

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (10): 2216.doi: 10.7503/cjcu20200519
陈相孟, 张雅琪, 梁豪, 陈彬, 欧阳嘉盛, 和晓波, 钱旭, 普晓云, 潘本都, 邱立勤( )
)
收稿日期:2020-08-03
出版日期:2020-10-10
发布日期:2020-09-14
通讯作者:
邱立勤
E-mail:qiuliqin@mail.sysu.edu.cn
基金资助:
CHEN Xiangmeng, ZHANG Yaqi, LIANG Hao, CHEN Bin, OUYANG Jiasheng, HE Xiaobo, QIAN Xu, PU Xiaoyun, PAN Bendu, QIU Liqin( )
)
Received:2020-08-03
Online:2020-10-10
Published:2020-09-14
Contact:
QIU Liqin
E-mail:qiuliqin@mail.sysu.edu.cn
Supported by:摘要:
研究了一种新的铱催化的Morita-Baylis-Hillman(MBH)乙酸酯与3-苯基取代的吲哚酮的烯丙基烷基化反应, 发现铱催化的区域选择明显不同于钯催化的反应, 直接、 高效地合成了一类未见报道的具有新结构的 3,3-二取代的吲哚酮类化合物. 通过对亚磷酰胺、 双膦及单膦等配体、 金属源、 溶剂、 碱以及反应温度的筛选, 获得了适合该反应的催化体系及最优条件: 以[Ir(COD)Cl]2(摩尔分数5%)和亚磷酰胺配体(L6, 摩尔分数10%)为催化剂, CH3CN为溶剂, Cs2CO3为碱, 于?30 ℃反应25 h. 在最优条件下, 对不同类型取代基的底物进行了考察, 发现底物普适性良好, 产率最低为84%, 最高可达98%. 同时还发现, 底物取代基的电性对反应产率影响不大, 一些其它类型的双膦和单膦配体对催化反应也有较好的催化效果.
中图分类号:
TrendMD:
陈相孟, 张雅琪, 梁豪, 陈彬, 欧阳嘉盛, 和晓波, 钱旭, 普晓云, 潘本都, 邱立勤. 铱催化的MBH乙酸酯与吲哚酮类化合物的烯丙基化反应. 高等学校化学学报, 2020, 41(10): 2216.
CHEN Xiangmeng, ZHANG Yaqi, LIANG Hao, CHEN Bin, OUYANG Jiasheng, HE Xiaobo, QIAN Xu, PU Xiaoyun, PAN Bendu, QIU Liqin. Iridium-catalyzed Allylation of Morita-Baylis-Hillman Acetates with Indolinone Compounds†. Chem. J. Chinese Universities, 2020, 41(10): 2216.
| Compound | R1 | R2 | R3 | Yieldb(%) | HRMS[M+H]+ |
|---|---|---|---|---|---|
| 3a | H | H | Ph | 98% | 488.2219 |
| 3b | H | H | 2?NO2Ph | 93% | 533.2057 |
| 3c | H | H | 3,4?Cl2Ph | 91% | 556.1433 |
| 3d | H | H | 3?BrPh | 93% | 566.1335 |
| 3e | H | H | 3?ClPh | 94% | 522.1822 |
| 3f | H | H | 3?FPh | 92% | 506.2127 |
| 3g | H | H | 4?CF3Ph | 92% | 556.2082 |
| 3h | H | H | 4?CNPh | 93% | 513.2169 |
| 3i | H | H | 3?MePh | 96% | 502.2371 |
| 3j | H | H | 2?Thiofuran | 94% | 494.1783 |
| 3k | H | 3,5?(OMe)2 | Ph | 96% | 548.2436 |
| 3l | H | 4?OMe | Ph | 98% | 518.2320 |
| 3m | 5?Cl | H | Ph | 95% | 522.1831 |
| 3n | 5?F | H | Ph | 95% | 506.2131 |
| 3o | 5?OMe | H | Ph | 97% | 518.2330 |
| 3p | 5?Me | H | Ph | 96% | 502.2368 |
| 3q | 6?F | H | Ph | 90% | 506.2114 |
| 3r | 7?F | H | Ph | 84% | 506.2134 |
Table 1 Yields and HRMS data of compounds 3a—3ra
| Compound | R1 | R2 | R3 | Yieldb(%) | HRMS[M+H]+ |
|---|---|---|---|---|---|
| 3a | H | H | Ph | 98% | 488.2219 |
| 3b | H | H | 2?NO2Ph | 93% | 533.2057 |
| 3c | H | H | 3,4?Cl2Ph | 91% | 556.1433 |
| 3d | H | H | 3?BrPh | 93% | 566.1335 |
| 3e | H | H | 3?ClPh | 94% | 522.1822 |
| 3f | H | H | 3?FPh | 92% | 506.2127 |
| 3g | H | H | 4?CF3Ph | 92% | 556.2082 |
| 3h | H | H | 4?CNPh | 93% | 513.2169 |
| 3i | H | H | 3?MePh | 96% | 502.2371 |
| 3j | H | H | 2?Thiofuran | 94% | 494.1783 |
| 3k | H | 3,5?(OMe)2 | Ph | 96% | 548.2436 |
| 3l | H | 4?OMe | Ph | 98% | 518.2320 |
| 3m | 5?Cl | H | Ph | 95% | 522.1831 |
| 3n | 5?F | H | Ph | 95% | 506.2131 |
| 3o | 5?OMe | H | Ph | 97% | 518.2330 |
| 3p | 5?Me | H | Ph | 96% | 502.2368 |
| 3q | 6?F | H | Ph | 90% | 506.2114 |
| 3r | 7?F | H | Ph | 84% | 506.2134 |
| Compound | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(101 MHz, CDCl3), δ |
|---|---|---|
3a | 7.47(m, 3H, PhH), 7.35—7.21(m, 12H, PhH), 7.20—7.08(m, 5H, PhH), 6.96—6.70(m, 2H, PhH, CH), 4.97—4.78(m, 2H, Ph—CH2), 3.89(t, J=7.0 Hz, 2H, CH2), 3.87—3.78(m, 2H, CH2), 1.22—1.06(m, 3H, CH3) | 177.83, 168.67, 143.09, 140.58, 140.02, 135.97, 135.27, 130.20, 129.93, 128.84, 128.70, 128.65, 128.40, 128.37, 128.15, 127.89, 127.44, 127.28, 127.21, 126.94, 126.85, 122.01, 109.09, 60.82, 56.06, 43.90, 34.91, 14.06 |
