

Chem. J. Chinese Universities ›› 2020, Vol. 41 ›› Issue (10): 2230.doi: 10.7503/cjcu20200383
• Article • Previous Articles Next Articles
QIN Wenbing, LIN Weifeng, LI Xin, XIONG Wei, LIU Guokai( )
)
Received:2020-06-23
Online:2020-10-10
Published:2020-10-08
Contact:
LIU Guokai
E-mail:gkliu@szu.edu.cn
Supported by:CLC Number:
TrendMD:
QIN Wenbing, LIN Weifeng, LI Xin, XIONG Wei, LIU Guokai. Difluoromethylation of Dicyanoalkylidenes by Electrophilic S-(Difluoromethyl)sulfonium Salt: Efficient Construction of Difluoromethylated All-carbon-substituted Centers†[J]. Chem. J. Chinese Universities, 2020, 41(10): 2230.
| Compd. | Appearance | m.p./℃(ref.) | MSa(calcd.), m/z |
|---|---|---|---|
| 2a | White solid | 115.1—117.5(115.3—117.8)[ | 194(194) |
| 2b | Yellow solid | — | 225.0(225.1) |
| 2c | Light yellow solid | — | 225.0(225.1) |
| 2d | White solid | — | 225.0(225.1) |
| 2e | Brown solid | — | 255.0(255.1) |
| 2f | White solid | — | 272.9(273.0), 275.0(275.0)b |
| 2g | White solid | — | 208(208) |
| 2h | Off?white solid | 171.9—173.8(172.9—174.0)[ | 208(208) |
| 2i | Colorless oil | — | 146(146) |
| 2j | Yellow solid | 121.0—123.0(120.9—123.2)[ | 274(274), 276(276)b |
| 2k | Yellow solid | 122.6—125.1(122.2—125.6)[ | 212(212) |
| 2m | White solid | 157.1—159.5(157.2—159.3)[ | 180(180) |
| 2n | White solid | 70.2—71.9(70.2—71.9)[ | 194(194) |
| 2o | White solid | — | 194(194) |
| 4a | White solid | — | 168(168) |
| 4b | Light yellow solid | 93.2—94.(93.5—94.5)[ | 246(246), 248(248)b |
| 4c | White solid | 113.2—115.1(113—115)[ | 260(260), 262(262)b |
| 4d | White solid | — | 196(196) |
| 4e | White solid | — | 210(210) |
| 4f | White solid | 84.5—86.8(85.4—88.9)[ | 244(244) |
| 4g | Yellow solid | 130.2—131.9(130—132)[ | 268(268) |
| 4h | Light yellow solid | 114.0—116.3(114.1—116.1)[ | 218(218) |
| 4i | White solid | 86.8—88.3(87—88)[ | 174(174) |
| 4j | Colorless oil | — | 182(182) |
| Compd. | Appearance | m.p./℃(ref.) | MSa(calcd.), m/z |
|---|---|---|---|
| 2a | White solid | 115.1—117.5(115.3—117.8)[ | 194(194) |
| 2b | Yellow solid | — | 225.0(225.1) |
| 2c | Light yellow solid | — | 225.0(225.1) |
| 2d | White solid | — | 225.0(225.1) |
| 2e | Brown solid | — | 255.0(255.1) |
| 2f | White solid | — | 272.9(273.0), 275.0(275.0)b |
| 2g | White solid | — | 208(208) |
| 2h | Off?white solid | 171.9—173.8(172.9—174.0)[ | 208(208) |
| 2i | Colorless oil | — | 146(146) |
| 2j | Yellow solid | 121.0—123.0(120.9—123.2)[ | 274(274), 276(276)b |
| 2k | Yellow solid | 122.6—125.1(122.2—125.6)[ | 212(212) |
| 2m | White solid | 157.1—159.5(157.2—159.3)[ | 180(180) |
| 2n | White solid | 70.2—71.9(70.2—71.9)[ | 194(194) |
| 2o | White solid | — | 194(194) |
| 4a | White solid | — | 168(168) |
| 4b | Light yellow solid | 93.2—94.(93.5—94.5)[ | 246(246), 248(248)b |
| 4c | White solid | 113.2—115.1(113—115)[ | 260(260), 262(262)b |
| 4d | White solid | — | 196(196) |
| 4e | White solid | — | 210(210) |
| 4f | White solid | 84.5—86.8(85.4—88.9)[ | 244(244) |
| 4g | Yellow solid | 130.2—131.9(130—132)[ | 268(268) |
| 4h | Light yellow solid | 114.0—116.3(114.1—116.1)[ | 218(218) |
| 4i | White solid | 86.8—88.3(87—88)[ | 174(174) |
| 4j | Colorless oil | — | 182(182) |
| Compd. | Appearance | m.p./℃ | HRMS(calcd.)a, m/z |
|---|---|---|---|
| 3a | White solid | 95.0—97.1 | 277.1148(277.1147) |
| 3b | White solid | 59.5—62.0 | 275.0987(275.0990) |
| 3c | White solid | 47.0—49.1 | 275.0987(275.0990) |
| 3d | White solid | 79.0—81.2 | 307.1248(307.1253) |
| 3e | White solid | 124.2—126.3 | 305.1098(305.1096) |
| 3f | White solid | 110.2—112.4 | 355.0246(355.0252) |
| 3g | White solid | 84.0—85.6 | 291.1308(291.1303) |
| 3h | White solid | 66.1—67.8 | 291.1306(291.1303) |
| 3i | Colorless oil | — | 229.1147(229.1147) |
| 3j | White solid | 135.0—137.2 | 357.0044(357.0045) |
| 3k | Light yellow solid | 97.1—99.8 | 295.0714(295.0711) |
