

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (1): 95.doi: 10.7503/cjcu20170458
• Physical Chemistry • Previous Articles Next Articles
HU Xianzhong, YU Qingbo*( ), LI Yanming
), LI Yanming
Received:2017-07-14
Online:2018-01-10
Published:2017-12-18
Contact:
YU Qingbo
E-mail:yuqb@smm.neu.edu.cn
Supported by:CLC Number:
TrendMD:
HU Xianzhong, YU Qingbo, LI Yanming. Skeletal and Reduced Mechanisms of Methane at O2/CO2 Atmosphere†[J]. Chem. J. Chinese Universities, 2018, 39(1): 95.
| Model | Temperature/K | Pressure/Pa | Molar ratio of CH4/O2 | Molar ratio of O2/CO2 | Number of sample points |
|---|---|---|---|---|---|
| PSR | 300, 400, 500, 600 | 1.0×101325 | 0.30, 0.35, 0.40, 0.45, 0.50, 0.55, 0.60, 0.65, 0.70 | 0.21/0.79, 0.25/0.75, 0.29/0.71, 0.33/0.67 | 144 |
| PREMIX | 300, 500, 700, 900 | 1.0×101325 | 0.30, 0.35, 0.40, 0.45, 0.50, 0.55, 0.60, 0.65, 0.70 | 0.21/0.79, 0.25/0.75, 0.29/0.71, 0.33/0.67 | 144 |
| PFR | 1000, 1200, 1400, 1600, 1800, 2000 | 0.8×101325, 1.0×101325, 1.2×101325 | 0.30, 0.35, 0.40, 0.45, 0.50, 0.55, 0.60, 0.65, 0.70 | 0.21/0.79, 0.25/0.75, 0.29/0.71, 0.33/0.67 | 648 |
| 0D | 1000, 1200, 1400, 1600, 1800, 2000 | 1.0×101325, 1.5×101325, 2.0×101325 | 0.30, 0.35, 0.40, 0.45, 0.50, 0.55, 0.60, 0.65, 0.70 | 0.21/0.79, 0.25/0.75, 0.29/0.71, 0.33/0.67 | 648 |
Table 1 Parameters of sample points
| Model | Temperature/K | Pressure/Pa | Molar ratio of CH4/O2 | Molar ratio of O2/CO2 | Number of sample points |
|---|---|---|---|---|---|
| PSR | 300, 400, 500, 600 | 1.0×101325 | 0.30, 0.35, 0.40, 0.45, 0.50, 0.55, 0.60, 0.65, 0.70 | 0.21/0.79, 0.25/0.75, 0.29/0.71, 0.33/0.67 | 144 |
| PREMIX | 300, 500, 700, 900 | 1.0×101325 | 0.30, 0.35, 0.40, 0.45, 0.50, 0.55, 0.60, 0.65, 0.70 | 0.21/0.79, 0.25/0.75, 0.29/0.71, 0.33/0.67 | 144 |
| PFR | 1000, 1200, 1400, 1600, 1800, 2000 | 0.8×101325, 1.0×101325, 1.2×101325 | 0.30, 0.35, 0.40, 0.45, 0.50, 0.55, 0.60, 0.65, 0.70 | 0.21/0.79, 0.25/0.75, 0.29/0.71, 0.33/0.67 | 648 |