| 3b | 8.19—8.16(m, 1H, PhH), 7.87(s, 1H, PhH), 7.67—7.63(m, 2H, PhH), 7.31—7.23(m, 8H, PhH, Ph—CH), 7.21—7.13(m, 6H, PhH), 7.00—6.96(m, 1H), 6.71(d, J=8 Hz, 1H, PhH), 4.99—4.66(dd, J=116, 16 Hz, 2H, BnH2), 4.01—3.83(m, 2H), 3.75—3.60(dd, J=48, 12 Hz, 2H), 1.11(t, J=7.1 Hz, 3H) | 177.41, 167.99, 146.91, 143.01, 139.06, 138.82, 135.92, 133.76, 132.44, 130.78, 129.83, 129.39, 128.93, 128.64, 128.59, 128.33, 128.29, 127.40, 127.20, 127.16, 126.92, 126.85, 125.34, 121.98, 109.38, 61.07, 56.29, 43.64, 33.92, 14.01 |
| 3c | 7.49—7.43(m, 2H, PhH), 7.35—7.25(m, 11H, PhH), 7.22—7.13(m, 3H, PhH), 7.01—6.94(m, 2H, PhH), 6.93—6.86(m, 1H, PhH), 6.89—6.73(m, 1H, PhH), 4.91—4.80(q, J=16 Hz, 2H, PhH), 3.97—3.86(m, 2H, CH2), 3.74(s, 2H, CH2),1.15(t, J=7.1 Hz, 3H, CH3) | 177.46, 168.24, 143.05, 139.26, 137.93, 135.92, 135.28, 132.51, 132.08, 131.91, 130.51, 130.28, 129.74, 128.70, 128.45, 128.36, 127.72, 127.58, 127.48, 127.26, 127.20, 126.85, 122.05, 109.24, 61.11, 56.01, 43.89, 34.88, 14.01 |
| 3d | 7.49—7.42(m, 2H, PhH), 7.41—7.35(m, 2H, PhH), 7.34—7.22(m, 8H, PhH, CH), 7.22—7.16(m, 1H, PhH), 7.16—7.10(m, 2H, PhH), 7.07(d, J=8.1 Hz, 1H, PhH), 7.03(dd, J=9.9, 2.8 Hz, 1H), 6.99— 6.93(m, 3H, PhH ), 6.72(d, J=7.8 Hz, 1H, PhH), 4.91—4.79(m, 2H, BnH), 3.97—3.87(m, 2H, CH2), 3.77(s, 2H), 1.15(t, J=7.1 Hz, 3H, CH3) | 177.53, 168.33, 143.04, 139.45, 138.88, 137.38, 135.93, 131.54, 131.50, 130.97, 129.83, 129.76, 128.67, 128.40, 128.26, 127.48, 127.39, 127.21, 127.03, 126.83, 122.44, 122.06, 109.20, 61.01, 56.01, 43.91, 34.86, 14.02 |
| 3e | 7.47—7.44(m, 2H, PhH), 7,38(s, 1H, PhH), 7.30—7.13(m, 10H, PhH), 6.99—6.95(m, 5H, PhH), 6.93(d, J=1.9 Hz, 1H, PhH), 4.91—4.79(m, 2H, BnH2), 4.01—3.84(m, 2H, CH2), 3.84—3.74(m, 2H, CH2), 1.15(t, J=7.1 Hz, 3H, CH3) | 177.58, 168.38, 143.07, 139.50, 139.01, 137.11, 135.95, 134.25, 131.48, 129.81, 129.59, 128.68, 128.42, 128.27, 128.09, 127.50, 127.40, 127.22, 126.85, 126.63, 122.07, 109.20, 61.02, 56.03, 43.90, 34.87, 14.02 |
| 3f | 7.46—7.40(m, 3H, PhH), 7.30—7.25(m, 9H, PhH, CH), 7.22—7.13(m, 3H), 6.98—6.94(m, 2H, PhH), 6.88(d, J=7.7 Hz, 1H, PhH), 6.72—6.69(m, 2H, PhH), 4.85(s, 2H, BnH), 3.95—3.90(m, 2H, CH2), 3.79(q, J=13.6 Hz, 2H, CH2), 1.14(t, J=7.1 Hz, 3H, CH3) | 177.58, 168.41, 163.78, 161.33, 143.08, 139.61, 139.16, 137.48, 137.40, 135.91, 131.35, 129.89, 129.83, 129.80, 128.65, 128.41, 128.24, 127.48, 127.37, 127.18, 126.93, 126.85, 124.37, 124.34, 122.03, 115.67, 115.45, 115.08, 114.87, 109.16, 60.98, 56.03, 43.88, 34.84, 14.01 |
| 3g | 7.49—7.42(m, 5H, PhH), 7.32—7.26(m, 9H, PhH, CH), 7.19—7.10(m, 5H, PhH), 6.97—6.93(m, 1H, PhH), 6.74—6.72(m, 1H, PhH), 4.86(dd, J=42.4, 15.8 Hz, 2H, BnH), 3.97—3.86(m, 2H, CH2), 3.77(s, 2H, CH2), 1.15(t, J=7.1 Hz, 3H, CH3) | 177.53, 168.32, 143.01, 139.35, 138.93, 138.86, 135.89, 132.12, 129.77, 128.82, 128.68, 128.44, 128.33, 127.58, 127.44, 127.25, 127.17, 126.83, 125.28, 125.25, 122.13, 109.21, 77.38, 77.06, 76.74, 61.10, 55.99, 43.89, 34.84, 14.00 |
| 3h | 7.52—7.50(m, 2H, PhH), 7.43—7.39(m, 3H, PhH), 7.32—7.27(m, 8H, PhH), 7.19—7.16(m, 3H, PhH), 7.12—7.02(m, 3H, PhH), 6.99—6.96(m, 1H, PhH), 6.75—6.73(m, 1H), 4.85(dd, J=52, 16 Hz, 2H, BnH), 3.92—3.88(m, 2H, CH2), 3.9—3.71(m, 2H, CH2), 1.14(t, J=7.1 Hz, 3H, CH3) | 177.35, 168.13, 143.06, 140.01, 139.25, 138.28, 135.88, 132.78, 132.04, 131.67, 129.63, 129.20, 128.77, 128.69, 128.61, 128.50, 128.43, 127.64, 127.51, 127.25, 127.08, 126.91, 126.75, 122.13, 118.65, 111.49, 109.23, 61.19, 56.02, 43.86, 34.74, 13.99 |