| 3l | Light yellow solid | 144.7—146.8 | 313.0614(313.0617) |
| 3m | White solid | 85.7—88.0 | 263.0987(263.0990) |
| 3n | White solid | 100.7—102.4 | 277.1144(277.1147) |
| 3o | White solid | 76.5—78.6 | 277.1145(277.1147) |
| 5a | White solid | 37.3—39.0 | 251.0987(251.0990) |
| 5b | White solid | 53.0—55.1 | 329.0096(329.0096) |
| 5c | White solid | 91.7—94.0 | 343.0246(343.0252) |
| 5d | Colorless oil | — | 279.1315(279.1303) |
| 5e | Colorless oil | — | 293.1458(293.1460) |
| 5f | White solid | 81.3—82.4 | 327.1300(327.1303) |
| 5g | White solid | 115.3—117.2 | 351.1301(351.1303) |
| 5h | White solid | 86.8—88.0 | 301.1144(301.1147) |
| 5i | Colorless oil | — | 257.0551(257.0555) |
| 5j | White solid | 37.0—39.3 | 265.1148(265.1147) |
| Compd. | Appearance | m.p./℃ | HRMS(calcd.)a, m/z |
|---|---|---|---|
| 3a | White solid | 95.0—97.1 | 277.1148(277.1147) |
| 3b | White solid | 59.5—62.0 | 275.0987(275.0990) |
| 3c | White solid | 47.0—49.1 | 275.0987(275.0990) |
| 3d | White solid | 79.0—81.2 | 307.1248(307.1253) |
| 3e | White solid | 124.2—126.3 | 305.1098(305.1096) |
| 3f | White solid | 110.2—112.4 | 355.0246(355.0252) |
| 3g | White solid | 84.0—85.6 | 291.1308(291.1303) |
| 3h | White solid | 66.1—67.8 | 291.1306(291.1303) |
| 3i | Colorless oil | — | 229.1147(229.1147) |
| 3j | White solid | 135.0—137.2 | 357.0044(357.0045) |
| 3k | Light yellow solid | 97.1—99.8 | 295.0714(295.0711) |
| 3l | Light yellow solid | 144.7—146.8 | 313.0614(313.0617) |
| 3m | White solid | 85.7—88.0 | 263.0987(263.0990) |
| 3n | White solid | 100.7—102.4 | 277.1144(277.1147) |
| 3o | White solid | 76.5—78.6 | 277.1145(277.1147) |
| 5a | White solid | 37.3—39.0 | 251.0987(251.0990) |
| 5b | White solid | 53.0—55.1 | 329.0096(329.0096) |
| 5c | White solid | 91.7—94.0 | 343.0246(343.0252) |
| 5d | Colorless oil | — | 279.1315(279.1303) |
| 5e | Colorless oil | — | 293.1458(293.1460) |
| 5f | White solid | 81.3—82.4 | 327.1300(327.1303) |
| 5g | White solid | 115.3—117.2 | 351.1301(351.1303) |
| 5h | White solid | 86.8—88.0 | 301.1144(301.1147) |
| 5i | Colorless oil | — | 257.0551(257.0555) |
| 5j | White solid | 37.0—39.3 | 265.1148(265.1147) |
| Compd. | 1H NMR(500 MHZ, CDCl3), δ | 13C NMR(126 MHZ, CDCl3), δ | 19F NMR(471 MHZ, CDCl3), δ |
|---|---|---|---|
| 3a | 7.57—7.52(m, 1H), 7.32—7.26(m, 3H), 6.86(t, J=4.9 Hz, 1H), 6.39(t, J=54.3 Hz, 1H), 2.84—2.77(m, 2H), 2.51—2.44(m, 2H) | 137.2, 136.5, 129.1, 129.0, 128.5, 127.0, 123.2, 122.5, 110.1(t, J=3.0 Hz), 109.9(t, J=259.3 Hz),46.8(t, J=24.7 Hz), 27.3, 23.5 | -119.1(d, J=54.4 Hz) |
| 3b | 7.26(t, J=8.1 Hz, 1H), 7.18(d, J=7.9 Hz, 1H), 6.92(d, J=8.2 Hz, 1H), 6.86(t, J=4.9 Hz, 1H), 6.40(t, J=54.4 Hz, 1H), 3.86(s, 3H), 2.79(t, J=8.2 Hz, 2H), 2.41(td, J=8.2, 5.0 Hz, 2H) | 156.8, 136.7, 129.4, 127.2, 125.5, 123.1, 115.0, 111.6, 110.2(t, J=2.8 Hz), 109.9(d, J=259.1 Hz), 55.7, 47.1(t, J=24.8 Hz), 23.0, 18.9 | -119.3(d, J=54.5 Hz) |
| Compd. | 1H NMR(500 MHZ, CDCl3), δ | 13C NMR(126 MHZ, CDCl3), δ | 19F NMR(471 MHZ, CDCl3), δ |
| 3c | 7.50(d, J=8.3 Hz, 1H), 6.82(d, J=8.1 Hz, 2H), 6.73(t, J=4.9 Hz, 1H), 6.39(t, J=54.3 Hz, 1H), 3.85(s, 3H), 2.79(t, J=8.0 Hz, 2H), 2.46(td, J=8.0, 4.9 Hz, 2H) | 159.8, 139.4, 133.5, 123.9, 122.9, 121.4, 115.3, 111.3, 110.2(t, J=2.9 Hz), 109.9(t, J=259.3 Hz), 55.4, 46.8(t, J=24.7 Hz), 27.9, 23.4 | -118.6(d, J=53.9 Hz) |
| 3d | 7.18(d, J=8.3 Hz, 1H), 7.13(d, J=1.7 Hz, 1H), 6.87(t, J=4.8 Hz, 1H), 6.83(dd, J=8.3, 2.1 Hz, 1H), 6.40(t, J=54.3 Hz, 1H), 3.82(s, 3H), 2.72(t, J=7.9 Hz, 2H), 2.47—2.41(m, 2H) | 158.5, 137.3, 129.7, 129.4, 129.1, 123.2, 113.5, 110.2(t, J=2.7 Hz), 110.0(t, J=259.4 Hz), 109.9, 55.6, 47.0(t, J=24.7 Hz), 26.5, 24.0 | -119.1(d, J=54.3 Hz) |
| 3e | 7.15(s, 1H), 6.77(s, 1H), 6.71(t, J=4.9 Hz, 1H), 6.32(t, J=54.3 Hz, 1H), 3.91(s, 3H), 3.89(s, 3H), 2.72(t, J=8.1 Hz, 2H), 2.43(td, J=8.1, 7.7, 5.0 Hz, 2H) | 149.2, 147.4, 133.8, 130.6, 122.9, 120.9, 112.1, 110.3(t, J=259.8 Hz), 110.2(t, J=2.8 Hz), 107.2, 56.3, 56.0, 46.7(t, J=24.7 Hz), 27.1, 23.6 | -118.6(d, J=54.4 Hz) |