| 0D | 1000, 1200, 1400, 1600, 1800, 2000 | 1.0×101325, 1.5×101325, 2.0×101325 | 0.30, 0.35, 0.40, 0.45, 0.50, 0.55, 0.60, 0.65, 0.70 | 0.21/0.79, 0.25/0.75, 0.29/0.71, 0.33/0.67 | 648 |
| Rank | PREMIX | Rank | PSR | Rank | PFR | Rank | 0D |
|---|---|---|---|---|---|---|---|
| 1 | C | 1 | CH3O | 1 | C | 1 | CH |
| 2 | CH2(S) | 2 | CH2(S) | 2 | CH | 2 | C |
| 3 | CH | 3 | CH | 3 | HCCO | 3 | HCCO |
| 4 | CH2OH | 4 | C | 4 | CH2OH | 4 | CH2(S) |
| 5 | HCCO | 5 | HCCO | 5 | CH2(S) | 5 | CH2OH |
| 6 | HCO | 6 | CH2OH | 6 | CH2 | 6 | CH2 |
| 7 | CH2 | 7 | HCO | 7 | HCO | 7 | CH3O |
| 8 | CH3O | 8 | H2O2 | 8 | CH3O | 8 | HCO |
| 9 | CH2CO | 9 | CH2 | 9 | H | 9 | CH2CO |
| 10 | HO2 | 10 | CH2CO | 10 | CH2CO | 10 | C2H4 |
Table 2 Order of quasi-steady-state components in four reactor models
| Rank | PREMIX | Rank | PSR | Rank | PFR | Rank | 0D |
|---|---|---|---|---|---|---|---|
| 1 | C | 1 | CH3O | 1 | C | 1 | CH |
| 2 | CH2(S) | 2 | CH2(S) | 2 | CH | 2 | C |
| 3 | CH | 3 | CH | 3 | HCCO | 3 | HCCO |
| 4 | CH2OH | 4 | C | 4 | CH2OH | 4 | CH2(S) |
| 5 | HCCO | 5 | HCCO | 5 | CH2(S) | 5 | CH2OH |
| 6 | HCO | 6 | CH2OH | 6 | CH2 | 6 | CH2 |
| 7 | CH2 | 7 | HCO | 7 | HCO | 7 | CH3O |
| 8 | CH3O | 8 | H2O2 | 8 | CH3O | 8 | HCO |
| 9 | CH2CO | 9 | CH2 | 9 | H | 9 | CH2CO |
| 10 | HO2 | 10 | CH2CO | 10 | CH2CO | 10 | C2H4 |
| No. | Global reaction | No. | Global reaction |
|---|---|---|---|
| 1 | 2O | 8 | CH3+O |
| 2 | H+O | 9 | CH4+O |
| 3 | H2+O | 10 | CO+O |
| 4 | HO2+O | 11 | CH2O+O |
| 5 | H2O2+O | 12 | C2H4+O |
| 6 | CH3+OH+O | 13 | CH2CO+OH+O |
| 7 | CH3+OH+O | 14 | 2CH3 |
Table 3 14-step global reaction mechanisms
| No. | Global reaction | No. | Global reaction |
|---|---|---|---|
| 1 | 2O | 8 | CH3+O |
| 2 | H+O | 9 | CH4+O |
| 3 | H2+O | 10 | CO+O |
| 4 | HO2+O | 11 | CH2O+O |
| 5 | H2O2+O | 12 | C2H4+O |
| 6 | CH3+OH+O | 13 | CH2CO+OH+O |
| 7 | CH3+OH+O | 14 | 2CH3 |
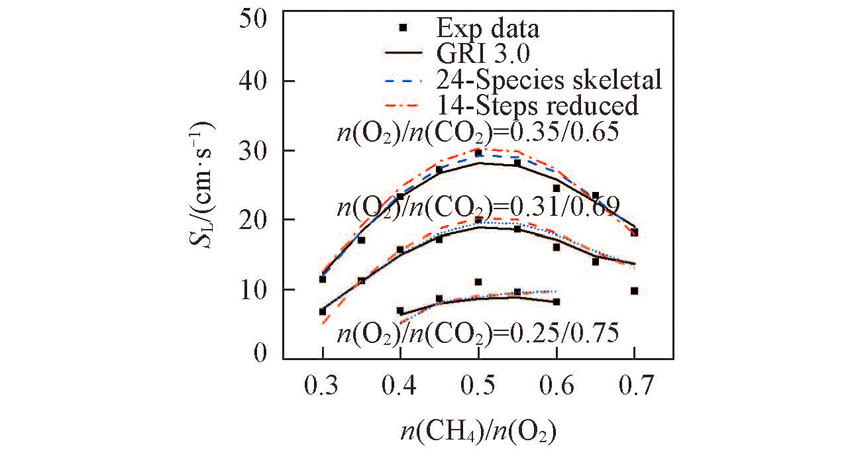
Fig.2 Laminar flame speeds of CH4 as a function of equivalence ratios with 24 species skeletal mechanism, 14 steps global reduced mechanism, detailed mechanism and experimental measurementsT=300 K, p=101315 Pa.
