wen J., Low J., Fang Y., Yu J., Sci. China Mater., 2014, 57, 70—100 |
| 14 | Low J., Cheng B., Yu J., Appl. Surf. Sci., 2017, 392, 658—686 |
| 15 | Mao J., Li K., Peng T. Y., Catal. Sci. Technol., 2013, 3, 2481—2498 |
| 16 | Ran J., Jaroniec M., Qiao S. Z., Adv. Mater., 2018, 30(7), 1704649 |
| 17 | Yang C., Li Q., Xia Y., Lv K., Li M., Appl. Surf. Sci., 2019, 464, 388—395 |
| 18 | Chu W., Zheng Q., Prezhdo O. V., Zhao J., J. Am. Chem. Soc., 2020, 142, 3214—3221 |
| 19 | Wang H., Peng R., Hood Z. D., Naguib M., Adhikari S. P., Wu Z., ChemSusChem, 2016, 9, 1490—1497 |
| 20 | An X., Wang W., Wang J., Duan H., Shi J., Yu X., Phys. Chem. Chem. Phys., 2018, 20, 11405—11411 |
| 21 | Ola O., Maroto⁃Valer M. M., J. Photochem. Photobiol. C, 2015, 24, 16—42 |
| 22 | Patial S., Kumar R., Raizada P., Singh P., Quyet Van L., Lichtfouse E., Dang Le Tri N., Van⁃Huy N., Environ. Res., 2021, 197, 111134 |
| 23 | Wen Z. Y., Zhan Z. G., Deng J. H., Feng Y. X., Lei Z., Xiong Z., Zhao Y. C., Zhang J. Y., Advances in New Renewable Energy, 2017, 5, 352—357 |
| 温智勇, 湛志刚, 邓剑华, 冯永新, 雷泽, 熊卓, 赵永椿, 张军营. 新能源进展, 2017, 5, 352—357 | |
| 24 | Li K., Teng C., Wang S., Min Q. H., Front. Chem., 2021, 9, 637501 |
| 25 | Wang Q., Domen K., Chem. Rev., 2020, 120, 919—985 |
| 26 | Ong W. J., Tan L. L., Ng Y. H., Yong S. T., Chai S. P., Chem. Rev., 2016, 116, 7159—7329 |
| 27 | Marschall R., Adv. Funct. Mater., 2014, 24, 2421—2440 |
| 28 | Lin J., Tian W., Zhang H., Duan X., Sun H., Wang S., Energy Fuels, 2021, 35, 7—24 |
| 29 | Sun S., Watanabe M., Wu J., An Q., Ishihara T., J. Am. Chem. Soc., 2018, 140, 6474—6482 |
| 30 | Stratakis M., Garcia H., Chem. Rev., 2012, 112, 4469—4506 |
| 31 | Ciriminna R., Falletta E., Della Pina C., Teles J. H., Pagliaro M., Angew. Chem. Int. Ed., 2016, 55, 14209—14216 |
| 32 | van Deelen T. W., Mejia C. H., de Jong K. P., Nat. Catal., 2019, 2, 955—970 |
| 33 | Zhang F., Li Y. H., Qi M. Y., Tang Z. R., Xu Y. J., Appl. Catal. B, 2020, 268, 118380 |
| 34 | Wang H., Cheng S., Cai X., Cheng L., Zhou R., Hou T., Li Y., Catal. Commun., 2022, 162, 106372 |
| 35 | Xie S., Wang Y., Zhang Q., Deng W., Wang Y., ACS Catal., 2014, 4, 3644—3653 |
| 36 | Ma X., Li D., Jiang Y., Jin H., Bai L., Qi J., You F., Yuan F., J. Colloid Interf. Sci., 2022, 628, 768—776 |