| 3f | 7.65(s, 1H), 7.43(dd, J=8.0, 1.7 Hz, 1H), 7.14(d, J=8.0 Hz, 1H), 6.92(t, J=4.9 Hz, 1H), 6.35(t, J=54.1 Hz, 1H), 2.75(t, J=8.0 Hz, 2H), 2.47(td, J=8.0, 5.0 Hz, 2H) | 138.2, 136.2, 132.1, 130.6, 130.5, 125.8, 122.6, 120.6, 109.9(t, J=259.8 Hz), 109.9(d, J=4.7 Hz), 26.9, 23.5 | -118.8(d, J=54.1 Hz) |
| 3g | 7.57(d, J=7.5 Hz, 1H), 7.37—7.27(m, 3H), 6.82—6.74(m, 1H), 6.41(t, J=54.3 Hz, 1H), 3.00—2.92(m, 1H), 2.61(ddd, J=17.3, 6.6, 4.2 Hz, 1H), 2.32(dt, J=17.2, 6.1 Hz, 1H), 1.21(d, J=7.0 Hz, 3H) | 142.2, 135.0, 129.5, 127.8, 127.6, 126.8, 122.6, 122.5, 110.1(dd, J=51.3, 4.3 Hz), 109.8(t, J=259.2 Hz), 46.8(t, J=24.8 Hz), 31.5, 31.0, 19.5 | -118.2(d, J=54.1 Hz), -118.8(d, J=54.6 Hz), -118.9(d, J=54.4 Hz), -119.5(d, J=54.0 Hz) |
| 3h | 7.67(dd, J=5.6, 3.5 Hz, 1H), 7.35(dt, J=7.4, 3.7 Hz, 2H), 7.32—7.28(m, 1H), 7.00(t, J=7.6 Hz, 1H), 5.87(t, J=54.1 Hz, 1H), 2.53(t, J=7.1 Hz, 2H), 2.15(p, J=7.1 Hz, 2H), 1.98(q, J=7.3 Hz, 2H) | 141.9, 138.6, 132.8, 129.9, 129.6, 127.1, 126.4, 125.2, 110.9(t, J=258.9 Hz), 110.3(t, J=3.0 Hz),47.7(t, J=24.9 Hz), 33.6, 31.4, 24.7 | -120.8(d, J=53.9 Hz) |
| 3i | 6.41(s, 1H), 5.94(t, J=54.4 Hz, 1H), 2.22(qd, J=8.5, 7.4, 3.2 Hz, 4H), 1.80—1.73(m, 2H), 1.69—1.62(m, 2H) | 134.5, 123.2, 111.2(t, J=260.1 Hz), 109.7(t, J=3.1 Hz), 48.7(t, J=24.2 Hz), 25.7, 25.5, 22.2, 20.9 | -118.8(d, J=54.3 Hz) |
| 3j | 7.62(d, J=2.1 Hz, 1H), 7.42(dd, J=8.7, 2.2 Hz, 1H), 6.88(d, J=8.6 Hz, 1H), 6.59(t, J=4.1 Hz, 1H), 6.34(t, J=54.0 Hz, 1H), 4.89(d, J=4.1 Hz, 2H) | 153.7, 134.3, 129.4, 125.6, 120.2, 119.5, 119.2, 114.2, 109.61(t, J=260.6 Hz), 108.97(t, J=2.9 Hz), 64.6, 45.71(t, J=25.7 Hz) | -117.7(d, J=54.0 Hz) |
| 3k | 7.73—7.69(m, 1H), 7.52—7.46(m, 1H), 7.30—7.25(m, 2H), 6.87(t, J=6.1 Hz, 1H), 6.24(t, J=54.1 Hz, 1H), 3.39(d, J=6.1 Hz, 2H) | 135.8, 129.9, 129.5, 129.12, 128.4, 126.3, 124.8, 124.5, 110.1(t, J=260.1 Hz), 109.9(t, J=2.9 Hz), 47.1(t, J=25.2 Hz), 24.9 | -118.4(d, J=54.0 Hz) |
| 3l | 7.52—7.44(m, 2H), 7.04(td, J=8.3, 2.5 Hz, 1H), 6.94(t, J=6.1 Hz, 1H), 6.23(t, J=54.1 Hz, 1H), 3.38(d, J=6.1 Hz, 2H) | 160.8(d, J=247.0 Hz), 131.6, 130.9(d, J=3.3 Hz), 130.6(d, J=7.9 Hz), 129.8(d, J=7.4 Hz), 124.6, 117.0(d, J=21.8 Hz), 112.3(d, J=24.9 Hz), 110.2(t, J=260.5 Hz), 109.7(t, J=2.9 Hz), 108.1, 47.1(t, J=25.5 Hz), 25.1 | -113.1(m, F), -118.5(d, J=54.1 Hz, 2F) |
| 3m | 7.69(d, J=7.6 Hz, 1H), 7.60(d, J=7.3 Hz, 1H), 7.44(t, J=7.3 Hz, 1H), 7.42—7.38(m, 1H), 7.13(t, J=2.1 Hz, 1H), 6.31(t, J=54.2 Hz, 1H), 3.65—3.59(m, 2H) | 144.2, 139.1, 138.2, 128.3, 127.1, 126.9, 124.8, 120.0, 110.5(t, J=260.7 Hz), 109.2(t, J=3.0 Hz), 43.1(t, J=25.8 Hz), 38.3 | -117.8(d, J=54.1 Hz) |
| 3n | 7.46—7.41(m, 2H), 7.18(d, J=7.8 Hz, 1H), 7.07(t, J=1.9 Hz, 1H), 6.29(t, J=54.2 Hz, 1H), 3.53(s, 2H), 2.45(s, 3H). | 141.4, 139.4, 138.6, 137.1, 128.2, 128.0, 124.6, 120.6, 110.5(t, J=260.6 Hz), 109.4(t, J=2.7 Hz), 43.3(t, J=25.9 Hz), 38.1, 21.8 | -118.0(d, J=54.2 Hz) |
| Compd. | 1H NMR(500 MHZ, CDCl3), δ | 13C NMR(126 MHZ, CDCl3), δ | 19F NMR(471 MHZ, CDCl3), δ |
| 3o | 7.68(d, J=7.8 Hz, 1H), 7.45(d, J=7.4 Hz, 1H), 7.34(t, J=7.6 Hz, 1H), 7.26(t, J=7.8 Hz, 1H), 6.23(t, J=54.4 Hz, 1H), 3.54(s, 2H), 2.47(s, 3H) | 150.5, 140.8, 140.7, 126.9, 125.8, 123.9, 119.2, 118.1, 110.6(t, J=260.4 Hz), 109.7(t, J=3.2 Hz), 45.6, 41.7(t, J=26.1 Hz), 15.3 | -118.9(d, J=54.4 Hz) |
| 5a | 7.49—7.42(m, 3H), 7.41—7.37(m, 2H), 6.14(s, 1H), 5.86(t, J=53.9 Hz, 1H), 5.73(s, 1H) | 134.9, 134.4, 130.0, 129.2, 128.7, 125.4, 110.5(t, J=259.2 Hz), 109.5(t, J=2.9 Hz),48.2(t, J=25.1 Hz) | -120.4(d, J=54.3 Hz) |
| 5b | 7.59(d, J=8.4 Hz, 2H), 7.27(d, J=8.3 Hz, 2H), 6.15(s, 1H), 5.87(t, J=53.9 Hz, 1H), 5.75(s, 1H) | 134.0, 133.2, 132.4, 130.3, 126.0, 124.6, 110.5(t, J=259.6 Hz), 109.3(t, J=2.9 Hz), 48.0(t, J=25.3 Hz) | -120.5(d, J=53.8 Hz) |