| 3i | 7.49—7.47(m, 3H, PhH), 7.31—7.26(m, 8H, PhH, CH), 7.17—7.16(m, 3H, PhH), 7.09—6.94(m, 2H), 6.83(s, 1H, PhH), 6.73—6.71(m, 1H, PhH), 4.87(q, J=15.8 Hz, 2H, BnH), 3.93—3.86(m, 2H, CH2), 3.83(s, 2H, CH2), 2.23(s, 3H, CH3), 1.13(t, J=7.1 Hz, 3H, CH3) | 177.99, 168.65, 143.14, 140.77, 140.10, 137.89, 136.02, 135.19, 130.06, 129.98, 129.49, 128.94, 128.65, 128.36, 128.25, 128.08, 127.43, 127.30, 127.26, 127.23, 127.21, 127.00, 125.92, 121.98, 109.07, 60.76, 55.95, 43.93, 35.31, 21.32, 14.06 |
| Compound | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(101 MHz, CDCl3), δ |
3j | 7.60—7.59(m, 3H, PhH), 7.35—7.26(m, 11H, PhH), 7.16—7.12(m, 1H, PhH), 6.99—6.91(m, 2H),, 6.71(d, J=7.7 Hz, 1H, CH), 4.97—4.81(m, 2H, BnH), 3.92(s, 2H, CH2), 3.90—3.79(m, 2H, CH2), 1.09(t, J=7.1 Hz, 3H, CH3) | 178.08, 168.44, 143.14, 140.08, 137.95, 136.00, 133.37, 132.20, 129.81, 128.71, 128.60, 128.38, 128.22, 127.43, 127.40, 127.34, 127.11, 126.97, 126.43, 121.84, 109.08, 77.38, 77.06, 76.74, 60.72, 55.88, 44.01, 36.70, 14.05 |
| 3k | 7.47(s, 1H, PhH), 7.32—7.28(m, 8H, PhH), 7.19—7.09(m, 4H, PhH), 7.04—6.97(m, 2H), 6.95—6.88(m, 4H, PhH), 6.71—6.69(m, 1H), 4.93—4.80(m, 2H, CH2), 3.91—3.83(m, 2H, PhH), 3.84(d, J=8.3 Hz, 2H), 2.25(s, 6H, CH3), 1.14(t, J=7.1 Hz, 3H, CH3) | 177.97, 168.72, 143.02, 140.44, 140.28, 137.80, 136.11, 135.36, 130.58, 130.28, 128.98, 128.91, 128.60, 128.34, 128.13, 127.94, 127.42, 127.31, 126.73, 124.81, 121.99, 108.92, 60.77, 56.14, 43.88, 34.47, 21.51, 14.05 |
| 3l | 7.47(s, 1H, PhH), 7.37—7.26(m, 10H), 7.15—7.10 (m, 4H, PhH), 6.96—6.92(m, 1H, PhH), 6.81—6.79 (m, 2H, PhH), 6.69(d, J=7.7 Hz, 1H, PhH), 4.92—4.78(m, 2H, CH2), 3.91—3.88(q, J=4 Hz, 2H, CH2), 3.79(s, 3H, CH3), 3.78(s, 2H, CH2), 1.13(t, J=7.1 Hz, 3H, CH3) | 178.07, 168.69, 158.72, 143.04, 140.51, 136.00, 135.33, 131.85, 130.25, 130.11, 128.81, 128.63, 128.39, 128.33, 128.08, 127.40, 127.17, 126.86, 121.95, 113.69, 109.07, 60.80, 55.37, 55.25, 43.84, 35.05, 14.03 |
| 3m | 7.52(s, 1H, PhH), 7.39—7.28(m, 12H), 7.24—7.12(m, 3H, PhH), 7.00(s, 1H, PhH), 6.59—6.57(d, J=8 Hz, 1H), 4.81(dd, J=48, 16 Hz, 2H, CH2), 4.07—3.96(m, 2H, CH2), 3.86(dd, J=44, 12 Hz, 2H, CH2), 1.23(t, J=7.1 Hz, 3H, CH3) | 177.24, 168.53, 141.66, 141.19, 139.49, 135.49, 135.13, 131.97, 129.64, 128.74, 128.68, 128.60, 128.49, 128.31, 128.19, 127.63, 127.54, 127.43, 127.17, 126.98, 110.00, 61.06, 56.56, 44.01, 34.30, 14.11 |
| 3n | 7.52(s, 1H, PhH), 7.40—7.42(m, 2H, PhH), 7.32—7.25(m, 12H, PhH), 7.15—7.12(m, 2H, PhH), 6.87—6.77(m, 2H, PhH), 6.64—6.54(m, 1H, PhH), 4.83(dd, J=36, 16 Hz, 2H, BnH), 4.06—3.95(m, 2H, CH2), 3.87(dd, J=40, 12 Hz, 2H, CH2), 1.21(t, J=7.1 Hz, 3H, CH3) | 177.46, 168.52, 159.84, 157.45, 141.03, 139.61, 139.03, 135.65, 135.17, 131.88, 131.80, 129.75, 128.77, 128.72, 128.70, 128.66, 128.56, 128.46, 128.44, 128.42, 128.40, 128.27, 127.58, 127.51, 127.19, 127.15, 127.01, 114.79, 114.65, 114.54, 114.42, 109.54, 109.46, 60.99, 56.67, 44.07, 34.49, 14.09 |
| 3o | 7.49(s, 1H, PhH), 7.47—7.49(m, 2H, PhH), 7.31—7.25(m, 12H, PhH, CH), 7.13—7.11(m, 2H, PhH), 6.73—6.67(m, 2H), 6.58(d, J=6 Hz, 1H), 4.83(q, J=12 Hz, 2H, PhH), 4.97—3.94(m, 2H, CH2), 3.86(q, J=12 Hz, 2H, CH2), 3.69(s, 3H, CH3), 1.17(t, J=7.1 Hz, 3H, CH3) | 177.50, 168.73, 155.36, 140.58, 139.98, 136.59, 136.08, 135.31, 131.29, 130.21, 128.77, 128.64, 128.42, 128.37, 128.10, 127.43, 127.30, 127.23, 113.89, 112.94, 109.42, 60.87, 56.62, 55.61, 43.99, 34.73, 14.06 |
