

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (2): 253.doi: 10.7503/cjcu20190493
收稿日期:2019-09-16
出版日期:2020-02-10
发布日期:2019-10-29
通讯作者:
李夏
E-mail:xiali@cnu.edu.cn
基金资助:
LIU Dongmei,SU Yajing,LI Shanshan,XU Qiwei,LI Xia( )
)
Received:2019-09-16
Online:2020-02-10
Published:2019-10-29
Contact:
Xia LI
E-mail:xiali@cnu.edu.cn
Supported by:摘要:
采用水热法合成了4个配位聚合物[Zn(Hcpoia)(2,2'-bpy)·H2O]n(1)和[M(Hcpoia)(phen)]n·nH2O[M=Zn(2), Mn(3), Co(4); H3cpoia=4-(4-羧基苯氧基)间苯二甲酸; 2,2'-bpy=2,2'-联吡啶; phen=1,10-邻菲罗啉], 利用X射线单晶衍射分析确定了配合物的晶体结构. 配合物1为一维链状结构, 中心Zn 2+离子的配位环境为[ZnO4N2]扭曲的八面体构型, 配体Hcpoia 2-以μ1∶η 1η 0和μ1∶η 1η 1配位模式桥连相邻的Zn 2+离子. 配合物2和4的结构与配合物1类似, 是由配体Hcpoia 2-以μ1∶η 1η 0和μ1∶η 1η 1配位模式联接[MO4N2]结构单元而形成的一维链状结构. 配合物1, 2和4中均存在分子间氢键(O—H…O), 氢键的存在使一维链连接形成二维超分子结构. 配合物3为二维网状结构, Mn 2+离子的配位环境为[MnO4N2]扭曲的八面体构型, 配体Hcpoia 2-以μ2∶η 1η 1配位模式桥连相邻Mn 2+离子形成[Mn2COO2]结构单元, 该结构单元被Hcpoia 2-连接形成二维结构. 在4个配合物中, 2,2'-bpy和phen配体均以端基的形式与金属离子螯合配位. 研究了水溶液中抗生素分子和Fe 3+离子对配合物1与荧光强度的影响, 实验结果表明, 甲硝唑、 Fe 3+离子对配合物1有荧光猝灭作用, 并进一步考察了甲硝唑浓度和Fe 3+离子浓度对配合物1荧光强度的影响. 基于荧光猝灭机理, 配合物1可以用作荧光传感器检测水溶液中的甲硝唑和Fe 3+离子. 研究了配合物4对罗丹明B(RhB)的催化降解性能, 发现在氙灯照射和H2O2存在条件下, 配合物4对RhB具有较好的光催化降解作用.
中图分类号:
TrendMD:
刘东枚,苏雅静,李姗姗,许奇炜,李夏. 4-(4-羧基苯氧基)间苯二甲酸构筑的过渡金属配位聚合物: 合成、 晶体结构、 荧光传感与光催化. 高等学校化学学报, 2020, 41(2): 253.
LIU Dongmei,SU Yajing,LI Shanshan,XU Qiwei,LI Xia. Transition Metal Coordination Polymers Constructed by 4-(4-Carboxyphenoxy)isophthalic Acid: Synthesis, Crystal Structure, Fluorescence Sensing and Photocatalysis †. Chem. J. Chinese Universities, 2020, 41(2): 253.