| 5c | 7.66—7.60(m, 2H), 7.17—7.10(m, 2H), 6.65(q, J=6.8 Hz, 1H), 5.78(t, J=53.9 Hz, 1H), 1.63(d, J=6.8 Hz, 3H) | 136.2, 132.8, 131.6, 131.0, 125.7, 124.2, 110.6(t, J=258.8 Hz), 109.5(t, J=2.9 Hz), 48.9(t, J=25.0 Hz), 15.3 | -120.8(d, J=53.8 Hz) |
| 5d | 7.46(dd, J=5.1, 1.9 Hz, 3H), 7.23(dd, J=6.6, 2.8 Hz, 2H), 6.52(t, J=7.4 Hz, 1H), 5.76(t, J=54.0 Hz, 1H), 1.93(p, J=7.5 Hz, 2H), 0.99(t, J=7.5 Hz, 3H) | 141.9, 132.2, 129.9, 129.6, 129.4, 125.5, 110.6(t, J=258.4 Hz), 109.7(t, J=2.8 Hz), 49.2(t, J=24.9 Hz), 22.9, 13.2 | -121.7(d, J=53.8 Hz) |
| 5e | 7.48—7.44(m, 3H), 7.23(dd, J=6.6, 2.9 Hz, 2H), 6.30(d, J=10.1 Hz, 1H), 5.75(t, J=54.0 Hz, 1H), 2.15(dp, J=10.1, 6.6 Hz, 1H), 0.97(d, J=6.6 Hz, 6H) | 146.9, 132.3, 129.8, 129.6, 129.3, 123.9, 110.6(t, J=258.4 Hz), 109.7(t, J=2.9 Hz), 49.2(t, J=24.8 Hz), 29.0, 22.0, 21.7 | -121.8(d, J=54.0 Hz) |
| 5f | 7.57—7.50(m, 3H), 7.40—7.33(m, 3H), 7.26(t, J=7.3 Hz, 1H), 7.19(t, J=7.6 Hz, 2H), 6.97(d, J=7.6 Hz, 2H), 5.89(t, J=53.9 Hz, 1H) | 137.3, 133.2, 132.7, 130.1, 130.1, 130.0, 129.8, 129.3, 128.5, 125.4, 110.8(t, J=258.9 Hz),109.7(t, J=2.8 Hz), 50.2(t, J=25.0 Hz) | -121.5(d, J=54.0 Hz) |
| 5g | 8.76(s, 1H), 8.69(d, J=8.2 Hz, 1H), 7.99(d, J=8.2 Hz, 1H), 7.96(d, J=7.8 Hz, 1H), 7.87(d, J=8.8 Hz, 1H), 7.79(d, J=8.8 Hz, 1H), 7.74(t, J=7.6 Hz, 1H), 7.69(t, J=7.0 Hz, 1H), 7.63(dd, J=8.2, 1.5 Hz, 2H), 6.31(s, 1H), 5.95(t, J=53.8 Hz, 1H), 5.92(s, 1H) | 135.2, 132.6, 132.4, 132.3, 130.3, 129.8, 129.6, 128.9, 128.8, 127.5, 127.3, 126.3, 126.1, 125.8, 123.3, 122.6, 110.7(t, J=259.3 Hz), 109.7(t, J=2.9 Hz), 48.5(t, J=25.1 Hz) | -120.8(d, J=53.8 Hz) |
| 5h | 7.91(d, J=8.5 Hz, 1H), 7.90—7.87(m, 3H), 7.61—7.54(m, 2H), 7.47(dd, J=8.4, 1.9 Hz, 1H), 6.22(d, J=1.0 Hz, 1H), 5.90(t, J=53.9 Hz, 1H), 5.83(s, 1H) | 135.0, 133.5, 132.9, 131.7, 129.2, 128.5, 128.4, 127.8, 127.6, 127.3, 125.7, 125.5, 110.6(t, J=259.3 Hz), 109.6(t, J=2.9 Hz), 48.3(t, J=25.2 Hz) | -120.8(d, J=53.9 Hz) |
| 5i | 7.43—7.39(m, 1H), 7.35(d, J=3.6 Hz, 1H), 7.10(dd, J=5.2, 3.7 Hz, 1H), 6.09(s, 1H), 6.08(t, J=54.2 Hz, 1H), 5.95(d, J=1.5 Hz, 1H) | 135.0, 128.2, 128.0, 127.9, 127.8, 124.9, 110.4(t, J=260.0 Hz), 109.4(t, J=2.9 Hz), 48.1(d, J=25.3 Hz) | -119.5(d, J=54.5 Hz) |
| 5j | 7.47—7.33(m, 5H), 6.17(t, J=54.2 Hz, 1H), 5.67—5.53(m, 1H), 2.47(d, J=1.4 Hz, 3H) | 150.9, 140.6, 129.4, 128.8, 126.1, 111.3(t, J=259.1 Hz), 109.7(t, J=3.1 Hz), 109.2(t, J=2.7 Hz), 40.1(t, J=25.1 Hz), 18.5 | -120.5(d, J=54.0 Hz) |
| Compd. | 1H NMR(500 MHZ, CDCl3), δ | 13C NMR(126 MHZ, CDCl3), δ | 19F NMR(471 MHZ, CDCl3), δ |
|---|---|---|---|
| 3a | 7.57—7.52(m, 1H), 7.32—7.26(m, 3H), 6.86(t, J=4.9 Hz, 1H), 6.39(t, J=54.3 Hz, 1H), 2.84—2.77(m, 2H), 2.51—2.44(m, 2H) | 137.2, 136.5, 129.1, 129.0, 128.5, 127.0, 123.2, 122.5, 110.1(t, J=3.0 Hz), 109.9(t, J=259.3 Hz),46.8(t, J=24.7 Hz), 27.3, 23.5 | -119.1(d, J=54.4 Hz) |
| 3b | 7.26(t, J=8.1 Hz, 1H), 7.18(d, J=7.9 Hz, 1H), 6.92(d, J=8.2 Hz, 1H), 6.86(t, J=4.9 Hz, 1H), 6.40(t, J=54.4 Hz, 1H), 3.86(s, 3H), 2.79(t, J=8.2 Hz, 2H), 2.41(td, J=8.2, 5.0 Hz, 2H) | 156.8, 136.7, 129.4, 127.2, 125.5, 123.1, 115.0, 111.6, 110.2(t, J=2.8 Hz), 109.9(d, J=259.1 Hz), 55.7, 47.1(t, J=24.8 Hz), 23.0, 18.9 | -119.3(d, J=54.5 Hz) |
| Compd. | 1H NMR(500 MHZ, CDCl3), δ | 13C NMR(126 MHZ, CDCl3), δ | 19F NMR(471 MHZ, CDCl3), δ |
| 3c | 7.50(d, J=8.3 Hz, 1H), 6.82(d, J=8.1 Hz, 2H), 6.73(t, J=4.9 Hz, 1H), 6.39(t, J=54.3 Hz, 1H), 3.85(s, 3H), 2.79(t, J=8.0 Hz, 2H), 2.46(td, J=8.0, 4.9 Hz, 2H) | 159.8, 139.4, 133.5, 123.9, 122.9, 121.4, 115.3, 111.3, 110.2(t, J=2.9 Hz), 109.9(t, J=259.3 Hz), 55.4, 46.8(t, J=24.7 Hz), 27.9, 23.4 | -118.6(d, J=53.9 Hz) |
| 3d | 7.18(d, J=8.3 Hz, 1H), 7.13(d, J=1.7 Hz, 1H), 6.87(t, J=4.8 Hz, 1H), 6.83(dd, J=8.3, 2.1 Hz, 1H), 6.40(t, J=54.3 Hz, 1H), 3.82(s, 3H), 2.72(t, J=7.9 Hz, 2H), 2.47—2.41(m, 2H) | 158.5, 137.3, 129.7, 129.4, 129.1, 123.2, 113.5, 110.2(t, J=2.7 Hz), 110.0(t, J=259.4 Hz), 109.9, 55.6, 47.0(t, J=24.7 Hz), 26.5, 24.0 | -119.1(d, J=54.3 Hz) |