| 37 | Xue H. R., Wang T., Gong H., Guo H., Fan X. L., Gao B., Feng Y. Y., Meng X. G., Huang X. L., He J. P., Chem. Asian J., 2018, 13, 577—583 |
| 38 | Li G., Sun Y., Zhang Q., Gao Z., Sun W., Zhou X., Chem. Eng. J., 2021, 410, 128397 |
| 39 | Ahmad I., Shukrullah S., Naz M. Y., Bhatti H. N., Dalton Trans., 2023, 52, 6343—6359 |
| 40 | Jiao J., Wei Y., Zhao Z., Zhong W., Liu J., Li J., Duan A., Jiang G., Catal. Today, 2015, 258, 319—326 |
| 41 | Feng S., Wang M., Zhou Y., Li P., Tu W., Zou Z., APL Mater., 2015, 3(10), 104416 |
| 42 | Liu H., Li M., Thang Duy D., Liu Y., Zhou W., Liu L., Meng X., Nagao T., Ye J., Nano Energy, 2016, 26, 398—404 |
| 43 | Song H., Meng X., Dao T. D., Zhou W., Liu H., Shi L., Zhang H., Nagao T., Kako T., Ye J., ACS Appl. Mater. Interf., 2018, 10, 408—416 |
| 44 | Tan H., Xiao S., Yao S. R., Xiong C. R., Fine Chemicals, 2019, 36, 1210—1216 |
| 谈恒, 肖洒, 姚淑荣, 熊春荣. 精细化工, 2019, 36, 1210—1216 | |
| 45 | Nogueira A. E., Silva G. T. S. T., Oliveira J. A., Lopes O. F., Torres J. A., Carmo M., Ribeiro C., ACS Appl. Energy Mater., 2020, 3, 7629—7636 |
| 46 | Nakajima K., Baba Y., Noma R., Kitano M., Kondo J. N., Hayashi S., Hara M., J. Am. Chem. Soc., 2011, 133, 4224—4227 |
| 47 | Dou Y., Zhou A., Yao Y., Lim S. Y., Li J. R., Zhang W., Appl. Catal. B, 2021, 286, 119876 |
| 48 | Matejova L., Koci K., Reli M., Capek L., Hospodkova A., Peikertova P., Matej Z., Obalova L., Wach A., Kustrowski P., Kotarba A., Appl. Catal. B, 2014, 152, 172—183 |
| 49 | Su J., Wei Y., Vayssieres L., J. Phys. Chem. Lett., 2017, 8, 5228—5238 |
| 50 | Island J. O., Blanter S. I., Buscema M., van der Zant H. S. J., Castellanos⁃Gomez A., Nano Lett., 2015, 15, 7853—7858 |
| 51 | Wang J., Chao D., Liu J., Li L., Lai L., Lin J., Shen Z., Nano Energy, 2014, 7, 151—160 |
| 52 | You F. F., Wan J. W., Qi J., Mao D., Yang N. L., Zhang Q. H., Gu L., Wang D., Angew. Chem. Int. Ed., 2020, 59, 721—724 |
| 53 | Li X., Xu A., Fan H., Liu X., Wang J., Cao J., Yang L., Wei M., J. Power Sources, 2022, 545, 231923 |
| 54 | Li J., Cai L., Shang J., Yu Y., Zhang L., Adv. Mater., 2016, 28, 4059—4064 |
| 55 | Liu Y., Ye S., Xie H., Zhu J., Shi Q., Ta N., Chen R., Gao Y., An H., Nie W., Jing H., Fan F., Li C., Adv. Mater., 2020, 32(7), 1906513 |
| 56 | Chen X., Wang J., Chai Y., Zhang Z., Zhu Y., Adv. Mater., 2021, 33(7), 2007479 |