| Complex | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C25H18N2O8Zn | C27H18N2O8Zn | C27H18MnN2O8 | C54H36Co2N4O16 |
| Formula weight | 539.78 | 563.80 | 553.37 | 1114.73 |
| Crystal system | Triclinic | Triclinic | Monoclinic | Triclinic |
| Space group | P | P | P21/c | P |
| a/nm | 0.97529(7) | 0.99103(5) | 1.42226(4) | 0.97396(3) |
| b/nm | 1.01915(5) | 1.00828(5) | 1.09657(3) | 1.00174(3) |
| c/nm | 1.34860(6) | 1.40052(6) | 1.53718(5) | 1.38980(4) |
| α/(°) | 69.525(4) | 96.263(2) | 90 | 97.159(2) |
| β/(°) | 89.020(5) | 106.8670(10) | 96.0640(10) | 107.019 |
| γ/(°) | 68.440(6) | 110.7200(10) | 90 | 107.595 |
| Volume/nm3 | 1.1583(12) | 1.2171(10) | 2.3840(12) | 1.2017(7) |
| Z | 2 | 2 | 4 | 1 |
| Dcalcd/(g·cm-3) | 1.548 | 1.538 | 1.542 | 1.540 |
| F(000) | 552.0 | 576.0 | 1132.0 | 570 |
| Crystal size/mm3 | 0.25×0.23×0.21 | 0.21×0.25×0.23 | 0.32×0.25×0.14 | 0.15×0.1×0.05 |
| 2θ range for data collection/(°) | 9.832—133.2 | 4.632—52.744 | 4.572—55.014 | 9.526—133.192 |
| Index range | -11≤h≤11, | -11≤h≤11, | -16≤h≤18, | -6≤h≤11 |
| -12≤k≤11, | -11≤k≤11, | -14≤k≤14, | -12≤k≤11, | |
| -15≤l≤16 | -14≤l≤16 | -19≤l≤19 | -16≤l≤17 | |
| Reflections collected | 9868 | 11800 | 27932 | 13362 |
| Independent reflections | 3999[Rint=0.0914] | 4166[Rint=0.0197] | 5473[Rint=0.0480] | 4700[Rint=0.0619] |
| Data/restraints/parameters | 3999/0/327 | 4166/7/345 | 5473/1/345 | 4700/0/345 |
| Goodness-of-fit on F2 | 1.113 | 1.063 | 1.084 | 1.113 |
| Final R indexes[I≥2σ(I)] | R1=0.0851, | R1=0.0334, | R1=0.0619, | R1=0.0547, |
| wR2=0.2576 | wR2=0.0847 | wR2=0.1583 | wR2=0.1582 | |
| Final R indexes(all data) | R1=0.0952, | R1=0.0386, | R1=0.0963, | R1=0.0560, |
| wR2=0.2929 | wR2=0.0875 | wR2=0.1747 | wR2=0.1593 | |
| CCDC No. | 1943993 | 1943994 | 1943992 | 1943991 |
Table 1 Crystallographic data of complexes 1—4
| Complex | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C25H18N2O8Zn | C27H18N2O8Zn | C27H18MnN2O8 | C54H36Co2N4O16 |
| Formula weight | 539.78 | 563.80 | 553.37 | 1114.73 |
| Crystal system | Triclinic | Triclinic | Monoclinic | Triclinic |
| Space group | P | P | P21/c | P |