| 3e | 7.15(s, 1H), 6.77(s, 1H), 6.71(t, J=4.9 Hz, 1H), 6.32(t, J=54.3 Hz, 1H), 3.91(s, 3H), 3.89(s, 3H), 2.72(t, J=8.1 Hz, 2H), 2.43(td, J=8.1, 7.7, 5.0 Hz, 2H) | 149.2, 147.4, 133.8, 130.6, 122.9, 120.9, 112.1, 110.3(t, J=259.8 Hz), 110.2(t, J=2.8 Hz), 107.2, 56.3, 56.0, 46.7(t, J=24.7 Hz), 27.1, 23.6 | -118.6(d, J=54.4 Hz) |
| 3f | 7.65(s, 1H), 7.43(dd, J=8.0, 1.7 Hz, 1H), 7.14(d, J=8.0 Hz, 1H), 6.92(t, J=4.9 Hz, 1H), 6.35(t, J=54.1 Hz, 1H), 2.75(t, J=8.0 Hz, 2H), 2.47(td, J=8.0, 5.0 Hz, 2H) | 138.2, 136.2, 132.1, 130.6, 130.5, 125.8, 122.6, 120.6, 109.9(t, J=259.8 Hz), 109.9(d, J=4.7 Hz), 26.9, 23.5 | -118.8(d, J=54.1 Hz) |
| 3g | 7.57(d, J=7.5 Hz, 1H), 7.37—7.27(m, 3H), 6.82—6.74(m, 1H), 6.41(t, J=54.3 Hz, 1H), 3.00—2.92(m, 1H), 2.61(ddd, J=17.3, 6.6, 4.2 Hz, 1H), 2.32(dt, J=17.2, 6.1 Hz, 1H), 1.21(d, J=7.0 Hz, 3H) | 142.2, 135.0, 129.5, 127.8, 127.6, 126.8, 122.6, 122.5, 110.1(dd, J=51.3, 4.3 Hz), 109.8(t, J=259.2 Hz), 46.8(t, J=24.8 Hz), 31.5, 31.0, 19.5 | -118.2(d, J=54.1 Hz), -118.8(d, J=54.6 Hz), -118.9(d, J=54.4 Hz), -119.5(d, J=54.0 Hz) |
| 3h | 7.67(dd, J=5.6, 3.5 Hz, 1H), 7.35(dt, J=7.4, 3.7 Hz, 2H), 7.32—7.28(m, 1H), 7.00(t, J=7.6 Hz, 1H), 5.87(t, J=54.1 Hz, 1H), 2.53(t, J=7.1 Hz, 2H), 2.15(p, J=7.1 Hz, 2H), 1.98(q, J=7.3 Hz, 2H) | 141.9, 138.6, 132.8, 129.9, 129.6, 127.1, 126.4, 125.2, 110.9(t, J=258.9 Hz), 110.3(t, J=3.0 Hz),47.7(t, J=24.9 Hz), 33.6, 31.4, 24.7 | -120.8(d, J=53.9 Hz) |
| 3i | 6.41(s, 1H), 5.94(t, J=54.4 Hz, 1H), 2.22(qd, J=8.5, 7.4, 3.2 Hz, 4H), 1.80—1.73(m, 2H), 1.69—1.62(m, 2H) | 134.5, 123.2, 111.2(t, J=260.1 Hz), 109.7(t, J=3.1 Hz), 48.7(t, J=24.2 Hz), 25.7, 25.5, 22.2, 20.9 | -118.8(d, J=54.3 Hz) |
| 3j | 7.62(d, J=2.1 Hz, 1H), 7.42(dd, J=8.7, 2.2 Hz, 1H), 6.88(d, J=8.6 Hz, 1H), 6.59(t, J=4.1 Hz, 1H), 6.34(t, J=54.0 Hz, 1H), 4.89(d, J=4.1 Hz, 2H) | 153.7, 134.3, 129.4, 125.6, 120.2, 119.5, 119.2, 114.2, 109.61(t, J=260.6 Hz), 108.97(t, J=2.9 Hz), 64.6, 45.71(t, J=25.7 Hz) | -117.7(d, J=54.0 Hz) |
| 3k | 7.73—7.69(m, 1H), 7.52—7.46(m, 1H), 7.30—7.25(m, 2H), 6.87(t, J=6.1 Hz, 1H), 6.24(t, J=54.1 Hz, 1H), 3.39(d, J=6.1 Hz, 2H) | 135.8, 129.9, 129.5, 129.12, 128.4, 126.3, 124.8, 124.5, 110.1(t, J=260.1 Hz), 109.9(t, J=2.9 Hz), 47.1(t, J=25.2 Hz), 24.9 | -118.4(d, J=54.0 Hz) |
| 3l | 7.52—7.44(m, 2H), 7.04(td, J=8.3, 2.5 Hz, 1H), 6.94(t, J=6.1 Hz, 1H), 6.23(t, J=54.1 Hz, 1H), 3.38(d, J=6.1 Hz, 2H) | 160.8(d, J=247.0 Hz), 131.6, 130.9(d, J=3.3 Hz), 130.6(d, J=7.9 Hz), 129.8(d, J=7.4 Hz), 124.6, 117.0(d, J=21.8 Hz), 112.3(d, J=24.9 Hz), 110.2(t, J=260.5 Hz), 109.7(t, J=2.9 Hz), 108.1, 47.1(t, J=25.5 Hz), 25.1 | -113.1(m, F), -118.5(d, J=54.1 Hz, 2F) |
| 3m | 7.69(d, J=7.6 Hz, 1H), 7.60(d, J=7.3 Hz, 1H), 7.44(t, J=7.3 Hz, 1H), 7.42—7.38(m, 1H), 7.13(t, J=2.1 Hz, 1H), 6.31(t, J=54.2 Hz, 1H), 3.65—3.59(m, 2H) | 144.2, 139.1, 138.2, 128.3, 127.1, 126.9, 124.8, 120.0, 110.5(t, J=260.7 Hz), 109.2(t, J=3.0 Hz), 43.1(t, J=25.8 Hz), 38.3 | -117.8(d, J=54.1 Hz) |
| 3n | 7.46—7.41(m, 2H), 7.18(d, J=7.8 Hz, 1H), 7.07(t, J=1.9 Hz, 1H), 6.29(t, J=54.2 Hz, 1H), 3.53(s, 2H), 2.45(s, 3H). | 141.4, 139.4, 138.6, 137.1, 128.2, 128.0, 124.6, 120.6, 110.5(t, J=260.6 Hz), 109.4(t, J=2.7 Hz), 43.3(t, J=25.9 Hz), 38.1, 21.8 | -118.0(d, J=54.2 Hz) |
| Compd. | 1H NMR(500 MHZ, CDCl3), δ | 13C NMR(126 MHZ, CDCl3), δ | 19F NMR(471 MHZ, CDCl3), δ |
| 3o | 7.68(d, J=7.8 Hz, 1H), 7.45(d, J=7.4 Hz, 1H), 7.34(t, J=7.6 Hz, 1H), 7.26(t, J=7.8 Hz, 1H), 6.23(t, J=54.4 Hz, 1H), 3.54(s, 2H), 2.47(s, 3H) | 150.5, 140.8, 140.7, 126.9, 125.8, 123.9, 119.2, 118.1, 110.6(t, J=260.4 Hz), 109.7(t, J=3.2 Hz), 45.6, 41.7(t, J=26.1 Hz), 15.3 | -118.9(d, J=54.4 Hz) |
| 5a | 7.49—7.42(m, 3H), 7.41—7.37(m, 2H), 6.14(s, 1H), 5.86(t, J=53.9 Hz, 1H), 5.73(s, 1H) | 134.9, 134.4, 130.0, 129.2, 128.7, 125.4, 110.5(t, J=259.2 Hz), 109.5(t, J=2.9 Hz),48.2(t, J=25.1 Hz) | -120.4(d, J=54.3 Hz) |