| 57 | Chen Q., Chen X., Jiang Q., Zheng Z., Song Z., Zhao Z., Xie Z., Kuang Q., Appl. Catal. B, 2021, 297, 120394 |
| 58 | Cao S., Wang C. J., Fu W. F., Chen Y., ChemSusChem, 2017, 10, 4306—4323 |
| 59 | Ji X. H., Wang Z. M., Chen X. Y., Yu R. B., Chem. J. Chinese Universities, 2021, 42(5), 1377—1394 |
| 季小好, 王祖民, 陈晓煜, 于然波. 高等学校化学学报, 2021, 42(5), 1377—1394 | |
| 60 | Von Schnering H. G., Hoenle W., Chem. Rev., 1988, 88(1), 243—273 |
| 61 | Blanchard P. E. R., Grosvenor A. P., Cavell R. G., Mar A., Chem. Mater., 2008, 20, 7081—7088 |
| 62 | Callejas J. F., Read C. G., Roske C. W., Lewis N. S., Schaak R. E., Chem. Mater., 2016, 28(1), 6017—6044 |
| 63 | Diplas S., Lovvik O. M., J. Phys. Condens. Matter, 2009, 21(24), 245503 |
| 64 | Xu Y. F., Duchesne P. N., Wang L., Tavasoli A., Jelle A. A., Xia M., Liao J. F., Kuang D. B., Ozin G. A., Nat. Commun., 2020, 11, 5149 |
| 65 | Marcì G., García⁃López E. I., Palmisano L., Catal. Commun., 2014, 53, 38—41 |
| 66 | Wang Y., Xu J., Wan J., Wang J., Wang L., J. Colloid Interface Sci., 2022, 616, 532—539 |
| 67 | Li Q., Feng W., Liu Y., Chen D., Wu Z., Wang H., J. Mater. Chem. A, 2022, 10, 15752—15765 |
| 68 | Balogun M. S., Huang Y., Qiu W., Yang H., Ji H., Tong Y., Mater. Today, 2017, 20, 425—451 |
| 69 | Nguyen N. T., Xia M., Duchesne P. N., Wang L., Mao C., Jelle A. A., Yan T., Li P., Lu Z. H., Ozin G. A., Nano Lett., 2021, 21, 1311—1319 |
| 70 | Yi H., Huang D., Qin L., Zeng G., Lai C., Cheng M., Ye S., Song B., Ren X., Guo X., Appl. Catal. B, 2018, 239, 408—424 |
| 71 | Zhang Y., Zeng G. M., Tang L., Chen J., Zhu Y., He X. X., He Y., Anal. Chem., 2015, 87, 989—996 |
| 72 | Kim J. K., Park G. D., Kim J. H., Park S. K., Kang Y. C., Small, 2017, 13(27), 1700068 |
| 73 | Sun Z., Guo J., Zhu S., Mao L., Ma J., Zhang D., Nanoscale, 2014, 6, 2186—2193 |
| 74 | Chen M., Zhou S., Zeng G., Zhang C., Xu P., Nano Today, 2018, 20, 7—9 |
| 75 | Ahmad I., Shukrullah S., Naz M. Y., Bhatti H. N., Ahmad M., Ahmed E., Ullah S., Hussien M., J. Environ. Chem. Eng., 2022, 10(3), 107762 |
| 76 | Faraz M., Naqvi F. K., Shakir M., Khare N., New J. Chem., 2018, 42, 2295—2305 |
| 77 | Zhu J., Jiang Z., Int. J. Electrochem. Sci., 2021, 16(3), 210318 |
| 78 | Zhang J., Shao S., Zhou D., Xu Q., Wang T., J. CO2 Util., 2021, 50, 101584 |