| 3p | 7.47—7.42(m, 3H, PhH) 7.29—7.26(m, 11H, PhH), 6.95(t, J=10.0 Hz, 1H), 7.12—7.10(m, 2H, PhH), 6.86—6.94(m, 2H, PhH), 6.57(d, J=8 Hz, 1H, PhH), 4.83(dd, J=36, 16 Hz, 2H, CH | 177.63, 168.75, 140.68, 140.57, 140.20, 136.10, 135.39, 131.33, 130.20, 130.15, 128.78, 128.61, 128.48, 128.38, 128.33, 128.03, 127.49, 127.38, 127.21, 108.82, 60.82, 56.34, 43.90, 34.54, 21.18, 14.07 |
| 3q | 7.50(s, 1H, PhH), 7.43—7.40(m, 2H, PhH), 7.33—7.26(m, 11H, PhH), 7.14—7.13(d, J=4 Mz, 2H, PhH), 7.04—7.00(m, 1H, PhH), 6.66—6.60(m, 1H, PhH), 6.44—6.41(m, 1H, PhH), 4.89—4.73(m, 2H, CH2), 3.98—3.78(m, 4H, CH2, CH2), 1.17(t, J=7.1 Hz, 3H, CH3) | 178.11, 168.56, 164.06, 161.62, 144.67, 144.56, 140.72, 139.92, 135.42, 135.19, 129.97, 128.77, 128.50, 128.43, 128.26, 127.67, 127.44, 127.20, 127.06, 108.33, 108.11, 97.81, 97.54, 60.93, 55.76, 44.09, 34.81, 14.08 |
| 3r | 7.51(s, 1H, PhH), 7.43—7.26(m, 14H), 7.14—7.13(d, J=4 Mz, 2H), 6.63—6.61(m, 1H), 6.41—6.44(m, 1H), 4.82(dd, J=28, 16 Mz, 2H, CH2), 3.98—3.78(m, 4H, CH2, CH2), 1.17(t, J=7.1 Hz, CH3) | 177.55, 168.50, 148.55, 146.12, 140.90, 139.72, 137.28, 135.20, 133.10, 133.08, 129.78, 128.82, 128.49, 128.42, 128.25, 127.47, 127.34, 127.06, 122.79, 122.76, 122.55, 122.49, 116.27, 116.08, 60.89, 56.38, 45.44, 34.76, 14.07 |
Table 2 1H NMR and 13C NMR data of compounds 3a—3r
| Compound | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(101 MHz, CDCl3), δ |
|---|---|---|
3a | 7.47(m, 3H, PhH), 7.35—7.21(m, 12H, PhH), 7.20—7.08(m, 5H, PhH), 6.96—6.70(m, 2H, PhH, CH), 4.97—4.78(m, 2H, Ph—CH2), 3.89(t, J=7.0 Hz, 2H, CH2), 3.87—3.78(m, 2H, CH2), 1.22—1.06(m, 3H, CH3) | 177.83, 168.67, 143.09, 140.58, 140.02, 135.97, 135.27, 130.20, 129.93, 128.84, 128.70, 128.65, 128.40, 128.37, 128.15, 127.89, 127.44, 127.28, 127.21, 126.94, 126.85, 122.01, 109.09, 60.82, 56.06, 43.90, 34.91, 14.06 |
| 3b | 8.19—8.16(m, 1H, PhH), 7.87(s, 1H, PhH), 7.67—7.63(m, 2H, PhH), 7.31—7.23(m, 8H, PhH, Ph—CH), 7.21—7.13(m, 6H, PhH), 7.00—6.96(m, 1H), 6.71(d, J=8 Hz, 1H, PhH), 4.99—4.66(dd, J=116, 16 Hz, 2H, BnH2), 4.01—3.83(m, 2H), 3.75—3.60(dd, J=48, 12 Hz, 2H), 1.11(t, J=7.1 Hz, 3H) | 177.41, 167.99, 146.91, 143.01, 139.06, 138.82, 135.92, 133.76, 132.44, 130.78, 129.83, 129.39, 128.93, 128.64, 128.59, 128.33, 128.29, 127.40, 127.20, 127.16, 126.92, 126.85, 125.34, 121.98, 109.38, 61.07, 56.29, 43.64, 33.92, 14.01 |
| 3c | 7.49—7.43(m, 2H, PhH), 7.35—7.25(m, 11H, PhH), 7.22—7.13(m, 3H, PhH), 7.01—6.94(m, 2H, PhH), 6.93—6.86(m, 1H, PhH), 6.89—6.73(m, 1H, PhH), 4.91—4.80(q, J=16 Hz, 2H, PhH), 3.97—3.86(m, 2H, CH2), 3.74(s, 2H, CH2),1.15(t, J=7.1 Hz, 3H, CH3) | 177.46, 168.24, 143.05, 139.26, 137.93, 135.92, 135.28, 132.51, 132.08, 131.91, 130.51, 130.28, 129.74, 128.70, 128.45, 128.36, 127.72, 127.58, 127.48, 127.26, 127.20, 126.85, 122.05, 109.24, 61.11, 56.01, 43.89, 34.88, 14.01 |
| 3d | 7.49—7.42(m, 2H, PhH), 7.41—7.35(m, 2H, PhH), 7.34—7.22(m, 8H, PhH, CH), 7.22—7.16(m, 1H, PhH), 7.16—7.10(m, 2H, PhH), 7.07(d, J=8.1 Hz, 1H, PhH), 7.03(dd, J=9.9, 2.8 Hz, 1H), 6.99— 6.93(m, 3H, PhH ), 6.72(d, J=7.8 Hz, 1H, PhH), 4.91—4.79(m, 2H, BnH), 3.97—3.87(m, 2H, CH2), 3.77(s, 2H), 1.15(t, J=7.1 Hz, 3H, CH3) | 177.53, 168.33, 143.04, 139.45, 138.88, 137.38, 135.93, 131.54, 131.50, 130.97, 129.83, 129.76, 128.67, 128.40, 128.26, 127.48, 127.39, 127.21, 127.03, 126.83, 122.44, 122.06, 109.20, 61.01, 56.01, 43.91, 34.86, 14.02 |