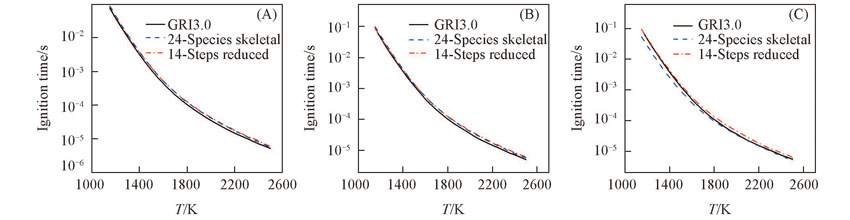
Fig.4 Predictions of ignition time of CH4/O2/CO2 mixtures with 24 species skeletal mechanism, 14 steps global reduced mechanism and detailed mechanismn(O2)/n(CO2)=0.24/0.76, T=300 K, p=101325 Pa. (A) n(CH4)/n(O2)=0.4; (B) n(CH4)/n(O2)=0.5;(C) n(CH4)/n(O2)=0.6.
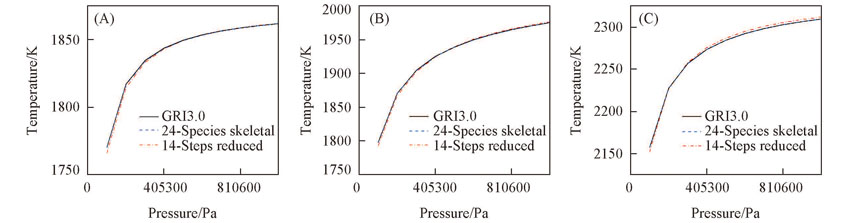
Fig.5 Predictions of flame temperature versus pressure of CH4/O2/CO2 mixtures with 24 species skeletal mechanism, 14 steps global reduced mechanism and detailed mechanism(A) n(CH4)/n(O2)=0.35, n(O2)/n(CO2)=0.31/0.69; (B) n(CH4)/n(O2)=0.50, n(O2)/n(CO2)=0.26/0.74;(C) n(CH4)/n(O2)=0.60, n(O2)/n(CO2)=0.35/0.65.
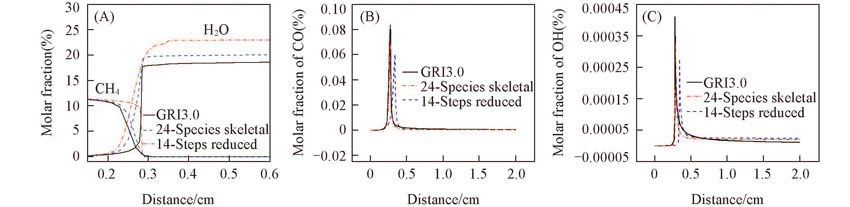
Fig.6 Predictions of flame structure of CH4/O2/CO2 mixtures with 24 species skeletal mechanism, 14 steps global reduced mechanism and detailed mechanism(A) CH4 and H2O; (B) CO; (C) OH. n(CH4)/n(O2)=0.50, n(O2)/n(CO2)=0.24/0.76, T=1200 K, p=101325 Pa.