| 5b | 7.59(d, J=8.4 Hz, 2H), 7.27(d, J=8.3 Hz, 2H), 6.15(s, 1H), 5.87(t, J=53.9 Hz, 1H), 5.75(s, 1H) | 134.0, 133.2, 132.4, 130.3, 126.0, 124.6, 110.5(t, J=259.6 Hz), 109.3(t, J=2.9 Hz), 48.0(t, J=25.3 Hz) | -120.5(d, J=53.8 Hz) |
| 5c | 7.66—7.60(m, 2H), 7.17—7.10(m, 2H), 6.65(q, J=6.8 Hz, 1H), 5.78(t, J=53.9 Hz, 1H), 1.63(d, J=6.8 Hz, 3H) | 136.2, 132.8, 131.6, 131.0, 125.7, 124.2, 110.6(t, J=258.8 Hz), 109.5(t, J=2.9 Hz), 48.9(t, J=25.0 Hz), 15.3 | -120.8(d, J=53.8 Hz) |
| 5d | 7.46(dd, J=5.1, 1.9 Hz, 3H), 7.23(dd, J=6.6, 2.8 Hz, 2H), 6.52(t, J=7.4 Hz, 1H), 5.76(t, J=54.0 Hz, 1H), 1.93(p, J=7.5 Hz, 2H), 0.99(t, J=7.5 Hz, 3H) | 141.9, 132.2, 129.9, 129.6, 129.4, 125.5, 110.6(t, J=258.4 Hz), 109.7(t, J=2.8 Hz), 49.2(t, J=24.9 Hz), 22.9, 13.2 | -121.7(d, J=53.8 Hz) |
| 5e | 7.48—7.44(m, 3H), 7.23(dd, J=6.6, 2.9 Hz, 2H), 6.30(d, J=10.1 Hz, 1H), 5.75(t, J=54.0 Hz, 1H), 2.15(dp, J=10.1, 6.6 Hz, 1H), 0.97(d, J=6.6 Hz, 6H) | 146.9, 132.3, 129.8, 129.6, 129.3, 123.9, 110.6(t, J=258.4 Hz), 109.7(t, J=2.9 Hz), 49.2(t, J=24.8 Hz), 29.0, 22.0, 21.7 | -121.8(d, J=54.0 Hz) |
| 5f | 7.57—7.50(m, 3H), 7.40—7.33(m, 3H), 7.26(t, J=7.3 Hz, 1H), 7.19(t, J=7.6 Hz, 2H), 6.97(d, J=7.6 Hz, 2H), 5.89(t, J=53.9 Hz, 1H) | 137.3, 133.2, 132.7, 130.1, 130.1, 130.0, 129.8, 129.3, 128.5, 125.4, 110.8(t, J=258.9 Hz),109.7(t, J=2.8 Hz), 50.2(t, J=25.0 Hz) | -121.5(d, J=54.0 Hz) |
| 5g | 8.76(s, 1H), 8.69(d, J=8.2 Hz, 1H), 7.99(d, J=8.2 Hz, 1H), 7.96(d, J=7.8 Hz, 1H), 7.87(d, J=8.8 Hz, 1H), 7.79(d, J=8.8 Hz, 1H), 7.74(t, J=7.6 Hz, 1H), 7.69(t, J=7.0 Hz, 1H), 7.63(dd, J=8.2, 1.5 Hz, 2H), 6.31(s, 1H), 5.95(t, J=53.8 Hz, 1H), 5.92(s, 1H) | 135.2, 132.6, 132.4, 132.3, 130.3, 129.8, 129.6, 128.9, 128.8, 127.5, 127.3, 126.3, 126.1, 125.8, 123.3, 122.6, 110.7(t, J=259.3 Hz), 109.7(t, J=2.9 Hz), 48.5(t, J=25.1 Hz) | -120.8(d, J=53.8 Hz) |
| 5h | 7.91(d, J=8.5 Hz, 1H), 7.90—7.87(m, 3H), 7.61—7.54(m, 2H), 7.47(dd, J=8.4, 1.9 Hz, 1H), 6.22(d, J=1.0 Hz, 1H), 5.90(t, J=53.9 Hz, 1H), 5.83(s, 1H) | 135.0, 133.5, 132.9, 131.7, 129.2, 128.5, 128.4, 127.8, 127.6, 127.3, 125.7, 125.5, 110.6(t, J=259.3 Hz), 109.6(t, J=2.9 Hz), 48.3(t, J=25.2 Hz) | -120.8(d, J=53.9 Hz) |
| 5i | 7.43—7.39(m, 1H), 7.35(d, J=3.6 Hz, 1H), 7.10(dd, J=5.2, 3.7 Hz, 1H), 6.09(s, 1H), 6.08(t, J=54.2 Hz, 1H), 5.95(d, J=1.5 Hz, 1H) | 135.0, 128.2, 128.0, 127.9, 127.8, 124.9, 110.4(t, J=260.0 Hz), 109.4(t, J=2.9 Hz), 48.1(d, J=25.3 Hz) | -119.5(d, J=54.5 Hz) |
| 5j | 7.47—7.33(m, 5H), 6.17(t, J=54.2 Hz, 1H), 5.67—5.53(m, 1H), 2.47(d, J=1.4 Hz, 3H) | 150.9, 140.6, 129.4, 128.8, 126.1, 111.3(t, J=259.1 Hz), 109.7(t, J=3.1 Hz), 109.2(t, J=2.7 Hz), 40.1(t, J=25.1 Hz), 18.5 | -120.5(d, J=54.0 Hz) |
| Entry | Product | Yieldb(%) | Entry | Product | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | 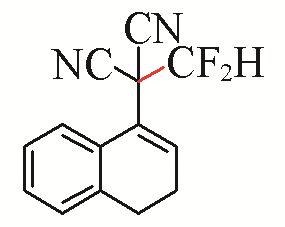 | 94 | 4 | 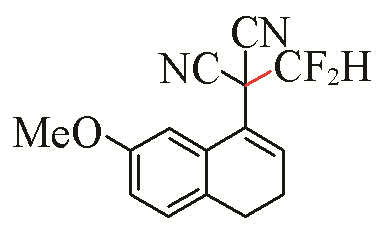 | 99 |
| 3a | 3d | ||||
| 2 | 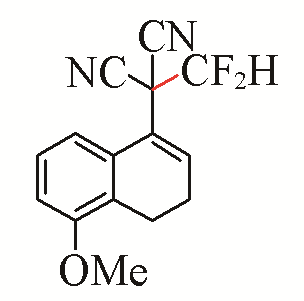 | 79 | 5 | 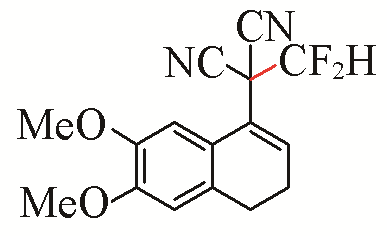 | 79 |
| 3b | 3e | ||||
| 3 | 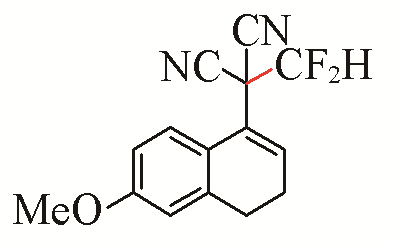 | 72 | 6 | 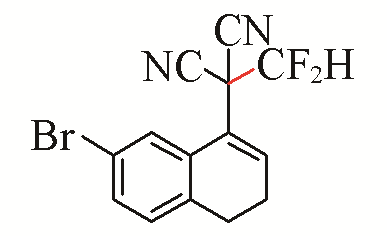 | 81 |
| 3c | 3f | ||||
| Entry | Product | Yieldb(%) | Entry | Product | Yieldb(%) |
| 7 | 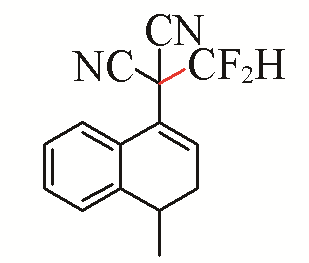 | 92 | 17 | 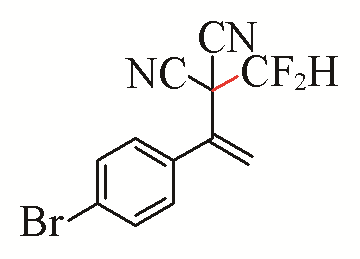 | 54 |
| 3g | 5b | ||||
| 8 | 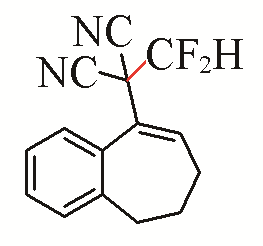 | 85 | 18 | 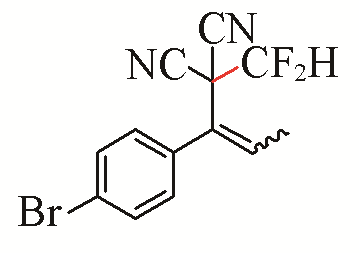 | 81 Z/E=4:1 |
| 3h | 5c | ||||
| 9 | 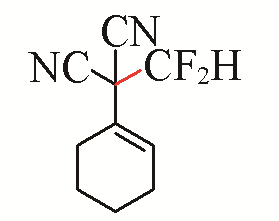 | 51 | 19 | 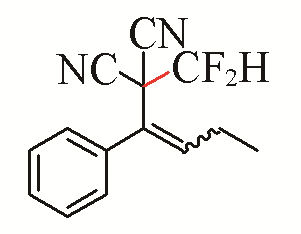 | 77 Z/E=9:1 |
| 3i | 5d | ||||
| 10 | 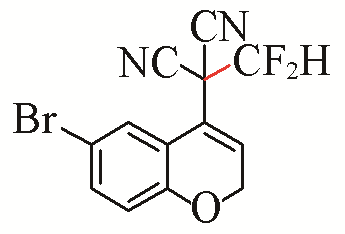 | 66 | 20 | 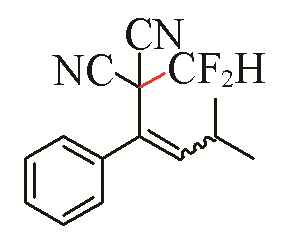 | 79 Z/E=8:1 |
| 3j | 5e | ||||
| 11 | 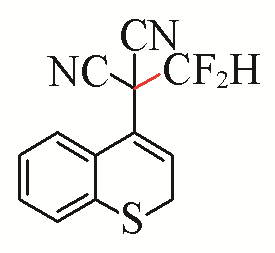 | 82 | 21 | 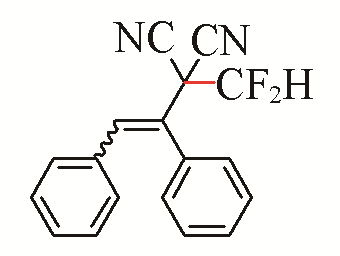 | 55 E/Z>99:1 |
| 3k | 5f | ||||
| 12 | 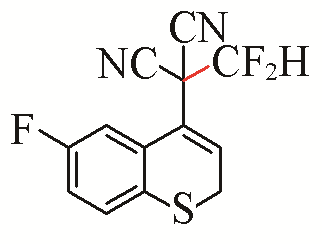 | 74 | 22 | 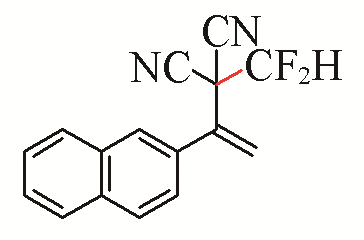 | 51 |
| 3l | 5g | ||||