| a/nm | 0.97529(7) | 0.99103(5) | 1.42226(4) | 0.97396(3) |
| b/nm | 1.01915(5) | 1.00828(5) | 1.09657(3) | 1.00174(3) |
| c/nm | 1.34860(6) | 1.40052(6) | 1.53718(5) | 1.38980(4) |
| α/(°) | 69.525(4) | 96.263(2) | 90 | 97.159(2) |
| β/(°) | 89.020(5) | 106.8670(10) | 96.0640(10) | 107.019 |
| γ/(°) | 68.440(6) | 110.7200(10) | 90 | 107.595 |
| Volume/nm3 | 1.1583(12) | 1.2171(10) | 2.3840(12) | 1.2017(7) |
| Z | 2 | 2 | 4 | 1 |
| Dcalcd/(g·cm-3) | 1.548 | 1.538 | 1.542 | 1.540 |
| F(000) | 552.0 | 576.0 | 1132.0 | 570 |
| Crystal size/mm3 | 0.25×0.23×0.21 | 0.21×0.25×0.23 | 0.32×0.25×0.14 | 0.15×0.1×0.05 |
| 2θ range for data collection/(°) | 9.832—133.2 | 4.632—52.744 | 4.572—55.014 | 9.526—133.192 |
| Index range | -11≤h≤11, | -11≤h≤11, | -16≤h≤18, | -6≤h≤11 |
| -12≤k≤11, | -11≤k≤11, | -14≤k≤14, | -12≤k≤11, | |
| -15≤l≤16 | -14≤l≤16 | -19≤l≤19 | -16≤l≤17 | |
| Reflections collected | 9868 | 11800 | 27932 | 13362 |
| Independent reflections | 3999[Rint=0.0914] | 4166[Rint=0.0197] | 5473[Rint=0.0480] | 4700[Rint=0.0619] |
| Data/restraints/parameters | 3999/0/327 | 4166/7/345 | 5473/1/345 | 4700/0/345 |
| Goodness-of-fit on F2 | 1.113 | 1.063 | 1.084 | 1.113 |
| Final R indexes[I≥2σ(I)] | R1=0.0851, | R1=0.0334, | R1=0.0619, | R1=0.0547, |
| wR2=0.2576 | wR2=0.0847 | wR2=0.1583 | wR2=0.1582 | |
| Final R indexes(all data) | R1=0.0952, | R1=0.0386, | R1=0.0963, | R1=0.0560, |
| wR2=0.2929 | wR2=0.0875 | wR2=0.1747 | wR2=0.1593 | |
| CCDC No. | 1943993 | 1943994 | 1943992 | 1943991 |
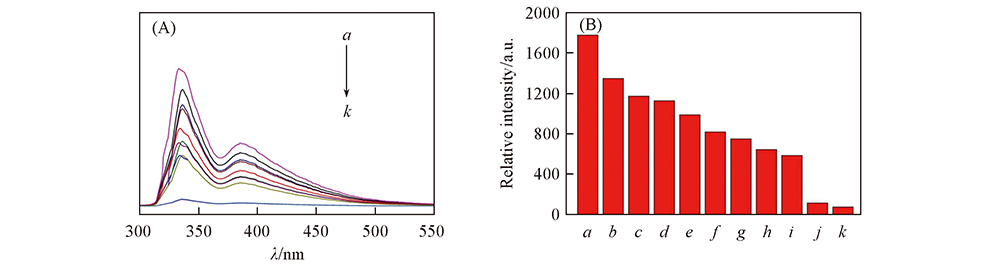
Fig.5 Emission spectra(A) and histogram of fluorescence intensity(B) of complex 1 in aqueous solution containing different antibiotics a. Thiamphenicol; b. H2O; c. amoxicillin; d. sulfathiazole; e. sulfadiazine; f. roxithromycin; g. azithromycin; h. penicillin; i. sulfadimidine; j. ornidazole; k. metronidazole.
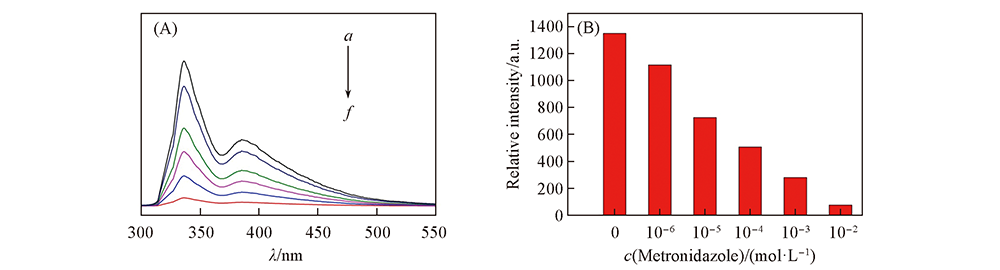
Fig.6 Emission spectra(A) and histogram of fluorescence intensity(B) of complex 1 in different concentrations of metronidazole solution c(Metronidazole)/(mol·L-1): a. 0; b. 10-6; c. 10-5; d. 10-4; e. 10-3; f. 10-2.