| 3e | 7.47—7.44(m, 2H, PhH), 7,38(s, 1H, PhH), 7.30—7.13(m, 10H, PhH), 6.99—6.95(m, 5H, PhH), 6.93(d, J=1.9 Hz, 1H, PhH), 4.91—4.79(m, 2H, BnH2), 4.01—3.84(m, 2H, CH2), 3.84—3.74(m, 2H, CH2), 1.15(t, J=7.1 Hz, 3H, CH3) | 177.58, 168.38, 143.07, 139.50, 139.01, 137.11, 135.95, 134.25, 131.48, 129.81, 129.59, 128.68, 128.42, 128.27, 128.09, 127.50, 127.40, 127.22, 126.85, 126.63, 122.07, 109.20, 61.02, 56.03, 43.90, 34.87, 14.02 |
| 3f | 7.46—7.40(m, 3H, PhH), 7.30—7.25(m, 9H, PhH, CH), 7.22—7.13(m, 3H), 6.98—6.94(m, 2H, PhH), 6.88(d, J=7.7 Hz, 1H, PhH), 6.72—6.69(m, 2H, PhH), 4.85(s, 2H, BnH), 3.95—3.90(m, 2H, CH2), 3.79(q, J=13.6 Hz, 2H, CH2), 1.14(t, J=7.1 Hz, 3H, CH3) | 177.58, 168.41, 163.78, 161.33, 143.08, 139.61, 139.16, 137.48, 137.40, 135.91, 131.35, 129.89, 129.83, 129.80, 128.65, 128.41, 128.24, 127.48, 127.37, 127.18, 126.93, 126.85, 124.37, 124.34, 122.03, 115.67, 115.45, 115.08, 114.87, 109.16, 60.98, 56.03, 43.88, 34.84, 14.01 |
| 3g | 7.49—7.42(m, 5H, PhH), 7.32—7.26(m, 9H, PhH, CH), 7.19—7.10(m, 5H, PhH), 6.97—6.93(m, 1H, PhH), 6.74—6.72(m, 1H, PhH), 4.86(dd, J=42.4, 15.8 Hz, 2H, BnH), 3.97—3.86(m, 2H, CH2), 3.77(s, 2H, CH2), 1.15(t, J=7.1 Hz, 3H, CH3) | 177.53, 168.32, 143.01, 139.35, 138.93, 138.86, 135.89, 132.12, 129.77, 128.82, 128.68, 128.44, 128.33, 127.58, 127.44, 127.25, 127.17, 126.83, 125.28, 125.25, 122.13, 109.21, 77.38, 77.06, 76.74, 61.10, 55.99, 43.89, 34.84, 14.00 |
| 3h | 7.52—7.50(m, 2H, PhH), 7.43—7.39(m, 3H, PhH), 7.32—7.27(m, 8H, PhH), 7.19—7.16(m, 3H, PhH), 7.12—7.02(m, 3H, PhH), 6.99—6.96(m, 1H, PhH), 6.75—6.73(m, 1H), 4.85(dd, J=52, 16 Hz, 2H, BnH), 3.92—3.88(m, 2H, CH2), 3.9—3.71(m, 2H, CH2), 1.14(t, J=7.1 Hz, 3H, CH3) | 177.35, 168.13, 143.06, 140.01, 139.25, 138.28, 135.88, 132.78, 132.04, 131.67, 129.63, 129.20, 128.77, 128.69, 128.61, 128.50, 128.43, 127.64, 127.51, 127.25, 127.08, 126.91, 126.75, 122.13, 118.65, 111.49, 109.23, 61.19, 56.02, 43.86, 34.74, 13.99 |
| 3i | 7.49—7.47(m, 3H, PhH), 7.31—7.26(m, 8H, PhH, CH), 7.17—7.16(m, 3H, PhH), 7.09—6.94(m, 2H), 6.83(s, 1H, PhH), 6.73—6.71(m, 1H, PhH), 4.87(q, J=15.8 Hz, 2H, BnH), 3.93—3.86(m, 2H, CH2), 3.83(s, 2H, CH2), 2.23(s, 3H, CH3), 1.13(t, J=7.1 Hz, 3H, CH3) | 177.99, 168.65, 143.14, 140.77, 140.10, 137.89, 136.02, 135.19, 130.06, 129.98, 129.49, 128.94, 128.65, 128.36, 128.25, 128.08, 127.43, 127.30, 127.26, 127.23, 127.21, 127.00, 125.92, 121.98, 109.07, 60.76, 55.95, 43.93, 35.31, 21.32, 14.06 |
| Compound | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(101 MHz, CDCl3), δ |
3j | 7.60—7.59(m, 3H, PhH), 7.35—7.26(m, 11H, PhH), 7.16—7.12(m, 1H, PhH), 6.99—6.91(m, 2H),, 6.71(d, J=7.7 Hz, 1H, CH), 4.97—4.81(m, 2H, BnH), 3.92(s, 2H, CH2), 3.90—3.79(m, 2H, CH2), 1.09(t, J=7.1 Hz, 3H, CH3) | 178.08, 168.44, 143.14, 140.08, 137.95, 136.00, 133.37, 132.20, 129.81, 128.71, 128.60, 128.38, 128.22, 127.43, 127.40, 127.34, 127.11, 126.97, 126.43, 121.84, 109.08, 77.38, 77.06, 76.74, 60.72, 55.88, 44.01, 36.70, 14.05 |
| 3k | 7.47(s, 1H, PhH), 7.32—7.28(m, 8H, PhH), 7.19—7.09(m, 4H, PhH), 7.04—6.97(m, 2H), 6.95—6.88(m, 4H, PhH), 6.71—6.69(m, 1H), 4.93—4.80(m, 2H, CH2), 3.91—3.83(m, 2H, PhH), 3.84(d, J=8.3 Hz, 2H), 2.25(s, 6H, CH3), 1.14(t, J=7.1 Hz, 3H, CH3) | 177.97, 168.72, 143.02, 140.44, 140.28, 137.80, 136.11, 135.36, 130.58, 130.28, 128.98, 128.91, 128.60, 128.34, 128.13, 127.94, 127.42, 127.31, 126.73, 124.81, 121.99, 108.92, 60.77, 56.14, 43.88, 34.47, 21.51, 14.05 |