| [1] | Wall T. F., Proc. Combust. Inst., 2007, 31(1), 31—47 |
| [2] | Habib M. A., Badr H. M., Ahmed S. F., Ben-Mansour R., Mezghani K., Imashuku S., la O' G. J., Shao-Horn Y., Mancini N. D., Mitsos A., Kirchen P., Ghoneim A. F., Int. J. Energ. Res., 2011, 35(9), 741—764 |
| [3] | Seepana S., Jayanti S., Fuel, 2012, 93(1), 52—58 |
| [4] | Liu C. Y., Chen G., Sipöcz N., Assadi M., Bai X. S., Appl. Energ., 2012, 89(1), 387—394 |
| [5] | Hu X. Z., Yu Q. B., Liu J. X., Sun N., Energy, 2014, 70(3), 626—634 |
| [6] | Chen L., Ghoniem A. F., Combust. Sci. Technol., 2014, 186(7), 829—848 |
| [7] | Leiser S., Schnell U., Scheffknecht G., 9th International Conference on Energy for a Clean Environment, Povoa de Varzim, Portugal, 2007 |
| [8] | Andersen J., Rasmussen C. L., Giselsson T., Glarborg P., Energy Fuels, 2009, 23(3), 1379—1389 |
| [9] | Frassoldati A., Cuoci A., Faravelli T., Ranzi E., Candusso C., Tolazzi D., Simplified Kinetic Schemes for Oxy-ful Combustion, The 1st International Conference on Sustainable Fossil Fuels for Future Energy, Rome, 2009 |
| [10] | Hjärtstam S., Normann F., Andersson K., Johnsson F., Ind. Eng. Chem. Res., 2012, 51(31), 10327—10337 |
| [11] | Turányi T., Reliab. Eng. Syst. Saf., 1997, 57(1), 41—48 |
| [12] | Turányi T., Bérces T., Vajda S., Int. J. Chem. Kinet., 1989, 21(2), 83—99 |
| [13] | Vajda S., Valko P., Turanyi T., Int. J. Chem. Kinet., 1985, 17(1), 55—81 |
| [14] | Esposito G., Chelliah H. K., Combust. Flame, 2011, 158(3), 477—489 |
| [15] | Sun W., Chen Z., Gou X., Ju Y., Combust. Flame, 2010, 157(7), 1298—1307 |
| [16] | Zhong B. J., Yao T., Wen F., Acta Phys. Chim. Sinica, 2014, 30(2), 210—216 |
| (钟北京, 姚通, 文斐.物理化学学报, 2014, 30(2), 210—216) | |
| [17] | Lu T. F., Law C. K., Combust. Flame, 2006, 144, 24—36 |
| [18] | Lu T. F., Law C. K., Proc. Combust. Inst., 2005, 30(1), 1333—1341 |
| [19] | Lu T., Law C. K., Proc. Combust. Inst., 2008, 154(1/2), 761—774 |
| [20] | Lu T., Law C. K., Proc. Combust. Inst., 2006, 146(3), 472—483 |
| [21] | Jiang Y., Qiu R., Acta Phys. Chim. Sinica, 2009, 25(5), 1019—1025 |
| (蒋勇, 邱榕.物理化学学报, 2009, 25(5), 1109—1025) | |
| [22] | Pepiotdesjardins P., Pitsch H., Combust. Flame, 2008, 154(1/2), 67—81 |
| [23] | Niemeyer K. E., Sung C. J., Raju M. P., Combust. Flame, 2010, 157(9), 1760—1770 |
| [24] | Løvs T., Nilsson D., Mauss F., Proc. Combust. Inst., 2000, 28(2), 1809—1815 |
| [25] | Turany T., Tomlin A. S., Pilling M. J., Int. J. Chem. Kinet., 1993, 97(1), 163—172 |
| [26] | Massias A., Diamantis D., Mastorakos E., Goussis D. A., Combust. Flame, 1999, 117(4), 685—708 |
| [27] | Maas U., Pope S. B., Combus. Flame, 1992, 88(3/4), 239—264 |
| [28] | Jiang Y., Qiu R., Chem. J. Chinese Universities, 2010, 31(2), 312—319 |
| (蒋勇, 邱榕.高等学校化学学报, 2010, 31(2), 312—319) | |
| [29] | Smith G.[last access: 09-30-2017] |
| [30] | Mazas A. N., Fiorina B., Lacoste D. A., Schuller T., Combust. Flame, 2011, 158(12), 2428—2440 |
| [31] | De Persis S., Foucher F., Pillier L., Osorio V., Gökalp I., Energy, 2013, 55(1523), 1055—1066 |
| [32] | Chen J. Y., Combust. Sci. Technol., 1988, 57(1—3), 89—94 |