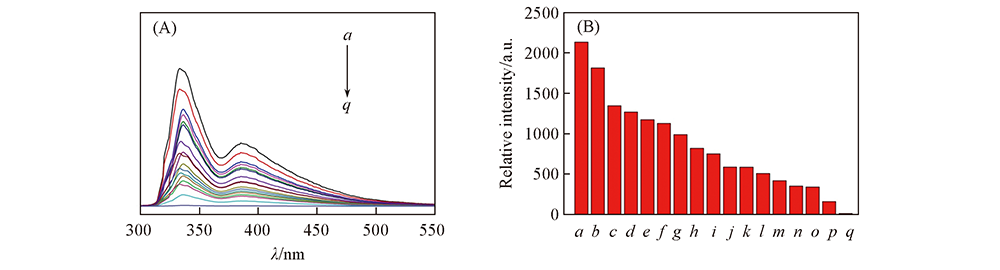
Fig.7 Emission spectra(A) and histogram of fluorescence intensity(B) of complex 1 in solution containing different metal ions a. Pb2+; b. Al3+; c. Cu2+; d. Na+; e. Ba2+; f. Ni2+; g. K+; h. Ag+; i. Mg2+; j. H2O; k. Li2+; l. Cd2+; m. Co2+; n. Ca2+; o. Zn2+; p. Cr3+; q. Fe3+.
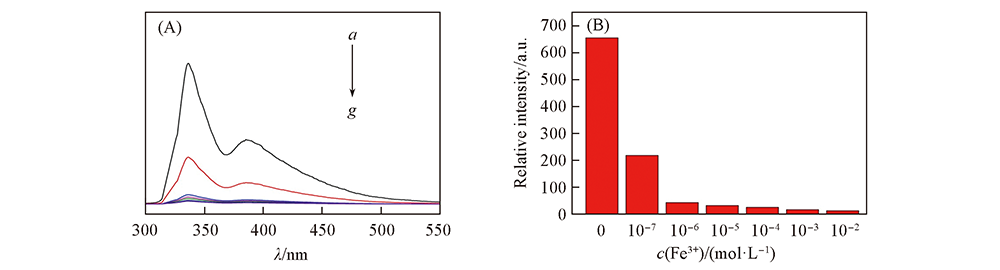
Fig.8 Emission spectra(A) and histogram of fluorescence intensity(B) of complex 1 in different concentrations of Fe3+ ions solution. c(Fe3+)/(mol·L-1): a. 0; b. 10-7; c. 10-6; d. 10-5; e. 10-4; f. 10-3; g. 10-2.
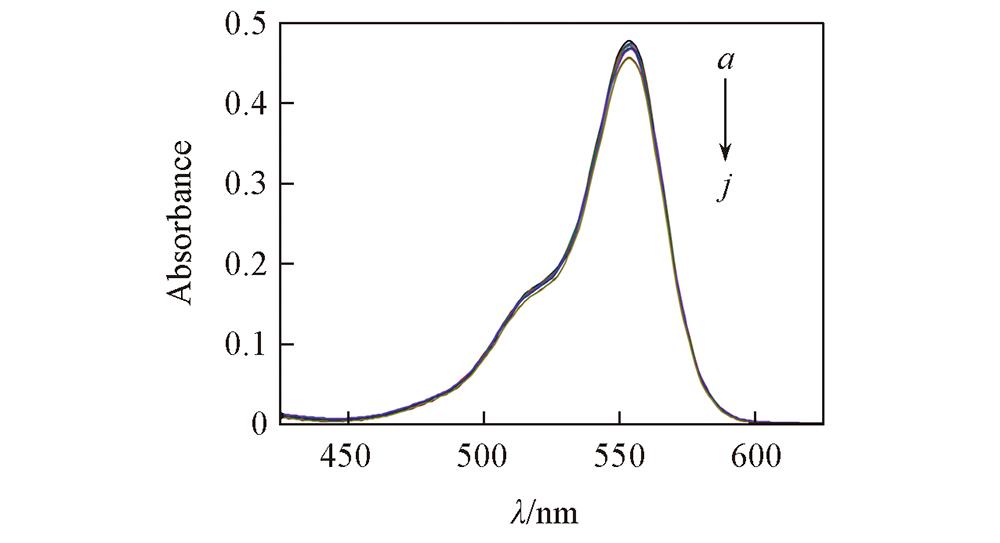
Fig.9 Time-dependent UV-Vis absorption spectra for degradation of RhB using complex 4 t/min: a. 0; b. 10; c. 20; d. 30; e. 40; f. 50; g. 60; h. 70; i. 80; j. 90.