| 3l | 7.47(s, 1H, PhH), 7.37—7.26(m, 10H), 7.15—7.10 (m, 4H, PhH), 6.96—6.92(m, 1H, PhH), 6.81—6.79 (m, 2H, PhH), 6.69(d, J=7.7 Hz, 1H, PhH), 4.92—4.78(m, 2H, CH2), 3.91—3.88(q, J=4 Hz, 2H, CH2), 3.79(s, 3H, CH3), 3.78(s, 2H, CH2), 1.13(t, J=7.1 Hz, 3H, CH3) | 178.07, 168.69, 158.72, 143.04, 140.51, 136.00, 135.33, 131.85, 130.25, 130.11, 128.81, 128.63, 128.39, 128.33, 128.08, 127.40, 127.17, 126.86, 121.95, 113.69, 109.07, 60.80, 55.37, 55.25, 43.84, 35.05, 14.03 |
| 3m | 7.52(s, 1H, PhH), 7.39—7.28(m, 12H), 7.24—7.12(m, 3H, PhH), 7.00(s, 1H, PhH), 6.59—6.57(d, J=8 Hz, 1H), 4.81(dd, J=48, 16 Hz, 2H, CH2), 4.07—3.96(m, 2H, CH2), 3.86(dd, J=44, 12 Hz, 2H, CH2), 1.23(t, J=7.1 Hz, 3H, CH3) | 177.24, 168.53, 141.66, 141.19, 139.49, 135.49, 135.13, 131.97, 129.64, 128.74, 128.68, 128.60, 128.49, 128.31, 128.19, 127.63, 127.54, 127.43, 127.17, 126.98, 110.00, 61.06, 56.56, 44.01, 34.30, 14.11 |
| 3n | 7.52(s, 1H, PhH), 7.40—7.42(m, 2H, PhH), 7.32—7.25(m, 12H, PhH), 7.15—7.12(m, 2H, PhH), 6.87—6.77(m, 2H, PhH), 6.64—6.54(m, 1H, PhH), 4.83(dd, J=36, 16 Hz, 2H, BnH), 4.06—3.95(m, 2H, CH2), 3.87(dd, J=40, 12 Hz, 2H, CH2), 1.21(t, J=7.1 Hz, 3H, CH3) | 177.46, 168.52, 159.84, 157.45, 141.03, 139.61, 139.03, 135.65, 135.17, 131.88, 131.80, 129.75, 128.77, 128.72, 128.70, 128.66, 128.56, 128.46, 128.44, 128.42, 128.40, 128.27, 127.58, 127.51, 127.19, 127.15, 127.01, 114.79, 114.65, 114.54, 114.42, 109.54, 109.46, 60.99, 56.67, 44.07, 34.49, 14.09 |
| 3o | 7.49(s, 1H, PhH), 7.47—7.49(m, 2H, PhH), 7.31—7.25(m, 12H, PhH, CH), 7.13—7.11(m, 2H, PhH), 6.73—6.67(m, 2H), 6.58(d, J=6 Hz, 1H), 4.83(q, J=12 Hz, 2H, PhH), 4.97—3.94(m, 2H, CH2), 3.86(q, J=12 Hz, 2H, CH2), 3.69(s, 3H, CH3), 1.17(t, J=7.1 Hz, 3H, CH3) | 177.50, 168.73, 155.36, 140.58, 139.98, 136.59, 136.08, 135.31, 131.29, 130.21, 128.77, 128.64, 128.42, 128.37, 128.10, 127.43, 127.30, 127.23, 113.89, 112.94, 109.42, 60.87, 56.62, 55.61, 43.99, 34.73, 14.06 |
| 3p | 7.47—7.42(m, 3H, PhH) 7.29—7.26(m, 11H, PhH), 6.95(t, J=10.0 Hz, 1H), 7.12—7.10(m, 2H, PhH), 6.86—6.94(m, 2H, PhH), 6.57(d, J=8 Hz, 1H, PhH), 4.83(dd, J=36, 16 Hz, 2H, CH | 177.63, 168.75, 140.68, 140.57, 140.20, 136.10, 135.39, 131.33, 130.20, 130.15, 128.78, 128.61, 128.48, 128.38, 128.33, 128.03, 127.49, 127.38, 127.21, 108.82, 60.82, 56.34, 43.90, 34.54, 21.18, 14.07 |
| 3q | 7.50(s, 1H, PhH), 7.43—7.40(m, 2H, PhH), 7.33—7.26(m, 11H, PhH), 7.14—7.13(d, J=4 Mz, 2H, PhH), 7.04—7.00(m, 1H, PhH), 6.66—6.60(m, 1H, PhH), 6.44—6.41(m, 1H, PhH), 4.89—4.73(m, 2H, CH2), 3.98—3.78(m, 4H, CH2, CH2), 1.17(t, J=7.1 Hz, 3H, CH3) | 178.11, 168.56, 164.06, 161.62, 144.67, 144.56, 140.72, 139.92, 135.42, 135.19, 129.97, 128.77, 128.50, 128.43, 128.26, 127.67, 127.44, 127.20, 127.06, 108.33, 108.11, 97.81, 97.54, 60.93, 55.76, 44.09, 34.81, 14.08 |
| 3r | 7.51(s, 1H, PhH), 7.43—7.26(m, 14H), 7.14—7.13(d, J=4 Mz, 2H), 6.63—6.61(m, 1H), 6.41—6.44(m, 1H), 4.82(dd, J=28, 16 Mz, 2H, CH2), 3.98—3.78(m, 4H, CH2, CH2), 1.17(t, J=7.1 Hz, CH3) | 177.55, 168.50, 148.55, 146.12, 140.90, 139.72, 137.28, 135.20, 133.10, 133.08, 129.78, 128.82, 128.49, 128.42, 128.25, 127.47, 127.34, 127.06, 122.79, 122.76, 122.55, 122.49, 116.27, 116.08, 60.89, 56.38, 45.44, 34.76, 14.07 |
| Entry | Ligand | Yieldb(%) | Entry | Ligand | Yieldb(%) | |