| 13 | 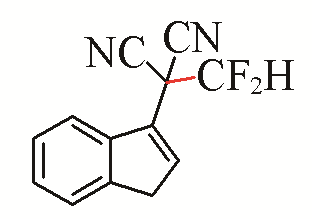 | 62 | 23 | 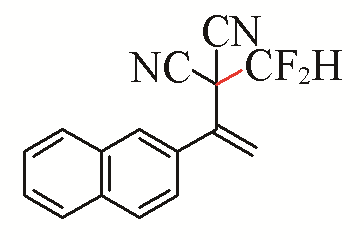 | 44 |
| 3m | 5h | ||||
| 14 | 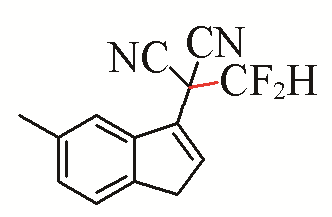 | 49 | 24 | 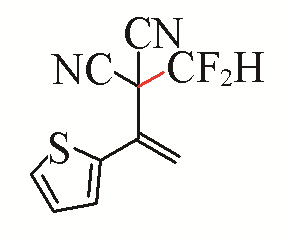 | 48 |
| 3n | 5i | ||||
| 15 | 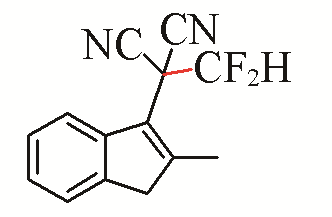 | 72 | 25 | 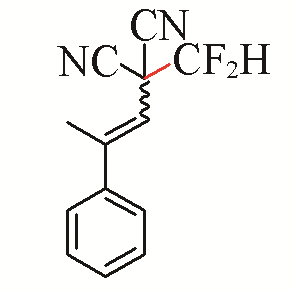 | 43 E/Z>99:1 |
| 3o | 5j | ||||
| 16 | 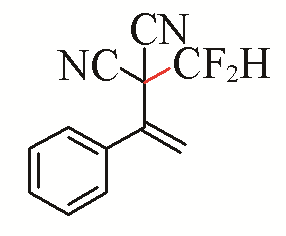 | 55 | |||
| 5a |
| Entry | Product | Yieldb(%) | Entry | Product | Yieldb(%) |
|---|---|---|---|---|---|
| 1 |  | 94 | 4 |  | 99 |
| 3a | 3d | ||||
| 2 |  | 79 | 5 |  | 79 |
| 3b | 3e | ||||
| 3 |  | 72 | 6 |  | 81 |
| 3c | 3f | ||||
| Entry | Product | Yieldb(%) | Entry | Product | Yieldb(%) |
| 7 |  | 92 | 17 |  | 54 |
| 3g | 5b | ||||
| 8 |  | 85 | 18 |  | 81 Z/E=4:1 |
| 3h | 5c | ||||
| 9 |  | 51 | 19 |  | 77 Z/E=9:1 |
| 3i | 5d | ||||
| 10 |  | 66 | 20 |  | 79 Z/E=8:1 |
| 3j | 5e | ||||
| 11 |  | 82 | 21 |  | 55 E/Z>99:1 |
| 3k | 5f | ||||
| 12 |  | 74 | 22 |  | 51 |
| 3l | 5g | ||||
| 13 |  | 62 | 23 |  | 44 |
| 3m | 5h | ||||
| 14 |  | 49 | 24 |  | 48 |
| 3n | 5i | ||||
| 15 |  | 72 | 25 |  | 43 E/Z>99:1 |
| 3o | 5j | ||||
| 16 |  | 55 | |||
| 5a |
| 1 | Smart B. E., Chem. Rev.,1996, 96, 1555—1556 |
| 2 | Furuya T., Kuttruff C., Ritter T., Curr. Opin. Drug Discovery Dev.,2008, 11, 803—819 |
| 3 | Ojima I., Fluorine in Medicinal Chemistry and Chemical Biology, Blackwell, Oxford, 2009,199—212 |
| 4 | Wang J., Sánchez⁃Roselló M., Aceña J. L., del Pozo C., Sorochinsky A. E., Fustero S., Soloshonok V. A., Liu H., Chem. Rev., 2014, 114, 2432—2506 |
| 5 | Bassetto M., Ferla S., Pertusati F., Future Med. Chem.,2015, 7, 527—546 |
| 6 | Goure W. F., Leschinsky K. L., Wratten S. J., Chupp J. P., J. Agric. Food Chem.,1991, 39, 981—986 |
| 7 | Pérez R. A., Sánchez⁃Brunete C., Miguel E., Tadeo J. L., J. Agric. Food Chem.,1998, 46, 1864—1869 |
| 8 | Kirsch P., Bremer B., Angew. Chem. Int. Ed.,2000, 39, 4216—4239 |
| 9 | Tasaka T., Takenaka S., Kabu K., Morita Y., Okamoto H., Ferroelectronics, 2002, 276, 83—92 |
| 10 | Boltalina O. V., Nakajima T., New Fluorinated Carbons: Fundamentals and Applications, Elsevier, Amsterdam, 2016, 305—323 |
| 11 | Prakash G. K. S., Mandal M., Schweizer S., Petasis N. A., Olah G. A., J. Org. Chem., 2002, 67, 3718—3723 |
| 12 | Narjes F., Koehler K. F., Koch U., Gerlach B., Colarusso S., Steink_hler C., Brunetti M., Altamura S., de Francesco R., Matassa V. G., Bioorg. Med. Chem. Lett., 2002, 12(4), 701—704 |
| 13 | Hu J., Zhang W., Wang F., Chem. Commun., 2009, 7465—7578 |
| 14 | Li Y., Hu J., Angew. Chem. Int. Ed., 2005, 44, 5882—5886 |
| 15 | Erickson J. A., McLoughlin J. I., J. Org. Chem., 1995, 60, 1626—1631 |
| 16 | Zafrani Y., Yeffet D., Sod⁃Moriah G., Berliner A., Amir D., Marciano D., Gershonov E., Saphier S., J. Med. Chem.,2017, 60, 797—804 |
| 17 | Prakash G. K. S., Weber C., Chacko S., Olah G. A., Org. Lett.,2007, 9(10), 1863—1866 |
| 18 | Prakash G. K. S., Zhang Z., Wang F., Ni C., Olah G. A., J. Fluorine Chem., 2011, 132(10), 792—798 |
| 19 | Zhang W., Wang F., Hu J., Org. Lett., 2009, 11(10), 2109—2112 |
| 20 | Zhu J., Liu Y., Shen Q., Angew. Chem. Int. Ed., 2016, 55, 9050—9054 |
| 21 | Lu S. L., Li X., Qin W. B., Liu J. J., Huang Y. Y., Wong H. N. C., Liu G. K, Org. Lett., 2018, 20, 6925—6929 |
| 22 | Liu G. K., Li X., Qin W. B., Peng X. S., Wong H. N. C., Zhang L., Zhang X., Chem. Commun., 2019, 55, 7446—7449 |
| 23 | Liu G. K., Qin W. B., Li X., Lin L. T., Wong H. N. C., J. Org. Chem., 2019, 84(24), 15948—15957 |
| 24 | Liu G. K., Li X., Qin W. B., Lin W. F., Lin L. T., Chen J. Y., Liu J. J., Chin. Chem. Lett., 2019, 30, 1515—1518 |
| 25 | Xue D., Chen Y. C., Cui X., Wang Q. W., Zhu J., Deng J. G., J. Org. Chem.,2005, 70, 3584—3591 |
| 26 | Liu G. K., Wang X., Lu X., Xu X. H., Tokunaga E., Shibata N., Chemistry Open, 2012, 1, 227—231 |