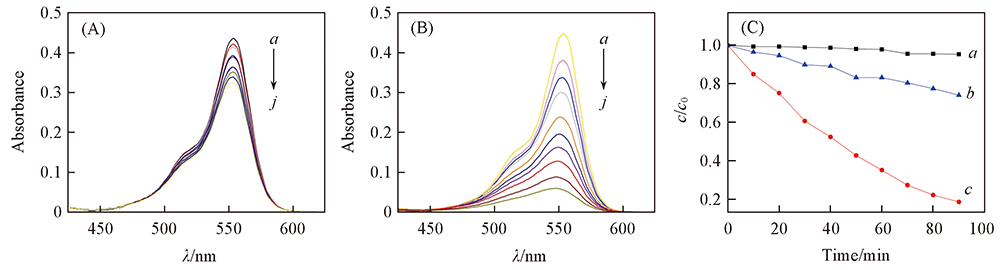
Fig.10 Time-dependent UV-Vis absorption spectra for degradation of RhB with H2O2(A), RhB with complex 4+H2O2(B) and the degradation rate of RhB under different conditions(C) (A), (B) t/min: a. 0; b. 10; c. 20; d. 30; e. 40; f. 50; g. 60; h. 70; i. 80; j. 90. (C) a. Complex 4+RhB; b. H2O2+RhB; c. complex 4+H2O2+RhB.
| [1] |
Zhou H. C., Long J. R., Yaghi O. M., Chem. Rev., 2012,112(2), 673— 674
doi: 10.1021/cr300014x URL |
| [2] |
Furukawa H., Cordova K. E., O’Keeffe M., Yaghi O. M., Science, 2013,341(6149), 1230444
doi: 10.1126/science.1230444 URL |
| [3] |
Kurmoo M., Chem. Soc. Rev., 2009,38(5), 1353— 1379
doi: 10.1039/b804757j URL |
| [4] |
Pal A., Chand S., Boquera J. C., Lloret F., Lin J. B., Pal S. C., Das M. C., Inorg. Chem., 2019,58(9), 6246— 6256
doi: 10.1021/acs.inorgchem.9b00471 URL |
| [5] |
Zhang Y. M., Yuan S., Day G., Wang X., Yang X. Y., Zhou H. C., Coord. Chem. Rev., 2018,354, 28— 45
doi: 10.1016/j.ccr.2017.06.007 URL |
| [6] | Wu S., Min H., Shi W., Cheng P., Adv. Mater., 2019,e1805871 |
| [7] |
Mason J. A., Oktawiec J., Taylor M. K., Hudson M. R., Rodriguez J., Bachman J. E., Gonzalez M. I., Cervellino A., Guagliardi A., Brown C. M., Llewellyn P. L., Masciocchi N., Long J. R., Nature, 2015,527(7578), 357— 361
doi: 10.1038/nature15732 URL |
| [8] | Li B., Wen H. M., Yu Y., Cui Y., Zhou W., Chen B., Qian G., Mater. Today Nano, 2018,2, 21— 49 |
| [9] | Jiao L., Wang Y., Jiang H. L., Xu Q., Adv. Mater., 2018,30(37), e1703663 |
| [10] |
Dhakshinamoorthy A., Li Z. H., Garcia H., Chem. Soc. Rev., 2018,47(22), 8134— 8172
doi: 10.1039/C8CS00256H URL |
| [11] |
Zhang P. F., Yang G. P., Li G. P., Yang F., Liu W. N., Li J. Y., Wang Y. Y., Inorg. Chem., 2019,58(20), 13969— 13978
doi: 10.1021/acs.inorgchem.9b01954 URL |
| [12] |
Wang W., Gong N., Yin H., Zhang B., Guo P., Liu B., Wang Y. Y., Inorg. Chem., 2019,58(15), 10295— 10303
doi: 10.1021/acs.inorgchem.9b01465 URL |
| [13] |
Ebrahim F. M., Nguyen T. N., Shyshkanov S., Gladysiak A., Favre P., Zacharia A., Itskos G., Dyson P. J., Stylianou K. C., J. Am. Chem. Soc., 2019,141(7), 3052— 3058
doi: 10.1021/jacs.8b11907 URL |
| [14] |