|---|---|---|---|---|---|---|
| 1 | 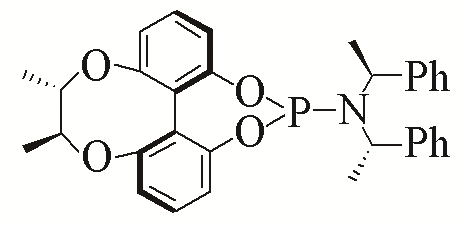 | 94% | 10 | 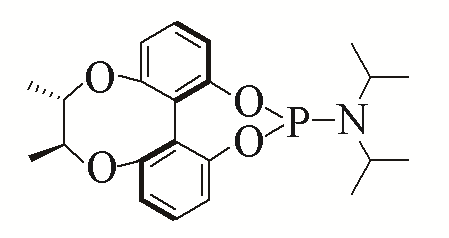 | 96% | |
| L1 | L10 | |||||
| 2 | 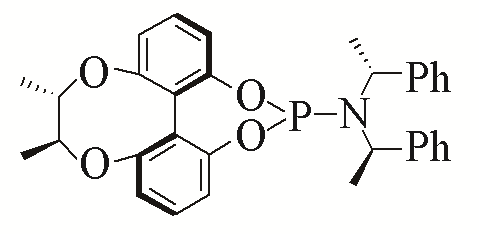 | 93% | 11 | 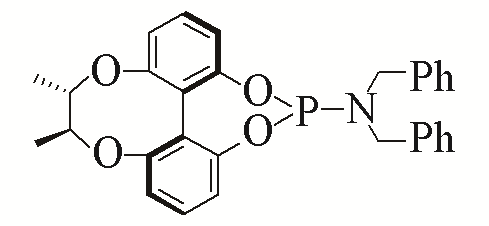 | 97% | |
| L2 | L11 | |||||
| 3 | 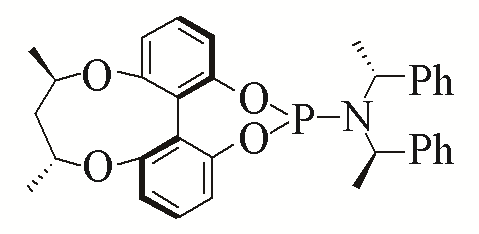 | 95% | 12 | 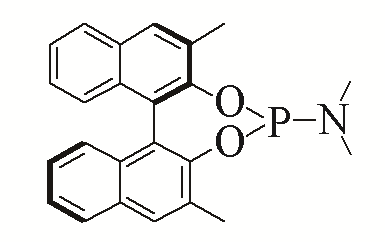 | 95% | |
| L3 | L12 | |||||
| 4 | 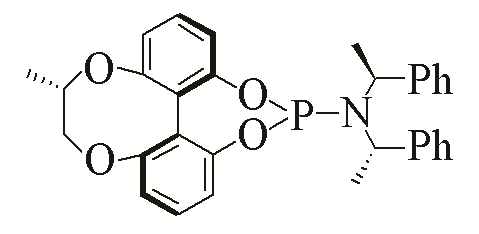 | 96% | 13 | 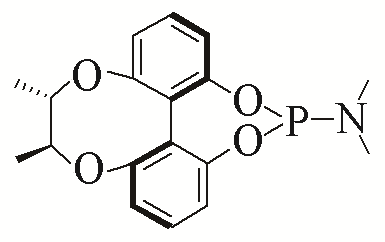 | 95% | |
| L4 | L13 | |||||
| 5 | 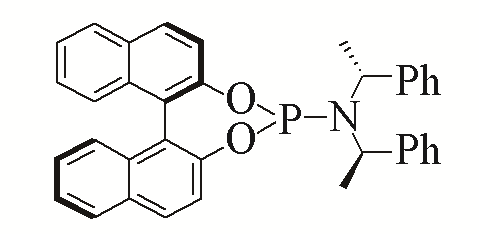 | 95% | 14 | 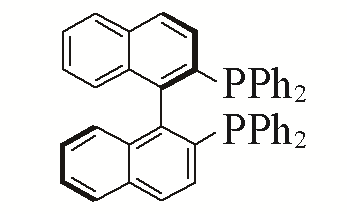 | 97% | |
| L5 | L14 | |||||
| 6 | 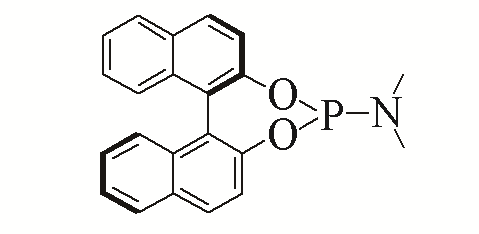 | 98% | 15 | 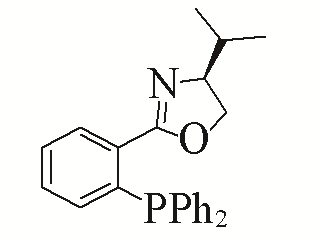 | 93% | |
| L6 | L15 | |||||
| 7 | 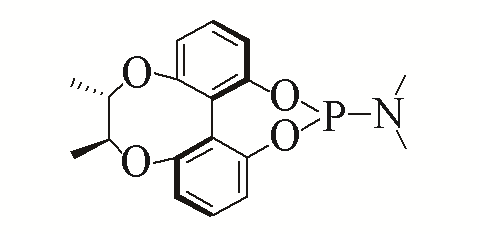 | 92% | 16 | 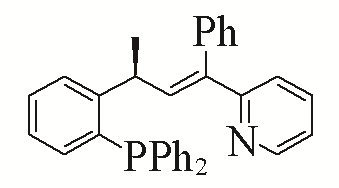 | 91% | |
| L7 | L16 | |||||
| 8 | 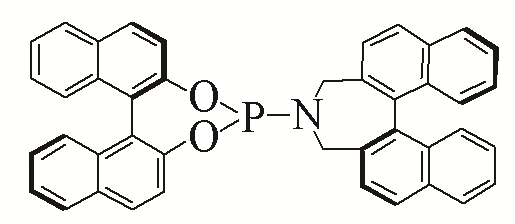 | 90% | 17 | 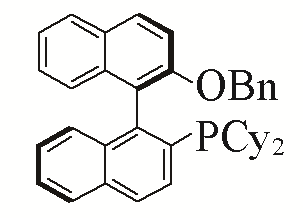 | 93% | |
| L8 | L17 | |||||
| 9 | 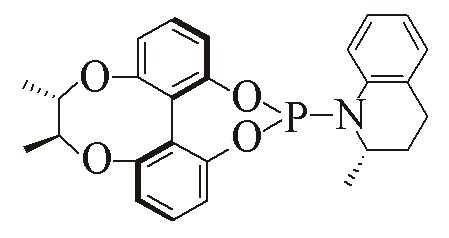 | 94% | ||||
| L9 | ||||||
Table 5 Influence of ligand on the yield of the reactiona
| Entry | Ligand | Yieldb(%) | Entry | Ligand | Yieldb(%) | |
|---|---|---|---|---|---|---|
| 1 |  | 94% | 10 |  | 96% | |
| L1 | L10 | |||||
| 2 |  | 93% | 11 |  | 97% | |
| L2 | L11 | |||||
| 3 |  | 95% | 12 |  | 95% | |
| L3 | L12 | |||||
| 4 |  | 96% | 13 |  | 95% | |
| L4 | L13 | |||||
| 5 |  | 95% | 14 |  | 97% | |
| L5 | L14 | |||||
| 6 |  | 98% | 15 |  | 93% | |
| L6 | L15 | |||||
| 7 |  | 92% | 16 |  | 91% | |
| L7 | L16 | |||||
| 8 |  | 90% | 17 |  | 93% | |
| L8 | L17 | |||||
| 9 |  | 94% | ||||
| L9 | ||||||
| 1 | Cui H., Feng X., Peng J., Lei J., Jiang K., Chen Y. H., Angew. Chem. Int. Ed., 2009, 48(31), 5737—5740 |
| 2 | Sun W. S., Ma X. Z., Hong L., Wang R., J. Org. Chem., 2011, 76(19), 7826—7833 |
| 3 | Li Y., Liang. F., Li Q., Xu Y. H., Wang Q., Jiang L., Org. Lett., 2011, 13(22), 6082—6085 |
| 4 | Ramachandran P. V., Madhi S., Bland⁃Berry L., Reddy M. R., J. O’Donnell M., J. Am. Chem. Soc., 2005, 127(39), 13450—13451 |
| 5 | Zhang S. J., Cui H., Jiang K., Li R., Ding Z. Y., Chen Y. C., Eur. J. Org. Chem., 2009, (33),5804—5809 |
| 6 | Cui H. L, Peng J., Feng X., Du W., Jiang K., Chen Y. C., Eur. J. Org. Chem., 2009, (15), 1574—1577 |
| 7 | Jiang K., Peng J., Cui H. L, Chen Y. C., Chem. Commun., 2009, (26),3955—3957 |
| 8 | Yang W., Wei X. L., Pan Y. H., Lee R., Zhu B., Liu. H. J., Yan L., Huang K. W., Jiang Z. Y., Tan C. H., Chem. Eur. J.,2011, 17(29), 8066—8070 |
| 9 | Furukawa T., Kawazoe J., Zhang W., Nishimine T., Tokunaga E., Matsumoto T., Shiro M., Shibata N., Angew. Chem. Int. Ed., 2011, 50(41), 9684—9688 |
| 10 | Companyó X., Valero G., Ceban V., Calvet T., Mercé F. B., Moyano A., Rios R., Org. Biomol. Chem., 2011, 9(23), 7986—7989 |
| 11 | Cui H. L., Huang J. R., Lei J., Wang Z. F., Chen S., Wu L., Chen Y. C., Org. Lett., 2010, 12(1), 4—7 |
| 12 | Peng J., Huang X., Cui H. L., Chen Y. C., Org. Lett., 2010, 12(9), 1924—1927 |
| 13 | Hong L., Sun W. S., Liu C. X., Zhao D. P., Wang R., Chem. Commun., 2010, 46(16), 2856—2858 |
| 14 | Chen G. Y., Zhong F. R., Lu Y. X., Org. Lett., 2011, 13(22), 6070—6073 |
| 15 | Jiang Y. Q., Shi Y. G., Shi M., J. Am. Chem. Soc., 2008, 130(23), 7202—7203 |
| 16 | Deng H. P., Shi M., Eur. J. Org. Chem., 2012,(1),183—187 |