| [33] | Liu J. W., Xiong S. W., Ma X. S., J. Prop. Tech., 2011, 32(4), 525—529 |
| (刘建文, 熊生伟, 马雪松.推进技术, 2011, 32(4), 525—529) |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [3] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| [4] | HU Chuanchuan, PANG Jingxiang, HE Chuangchuang, LI Wei, SUN Shutao. Sc(OTf)3 Catalyzed 1,6-Conjugate Allylation of δ-CN p-QMs: Synthesis of Allyl Substituted Diarylacetonitrile Compounds [J]. Chem. J. Chinese Universities, 2021, 42(9): 2805. |
| [5] | LIU Huazheng, PAN Xiaoguang, LI Hua, WAN Renzhong, LIU Xigong. Na2CO3-catalyzed 1,6-Conjugate Addition of Trimethylsilyl Azide to δ-CF3-δ-Aryl-disubstituted Para-Quinone Methides: Efficient Construction of Diarylmethanes Bearing CF3- and N3-Substituted Quaternary Stereocenters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2772. |
| [6] | LI Jian, YU Mingming, SUN Yuan, FENG Wenhua, FENG Zhaochi, WU Jianfeng. Effect of Aqueous Solution pH on the Oxidation of Methane to Methanol at Low Temperature [J]. Chem. J. Chinese Universities, 2021, 42(3): 776. |
| [7] | SHEN Wenjie. Molecular-fence Catalysts for Low-temperature Oxidation of Methane to Methanol [J]. Chem. J. Chinese Universities, 2020, 41(3): 375. |
| [8] | WU Hao, WANG Changzhen, QIU Yuan, TIAN Yani, ZHAO Yongxiang. Effect of Steric Confinement Dimension on Metal Site Anti-carbon Deposition Ability of Ni-SiO2 Catalysts in CH4-CO2 Reforming [J]. Chem. J. Chinese Universities, 2020, 41(11): 2488. |
| [9] | MA Jinyu, LIU Shuanglei, ZHANG Zhenguo, JIN Junyang, JIA Zhenhua. B(C6F5)3-Catalyzed Synthesis of 3,3′-Bisindolylmethane Derivatives [J]. Chem. J. Chinese Universities, 2020, 41(10): 2225. |
| [10] | RAN Shiya,SHEN Haifeng,LI Xiaonan,WANG Zilu,GUO Zhenghong,FANG Zhengping. Effect and Mechanism of Rare Earth Trifluoromethanesulfonate on the Thermal Stability of Polypropylene† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1333. |
| [11] | CHENG Wenmin,XIA Wensheng,WAN Huilin. Influence of Surface Reactivity of Lanthanum Oxide on the Activation of Methane and Oxygen† [J]. Chem. J. Chinese Universities, 2019, 40(5): 940. |
| [12] | CHEN Tao,FANG Lei,LUO Wei,MENG Yue,XUE Jilong,XIA Shengjie,NI Zheming. Theoretical Study of Dry Reforming of Methane Catalyzed by Bimetallic Alloy Cluster M12Ni(M=Pt, Sn, Cu) † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2135. |
| [13] | TONG Bo, ZHANG Zhongxiang, LIU Zhenjie, PENG Zhangquan, ZHOU Zhibin. Novel Electrolyte Containing Li[(CF3SO2)(n-C4F9SO2)N] for High Voltage LiNi0.5Mn1.5O4-based Cell† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1518. |
| [14] | DENG Guixian, LI Kongzhai, CHENG Xianming, GU Zhenhua, LU Chunqiang, ZHU Xing. Red Mud as Oxygen Carrier for Chemical Looping Combustion of Methane: Reactivity and Cyclic Performance† [J]. Chem. J. Chinese Universities, 2018, 39(2): 327. |
| [15] | BAI Yan, XIA Wensheng, WENG Weizheng, LIAN Mengshui, ZHAO Mingquan, WAN Huilin. Influence of Phosphate on La-based Catalysts for Oxidative Coupling of Methane† [J]. Chem. J. Chinese Universities, 2018, 39(2): 247. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||