Guo F., Inorg. Chem. Commun., 2019,102, 108— 112
doi: 10.1016/j.inoche.2019.02.026 URL |
| [15] | Li Y., Wei Z., Zhang Y., Guo Z., Chen D., Jia P., Chen P., Xing H ., ACS Sustainable Chemistry & Engineering, 2019,7(6), 6196— 6203 |
| [16] |
Tan L., Fan T., Xia T., Cui Y., Yang Y., Qian G ., J. Solid State Chem., 2019,272, 55— 61
doi: 10.1016/j.jssc.2019.01.027 URL |
| [17] |
Zhu X. D., Zhang K., Wang Y., Long W. W., Sa R. J., Liu T. F., Lu J., Inorg. Chem., 2018,57(3), 1060— 1065
doi: 10.1021/acs.inorgchem.7b02471 URL |
| [18] | Li Z., Li R., Li X., Chem. J. Chinese Universities, 2018,39(11), 2363— 2371 |
| ( 李铮, 李睿, 李夏 . 高等学校化学学报, 2018,39(11), 2363— 2371) | |
| [19] |
Xiao J., Liu J., Gao X., Ji G., Wang D., Liu Z ., Sens. Actuators B:Chem., 2018,269, 164— 172
doi: 10.1016/j.snb.2018.04.129 URL |
| [20] |
Yan X. L., Ma D. Y., Inorg. Chem. Commun., 2019,104, 31— 35
doi: 10.1016/j.inoche.2019.03.025 URL |
| [21] |
Sun Y. Q., Zhong J. C., Ding L., Chen Y. P., Dalton Trans., 2015,44(26), 11852— 11859
doi: 10.1039/C5DT01454A URL |
| [22] |
Zhang Y. Q., Blatov V. A., Lv X. X., Tang D. Y., Qian L. L., Li K., Li B. L., Acta Crystallogr. C: Struct. Chem., 2019,75(7), 960— 968
doi: 10.1107/S205322961900826X URL |
| [23] | Wang C., Liu X. M., Zhang M., Geng Y., Zhao L., Li Y. G., Su Z. M., ACS Sustainable Chemistry & Engineering, 2019,7(16), 14102— 14110 |
| [24] |
Munoz M., Garcia-Erce J. A., Remacha A. F., J. Clin. Pathol., 2011,64(4), 281— 286
doi: 10.1136/jcp.2010.079046 URL |
| [25] |
Zhang Q. Q., Ying G. G., Pan C. G., Liu Y. S., Zhao J. L., Environmental Science & Technology, 2015,49(11), 6772— 6782
doi: 10.1021/acs.est.5b00729 URL |
| [26] |
Liu W. N., Tong W. Q., Ma L. L., Wang Y., Wang J. M., Hou L., Wang Y. Y., Dalton Trans., 2019,48(22), 7786— 7793
doi: 10.1039/C9DT00933G URL |
| [27] |
Du Y., Yang H. Y., Shao C. Y., Liu J. W., Yan Y. T., Yu L. X., Zhu D. Q., Huang C. N., Yang L. R., J. Solid State Chem., 2019,277, 564— 574
doi: 10.1016/j.jssc.2019.07.012 URL |
| [28] |
Zhou Y., Yang Q., Zhang D., Gan N., Li Q., Cuan J ., Sens. Actuators B: Chem., 2018,262, 137— 143
doi: 10.1016/j.snb.2018.01.218 URL |
| [29] |