| 79 | Zhu X. W., Ji H. Y., Yi J. Y., Yang J. M., She X. J., Ding P. H., Li L., Deng J. J., Qian J. C., Xu H., Li H. M., Ind. Eng. Chem. Res., 2018, 57, 17394—17400 |
| 80 | Li X., Song X., Ma C., Cheng Y., Shen D., Zhang S., Liu W., Huo P., Wang H., ACS Appl. Nano Mater., 2020, 3, 1298—1306 |
| 81 | Wang L., Dong Y., Zhang J., Tao F., Xu J., J. Solid State Chem., 2022, 308, 122878 |
| 82 | Feng Z., Zeng L., Chen Y., Ma Y., Zhao C., Jin R., Lu Y., Wu Y., He Y., J. Mater. Res., 2017, 32, 3660—3668 |
| 83 | Rodriguez V., Camarillo R., Martinez F., Jimenez C., Rincon J., J. Supercrit Fluids, 2020, 163, 104876 |
| 84 | Jung H., Kim C., Yoo H. W., You J., Kim J. S., Jamal A., Gereige I., Ager J. W., Jung H. T., Energy Environ. Sci., 2023, 16(7), 2869—2878 |
| 85 | Ding C., Lu X., Tao B., Yang L., Xu X., Tang L., Chi H., Yang Y., Meira D. M., Wang L., Zhu X., Li S., Zhou Y., Zou Z., Adv. Funct. Mater., 2023, 33(35), 2302824 |
| [1] | 张立福, 王新康, 陈义旺. 用平衡相容性和相分离的新策略提高有机太阳电池效率[J]. 高等学校化学学报, 2023, 44(9): 20230177. |
| [2] | 曹圣哲, 黄欣, 杨志红. 直接Z型In2SSe/Sb范德华异质结光催化水分解的第一性原理研究[J]. 高等学校化学学报, 2023, 44(8): 20230145. |
| [3] | 文敏, 李豪杰, 李俊梁, 刘思奇, 胡笑添, 陈义旺. 准平面有机光伏器件的研究进展[J]. 高等学校化学学报, 2023, 44(7): 20230174. |
| [4] | 池丽萍, 牛壮壮, 廖洁, 唐凯斌, 高敏锐. 过渡金属氧化物插层化学及其电催化应用的新进展[J]. 高等学校化学学报, 2023, 44(5): 20220740. |
| [5] | 刘双红, 夏思玉, 刘世奇, 李旻, 孙嘉杰, 钟永, 张锋, 白锋. 中空全固态Z型异质结光催化剂的研究进展[J]. 高等学校化学学报, 2023, 44(1): 20220512. |
| [6] | 吴玉, 李轩, 杨恒攀, 何传新. 钴单原子的双重限域制备策略及高效CO2电还原性能[J]. 高等学校化学学报, 2022, 43(9): 20220343. |
| [7] | 滕镇远, 张启涛, 苏陈良. 聚合物单原子光催化剂的载流子分离和表面反应机制[J]. 高等学校化学学报, 2022, 43(9): 20220325. |
| [8] | 王新天, 李攀, 曹越, 洪文浩, 耿忠璇, 安志洋, 王昊宇, 王桦, 孙斌, 朱文磊, 周旸. 单原子材料在二氧化碳催化中的技术经济分析与产业化应用前景[J]. 高等学校化学学报, 2022, 43(9): 20220347. |
| [9] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [10] | 林治, 彭志明, 贺韦清, 沈少华. 单原子与团簇光催化: 竞争与协同[J]. 高等学校化学学报, 2022, 43(9): 20220312. |
| [11] | 赵盈喆, 张建玲. 金属-有机框架基材料在二氧化碳光催化转化中的应用[J]. 高等学校化学学报, 2022, 43(7): 20220223. |
| [12] | 王丽君, 李欣, 洪崧, 詹新雨, 王迪, 郝磊端, 孙振宇. 调节氧化镉-炭黑界面高效电催化CO2还原生成CO[J]. 高等学校化学学报, 2022, 43(7): 20220317. |
| [13] | 夏雾, 任颖异, 刘京, 王锋. 壳聚糖包裹CdSe量子点组装体的水相可见光催化CO2还原[J]. 高等学校化学学报, 2022, 43(7): 20220192. |
| [14] | 赵润瑶, 纪桂鹏, 刘志敏. 吡咯氮配位单原子铜催化剂的电催化二氧化碳还原性能[J]. 高等学校化学学报, 2022, 43(7): 20220272. |
| [15] | 邱丽琪, 姚向阳, 何良年. 可见光驱动丰产金属卟啉类配合物催化的二氧化碳选择性还原反应[J]. 高等学校化学学报, 2022, 43(7): 20220064. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||