| 27 | Barnes D. M., Haight A. R., Hameury T., McLaughlin M. A., Mei J., Tedrow J. S., Riva Toma J. D., Tetrahedron, 2006, 62, 11311—11319 |
| 28 | Chen H., Zhao S., Cheng S., Dai X., Xu X., Yuan W., Zhang X., J. Heterocyclic Chem.,2019, 56, 1672—1683 |
| 29 | Aksu K., Özgeriş B., Tümer F., Org. Commun.,2019, 12, 38—42 |
| 30 | Matsnev A., Noritake S., Nomura Y., Tokunaga E., Nakamura S., Shibata N., Angew. Chem. Int. Ed., 2010, 49, 572—576 |
| [1] | GE Yicong, NIE Wanli, SUN Guofeng, CHEN Jiaxuan, TIAN Chong. Silver-catalyzed [5+1] Cyclization of 2-Vinylanilines with Benzisoxazoles [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220142. |
| [2] | WANG Mingzhi, ZHENG Yanping, WENG Weizheng. Catalytic Methane Combustion over CeO2 Supported PdO and Ce1‒x Pd x O2‒δ Species [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210816. |
| [3] | GAO Jing, HE Wentao, WANG Xinxin, XIANG Yushu, LONG Lijuan, QIN Shuhao. Preparation of DOPO Derivative Modified Carbon Nanotubes and Their Effect on Flame Retardancy of Polylactic Acid [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210670. |
| [4] | LI Xiaohui, WEI Aijia, MU Jinping, HE Rui, ZHANG Lihui, WANG Jun, LIU Zhenfa. Effects of SmPO4 Coatingon Electrochemical Performance of High-voltage LiNi0.5Mn1.5O4 Cathode Materials [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210546. |
| [5] | ZHANG Liling, LIU Liu, ZHENG Mingqiu, FANG Wenkai, LIU Da, TANG Hongwu. Dual Signal Detection of HPV16 DNA by CRISPR/Cas12a Biosensing System Based on Upconversion Luminescent Resonance Energy Transfer [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220412. |
| [6] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [7] | LI Dan, XIAO Liping, FAN Jie. Inorganic-based Surface Materials with Anti-SARS-CoV-2 Properties and Their Mechanisms of Action [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220301. |
| [8] | ZHOU Yonghui, HUANG Rujun, YAN Jianyang, LI Yajun, QIU Huanhuan, YANG Jinxuan, ZHENG Youxuan. Synthesis and Electroluminescence Properties of Two Iridium(Ⅲ) Complexes with Nitrogen Heterocycle Structures [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210415. |
| [9] | LIANG Yu, LIU Huan, GONG Lige, WANG Chunxiao, WANG Chunmei, YU Kai, ZHOU Baibin. Synthesis and Supercapacitor Properties of Biimidazole-modified {SiW12O40} Hybrid [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210556. |
| [10] | LI Haibo, XIAO Changfa, JIANG Long, HUANG Yun, DAN Yi. Copolymerization of Methyl Acrylate and 1-Octene Catalyzed by the Loaded Aluminum Chloride on MCM-41 Molecular Sieve [J]. Chem. J. Chinese Universities, 2021, 42(9): 2974. |
| [11] | MENG Fanwei, GAO Qi, YE Qing, LI Chenxi. Potassium Poisoning Mechanism of Cu-SAPO-18 Catalyst for Selective Catalytic Reduction of NOx by Ammonia [J]. Chem. J. Chinese Universities, 2021, 42(9): 2832. |
| [12] | LUO Qiangqiang, JIN Shaoqing, SUN Hongmin, YANG Weimin. Post-synthesis of Ti-MWW Zeolite via Titanium Incorporation in Liquid Acid Solution [J]. Chem. J. Chinese Universities, 2021, 42(9): 2742. |
| [13] | WANG Meiyin, HUANG Daofeng, CHEN Xin, ZHOU Junfu, REN Yuanhang, YE Lin, YUE Bin, HE Heyong. Liquid Phase Assembly of Mesoporous CsxH3-xPW12O40 and Characterization of Their Acidity [J]. Chem. J. Chinese Universities, 2021, 42(9): 2734. |
| [14] | HU Chuanchuan, PANG Jingxiang, HE Chuangchuang, LI Wei, SUN Shutao. Sc(OTf)3 Catalyzed 1,6-Conjugate Allylation of δ-CN p-QMs: Synthesis of Allyl Substituted Diarylacetonitrile Compounds [J]. Chem. J. Chinese Universities, 2021, 42(9): 2805. |
| [15] | LIU Huazheng, PAN Xiaoguang, LI Hua, WAN Renzhong, LIU Xigong. Na2CO3-catalyzed 1,6-Conjugate Addition of Trimethylsilyl Azide to δ-CF3-δ-Aryl-disubstituted Para-Quinone Methides: Efficient Construction of Diarylmethanes Bearing CF3- and N3-Substituted Quaternary Stereocenters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2772. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||