Wei F. H., Chen D., Liang Z., Zhao S. Q., Luo Y., RSC Adv., 2017,7(73), 46520— 46528
doi: 10.1039/C7RA09243A URL |
| [30] |
Zheng T. R., Qian L. L., Li M., Wang Z. X., Li K., Zhang Y. Q., Li B. L., Wu B., Dalton Trans., 2018,47(27), 9103— 9113
doi: 10.1039/C8DT01685B URL |
| [31] |
Xu Q. W., Wang Q. S., Li S. S., Li X., RSC Adv., 2019,9(29), 16305— 16312
doi: 10.1039/C9RA01496A URL |
| [32] | Sheldrick G. M., SHELXS-97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen, 1997 |
| [33] | Sheldrick G. M., SHELXL-97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, 1997 |
| [34] | Hou B. W., Li K., Chinese J. Inorg. Chem., 2017,33(6), 1007— 1014 |
| ( 候不唯, 李恺. 无机化学学报, 2017,33(6), 1007— 1014) |
| [1] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [2] | 林治, 彭志明, 贺韦清, 沈少华. 单原子与团簇光催化: 竞争与协同[J]. 高等学校化学学报, 2022, 43(9): 20220312. |
| [3] | 滕镇远, 张启涛, 苏陈良. 聚合物单原子光催化剂的载流子分离和表面反应机制[J]. 高等学校化学学报, 2022, 43(9): 20220325. |
| [4] | 夏雾, 任颖异, 刘京, 王锋. 壳聚糖包裹CdSe量子点组装体的水相可见光催化CO2还原[J]. 高等学校化学学报, 2022, 43(7): 20220192. |
| [5] | 赵盈喆, 张建玲. 金属-有机框架基材料在二氧化碳光催化转化中的应用[J]. 高等学校化学学报, 2022, 43(7): 20220223. |
| [6] | 邱丽琪, 姚向阳, 何良年. 可见光驱动丰产金属卟啉类配合物催化的二氧化碳选择性还原反应[J]. 高等学校化学学报, 2022, 43(7): 20220064. |
| [7] | 龚妍熹, 王建兵, 柴歩瑜, 韩元春, 马云飞, 贾超敏. 钾掺杂g-C3N4薄膜光阳极的制备及光电催化氧化降解水中双氯芬酸钠性能[J]. 高等学校化学学报, 2022, 43(6): 20220005. |
| [8] | 刘晓磊, 陆永强, 游淇, 刘国辉, 姚伟, 胡日茗, 闫纪宪, 崔玉, 杨小凤, 孙国新, 蒋绪川. 基于3-羟基沙利度胺的比率型荧光探针对过氧化氢的检测[J]. 高等学校化学学报, 2022, 43(6): 20220070. |
| [9] | 蒋小康, 周琦, 周恒为. Gd2ZnTiO6∶Dy3+, Eu3+单基质白光荧光粉的制备与发光性能[J]. 高等学校化学学报, 2022, 43(6): 20220029. |
| [10] | 王广琦, 毕艺洋, 王嘉博, 石洪飞, 刘群, 张钰. 非贵金属三元复合Ni(PO3)2-Ni2P/CdS NPs异质结的构建及可见光高效催化产氢性能[J]. 高等学校化学学报, 2022, 43(6): 20220050. |
| [11] | 施耐克, 张娅, SANSON Andrea, 王蕾, 陈骏. Zn(NCN)单轴的负热膨胀性及机理研究[J]. 高等学校化学学报, 2022, 43(6): 20220124. |
| [12] | 宋颖颖, 黄琳, 李庆森, 陈立妙. CuO/BiVO4光催化剂的制备及光催化CO2还原性能[J]. 高等学校化学学报, 2022, 43(6): 20220126. |
| [13] | 王君旸, 刘争, 张茜, 孙春燕, 李红霞. DNA银纳米簇在功能核酸荧光生物传感器中的应用[J]. 高等学校化学学报, 2022, 43(6): 20220010. |
| [14] | 鲁聪, 李振华, 刘金露, 华佳, 李光华, 施展, 冯守华. 一种新的镧系金属有机骨架材料的合成、 结构及荧光检测性质[J]. 高等学校化学学报, 2022, 43(6): 20220037. |
| [15] | 陶雨, 欧鸿辉, 雷永鹏, 熊禹. 单原子催化剂在光催化二氧化碳还原中的研究进展[J]. 高等学校化学学报, 2022, 43(5): 20220143. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||