| 17 | Zhong F. R., Chen G. Y., Han X. Y., Yao W. J., Lu Y. X., Org. Lett.,2012, 14(14), 3764—3767 |
| 18 | Deng H. P., Wei Y., Shi M., Adv. Synth. Catal., 2012, 354(5), 783—789 |
| 19 | Wang Y., Liu L., Zhang T., Zhong N. J., Wang D., Chen Y. J., J. Org. Chem., 2012, 77(8), 4143—4147 |
| 20 | Yamashita Y., Gopalarathnam A., Hartwig J. F., J. Am. Chem. Soc., 2007, 129(24), 7508—7509 |
| 21 | Ueno S., Hartwig J. F., Angew. Chem. Int. Ed., 2008, 47(10), 1928—1931 |
| 22 | Defieber C., Ariger M. A., Moriel P., Carreira E. M., Angew. Chem. Int. Ed., 2007, 46(17), 3139—3143 |
| 23 | Kato M., Nakamura T., Ogata K., Fukuzawa S. I., Eur. J. Org. Chem.,2009,(30),5232—5238 |
| 24 | Ye K. Y., He H., Liu W. B., Dai L. X., Helmchen G., You S. L., J. Am. Chem. Soc.,2011, 133(46), 19006—19014 |
| 25 | Yang X. F., Yu W. H., Ding C. H., Ding Q. P., Wan S. L., Hou X. L., Dai L. X., Wang P. J., J. Org. Chem., 2013, 78(13), 6503—6509 |
| 26 | Zhang X., Yang Z. P., Huang L., You S. L., Angew. Chem. Int. Ed., 2015, 54(6), 1873—1876 |
| 27 | Wang X. M., Meng F. Y., Wang Y., Han Z. B., Chen Y. Z., Liu L., Wang Z., Ding K. L., Angew. Chem. Int. Ed.,2012, 51(37), 9276—9282 |
| 28 | Wang X. M., Guo P. H., Han Z. B., Wang X. B., Wang Z., Ding K. L., J. Am. Chem. Soc.,2014, 136(1), 405—411 |
| 29 | Wang X. B., Wang X. M., Han Z. B., Wang Z., Ding K. L., Angew. Chem. Int. Ed., 2017, 56(4), 1116—1119 |
| 30 | Trost B. M., Xie J., Sieber J. D., J. Am. Chem. Soc.,2011, 133(50), 20611—20622 |
| 31 | He R., Liu P., Huo X., Zhang W. B., Org. Lett., 2017, 19(20), 5513—5516 |
| 32 | Zhuang Y., He Y., Zhou Z., Xia W., Cheng C., Wang M., Chen B., Zhou Z., Pang J., Qiu L., J. Org. Chem., 2015, 80(14), 6968—6975 |
| 33 | Jiang X., Chen X., Li Y., Liang H., Zhang Y., He X., Chen B., Chan W. T. K., Chan A. S. C., Qiu L., Org. Lett., 2019, 21(3), 608—613 |
| [1] | 周永慧, 黄如军, 严健洋, 李亚军, 邱欢欢, 杨进轩, 郑佑轩. 两种基于氮杂环结构铱(Ⅲ)配合物的合成及有机电致发光性能[J]. 高等学校化学学报, 2022, 43(1): 20210415. |
| [2] | 赵可, 洪志, 张立明. 官能团化的手性联芳基单齿膦配体在不对称均相金催化反应中的应用[J]. 高等学校化学学报, 2021, 42(8): 2324. |
| [3] | 付志男, 谈云龙, 肖谷雨, 颜德岳. 含全氟联苯结构的磺化聚二氮杂萘酮醚氧膦质子交换膜的制备与性能[J]. 高等学校化学学报, 2021, 42(8): 2635. |
| [4] | 张慧东, 谷盼盼, 张芳, 都明旭, 叶开其, 刘宇. 窄光谱磷光配合物的设计及电致发光性能[J]. 高等学校化学学报, 2021, 42(12): 3571. |
| [5] | 周春妮, 郑子昂, 彭望明, 王洪波, 张玉敏, 王亮, 肖标. 微波辅助下铑催化二芳基膦酰胺与炔烃的C—H活化/环化反应[J]. 高等学校化学学报, 2020, 41(4): 726. |
| [6] | 陈秋宏, 叶艳春, 任孟然, 王凯民, 唐怀军, 汪正良, 周强. 含有三苯胺-三唑双极性单元的橙红光阳离子型铱(Ⅲ)配合物的合成及在LEDs中的应用[J]. 高等学校化学学报, 2020, 41(12): 2717. |
| [7] | 王晋宇, 柳春丽, 陈延辉. 胺基膦钌卡宾化合物的合成及催化烯烃复分解反应[J]. 高等学校化学学报, 2020, 41(12): 2766. |
| [8] | 张树辛, 冯宇, 范青华. 过渡金属催化的不对称氢化反应的国内研究进展[J]. 高等学校化学学报, 2020, 41(10): 2107. |
| [9] | 苏秋铭, 原安莹, 邝福儿, 陈志强, 白呈超, 辛伟贤. CM⁃Phos配体在钯催化交叉偶联反应中的应用[J]. 高等学校化学学报, 2020, 41(10): 2185. |
| [10] | 李世超,刘梦溪,裘晓辉. Ir(111)表面石墨烯中缺陷的原子结构确认[J]. 高等学校化学学报, 2020, 41(1): 49. |
| [11] | 邹晓川, 王跃, 王存, 胡世文, 石开云. 胺基功能化ZPS-PVPA固载手性MnⅢ(salen)的合成及不对称催化烯烃环氧化[J]. 高等学校化学学报, 2019, 40(7): 1488. |
| [12] | 张利荣, 王志鹏, 刘莹, 徐超, 陈靖, 丁颂东. 二(2-乙基己基)二硫代次膦酸对硝酸溶液中Am3+和Eu3+的萃取[J]. 高等学校化学学报, 2019, 40(1): 1. |
| [13] | 魏良晨, 胡伟康, 周世雄, 束俊, 周会东, 胡旭成, 姜毅, 童碧海, 张千峰. 含亚磷酸酯和联吡啶羧酸酯配体的铱配合物及其聚集诱导发光增强和电致发光性能[J]. 高等学校化学学报, 2018, 39(7): 1371. |
| [14] | 孙日勇, 陈泽宇, 叶艳春, 陈明先, 唐怀军, 王凯民, 汪正良. 六氟磷酸二(2-苯基吡啶)(2,2'-联噻唑)合铱(Ⅲ)的合成及在中性/暖白光二极管中的应用[J]. 高等学校化学学报, 2018, 39(5): 869. |
| [15] | 刘笑宇, 徐议, 唐良富. 3-烷基膦酸酯基取代的异吲哚啉酮衍生物的合成及生物活性[J]. 高等学校化学学报, 2018, 39(11): 2433. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||